Abstract
OBJECTIVE
Use of gastric bypass surgery is common and increasing. Over 40% of patients in diabetes remission after gastric bypass surgery may redevelop diabetes within 5 years. Metformin, the first-line drug for diabetes, has low bioavailability and slow, incomplete gastrointestinal absorption. We hypothesized that gastric bypass would further reduce the absorption and bioavailability of metformin.
RESEARCH DESIGN AND METHODS
In a nonblinded, single-dose pharmacokinetic study, 16 nondiabetic post–gastric bypass patients and 16 sex- and BMI-matched control subjects (mean age 40 years and BMI 39.2 kg/m2) were administered two 500-mg metformin tablets. Plasma metformin levels were sampled at 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 24 h. Metformin absorption, estimated by the area under the curve (AUC) of the plasma drug concentrations from time 0 to infinity (AUC0-∞), was the primary outcome, and metformin bioavailability, assessed by measuring 24-h urine metformin levels, was a secondary outcome.
RESULTS
Compared with control subjects, metformin AUC0–∞ was increased in gastric bypass subjects by 21% (13.7 vs. 11.4 μg/mL/h; mean difference 2.3 [95% CI −1.3 to 5.9]) and bioavailability was increased by 50% (41.8 vs. 27.8%; 14.0 [4.1–23.9]). Gastric bypass patients had significantly lower AUC glucose levels over 8 h compared with control subjects (35.8 vs. 41.7 μg/mL/h; 5.9 [3.1–8.8]), but this was likely a result of differences in baseline fasting glucose and not metformin absorption.
CONCLUSIONS
Metformin absorption and bioavailability seem to be higher after gastric bypass, and this may have implications on dosing and toxicity risk. Studies are needed to confirm these findings and delineate potential mechanisms.
Bariatric surgery currently is indicated in patients refractory to nonsurgical therapy with either severe obesity (BMI ≥40 kg/m2) or moderate obesity (BMI 35.0–39.9 kg/m2) and a major obesity-related comorbidity (1). The prevalence of moderate and severe obesity has increased fourfold in recent decades, and >5% of the total population in the U.S. and Canada currently meet criteria for surgical eligibility (2,3). The number of bariatric procedures performed globally has increased 70-fold in the past two decades, with nearly 350,000 procedures now performed annually (4). Because the effectiveness of nonsurgical treatments for obesity is widely considered to be limited, it is anticipated that the use of surgery will continue to rapidly increase (3).
Roux-en-Y gastric bypass (RYGB) comprises 40% of bariatric procedures (4). Gastric capacity is reduced by 95%, and bypass of the duodenum and proximal jejunum is performed. Because RYGB is well documented to lead to nutrient malabsorption, it is possible that clinically significant reductions in drug absorption also may occur after this procedure. However, this issue is poorly studied, and no controlled trials have been performed in RYGB patients (5).
Metformin hydrochloride currently is considered to be the preferred initial therapy for type 2 diabetes. Metformin possesses several characteristics that increase the potential for its malabsorption after gastric bypass surgery. The drug primarily is absorbed in the upper small intestine (6) but has a relatively low oral bioavailability that ranges between 29 and 60% (7,8). Furthermore, metformin absorption is the rate-limiting step in drug disposition because absorption is transporter dependent and saturatable, which causes bioavailability to diminish as dosage increases (7,9).
Over 40% of patients who initially develop remission of their diabetes after gastric bypass surgery may redevelop diabetes (10). Therefore, examining metformin absorption after gastric bypass surgery is of high clinical relevance. The purpose of this controlled study was to examine the single-dose pharmacokinetics, including absorption and bioavailability, of a standard-release preparation of metformin in RYGB subjects and matched control subjects. To our knowledge, this is the first controlled examination of metformin pharmacokinetics in post-RYGB subjects. Glycemic control over an 8-h period also was compared between groups to assess if any observed changes in bioavailability were concordant with pharmacodynamic response.
RESEARCH DESIGN AND METHODS
Sixteen post–gastric bypass patients and 16 sex- and BMI-matched (within 5 kg/m2) control subjects, aged 18–60 years, were recruited through local advertisements and from Edmonton Weight Wise, a joint medical and surgical regional obesity clinic. Informed consent was obtained prior to study enrollment, and ethics approval was granted by the University of Alberta Research Ethics Board. Surgical patients were ≥3 months after surgery and were free of major postoperative gastrointestinal complications (e.g., anastamotic leak or outlet obstruction). Patients currently receiving metformin or with contraindications to metformin treatment were excluded. Contraindications included allergy, history of lactic/metabolic acidosis, liver failure, baseline liver enzymes higher than threefold above the upper limit of normal, congestive heart failure, renal failure (glomerular filtration rate <60 mL/min), alcoholism, fatty liver disease, and acute illness. Pregnant or nursing mothers were excluded. Patients receiving furosemide or nifedipine were excluded because both drugs may increase metformin absorption by 15–20%.
All gastric bypass surgeries were performed in standardized fashion. The gastric pouch was formed using the pars flaccida technique. The anastamosis was conducted using a 25 French Orvil EEA device in an end-to-side fashion. Pouch volumes were 25–30 mL and the length of the Roux (bypass) limb was 90–110 cm.
End points
The primary end point was metformin absorption, estimated by the area under the curve (AUC) of the plasma drug concentration from time 0 extrapolated to infinity (AUC0–∞).
Secondary outcomes included the following:
Bioavailability of metformin, estimated from the amount of metformin excreted in the urine over 24 h;
AUC of metformin absorption from 0–24 h (AUC0–24 h);
Time to peak drug concentration (Tmax) and peak plasma drug concentration (Cmax); and
AUC of plasma glucose levels over the first 8 h of sampling (AUC0–8h).
Hypotheses and sample size calculation
We hypothesized that metformin absorption would be significantly reduced in RYGB subjects compared with control subjects. Assuming an AUC for a 1,000-mg metformin dose of 11.94 µg/h/mL (SD 2.71 µg/h/mL) (11) (α = 0.05, β = 0.9), we estimated that 13 patients would be required in each group to detect a 30% difference in absorption between groups. Sixteen patients were enrolled per group to account for variation in this estimate.
Pharmacokinetic and pharmacodynamic testing
Pharmacokinetic studies were performed in the clinical investigation unit of the University of Alberta Hospital. Baseline assessment included pregnancy testing on all female participants within 2 weeks of pharmacokinetic testing. Weight was measured using a calibrated scale to the nearest 0.1 kg, with the subject wearing indoor clothing with empty pockets and without shoes. BMI was calculated to the nearest 10th unit by dividing the weight in kilograms by the square of height in meters (kg/m2).
Subjects reported to the clinical investigation unit on the morning of their testing date. After intravenous catheter insertion, baseline fasting plasma metformin and glucose sampling were performed. Subjects then ingested two 500-mg metformin hydrochloride (glucophage) tablets at time 0. Twenty-four-hour urine collection for urinary metformin concentration also commenced at time 0. Plasma metformin and glucose sampling was performed 0.5, 1, 1.5, 2, 3, 4, 6, and 8 h after metformin ingestion. Standardized meals were administered at 2 and 6 h after drug administration, and a standardized snack was given 4 h after drug administration. Total caloric intake was 1,000 kcal (60% carbohydrates). Subjects were required to eat the entire meal. After the 8-h blood draw, the patient was discharged and returned the following morning for 24-h blood sampling and to turn in the remainder of the 24-h urine collection.
Samples were spun directly after collection and were immediately stored at −70°C. A validated high-pressure liquid chromatography assay was used to determine the plasma and urinary metformin concentrations (12). The lower limit of quantitation of the assay was 10 ng/mL. The concentration of potassium phosphate used in the mobile phase was 25 mM.
Data entry and statistical analyses
Paper-based standardized case report forms were populated, and data were entered into a Microsoft Excel spreadsheet using double-data entry. Analyses were performed using Microsoft Excel (version 2008; Microsoft), InStat (version 3.1a; GraphPad Software, San Diego, CA), and SAS (version 9.2; SAS Institute, Cary, NC). Noncompartmental analysis was used for calculation of pharmacokinetic parameters. The plasma metformin concentration (Cmax) and time at which Cmax occurred (Tmax) were determined directly from the data. The terminal elimination rate constant was estimated by applying linear regression to the log-transformed concentrations in the log-linear terminal portion of the concentration-versus-time curves. Terminal half-life was estimated as 0.693 divided by the terminal elimination rate constant. The AUC concentration-versus-time curves of metformin (up to 24 h after dose) and glucose (up to 8 h after dose) were determined using the linear trapezoidal rule. The AUC0–∞ of metformin concentrations was calculated by adding to the AUC0–24 h the last measured concentration divided by the elimination rate constant.
Because metformin is completely excreted unchanged in the urine, urinary recovery is indicative of the cumulative amount of systemically available drug. This allows estimation of bioavailability, calculated by dividing urinary recovery by total oral dose. Renal clearance was estimated by dividing urinary recovery by the AUC0–∞ of metformin concentrations. Between-group differences in the arithmetic means of continuous baseline variables were analyzed using a paired t test (if the normality assumption passed) or a Mann-Whitney test (if the normality assumption failed). A Fisher exact test was used for binary variables. Linear regression was performed to examine whether AUC0–∞ metformin levels independently predicted AUC0–8 glucose levels while controlling for baseline glucose and weight. The linearity assumption was verified using the runs test, and scatter and residual plots were used to verify model assumptions.
Two-tailed P values were considered significant at the 0.05 threshold.
RESULTS
Baseline characteristics
Mean age, BMI, and weight did not significantly differ between groups, although a 10.6-kg-higher weight was present in control subjects compared with gastric bypass subjects (104.0 vs. 114.6 kg; P = 0.3) (Table 1). Fasting glucose levels were significantly greater in control subjects (5.1 vs. 4.4 mmol/L; P = 0.0006), but HbA1c levels were virtually identical between groups (Table 1). LDL cholesterol was significantly higher in control subjects compared with gastric bypass subjects (3.0 vs. 2.4 mmol/L; P = 0.02). Although one gastric bypass subject had a preoperative history of diabetes treated with metformin, this condition was in remission postoperatively.
Table 1.
Baseline characteristics
| Variable | Gastric bypass subjects | Control subjects | P |
|---|---|---|---|
| n | 16 | 16 | |
| Age (years)* | 44.4 (10.0) | 43.5 (11.7) | 0.82 |
| Female sex† | 13 (82) | 13 (82) | NA‡ |
| BMI (kg/m2)* | 38.0 (7.9) | 40.5 (6.9) | 0.36 |
| Weight (kg)* | 104.0 (29.0) | 114.6 (26.1) | 0.30 |
| Preoperative BMI (kg/m2)* | 51.5 (10.3) | — | — |
| Preoperative weight (kg)* | 141.1 (35.9) | — | — |
| Time elapsed after bypass (months)* | 17 (13.5) | — | — |
| Creatinine (µmol/L)* | 62.9 (9.8) | 65.2 (11.8) | 0.56 |
| Creatinine clearance (mL/min)* | 91.3 (25.7) | 95.7 (25.4) | 0.63 |
| A1C (%)* | 5.5 (0.3) | 5.6 (0.6) | 0.28 |
| Fasting glucose (mmol/L)* | 4.4 (0.4) | 5.1 (0.6) | 0.0006 |
| AST (units/L)* | 22.6 (5.4) | 25.3 (6.6) | 0.22 |
| Total cholesterol (mmol/L)* | 3.96 (0.69) | 4.78 (1.08) | 0.02 |
| Triglycerides (mmol/L)* | 1.0 (0.33) | 1.5 (0.88) | 0.29§ |
| HDL cholesterol (mmol/L)* | 1.1 (0.25) | 1.1 (0.38) | 0.74 |
| LDL cholesterol (mmol/L)* | 2.4 (0.53) | 3.0 (1.02) | 0.03 |
| Hypertension† | 8 (50) | 7 (43.8) | 1.0 |
| Type 2 diabetes† | 1 (6.3)‖ | 0 (0) | 1.0 |
| Dyslipidemia† | 1 (6.3) | 4 (0.25) | 0.33 |
| Hypothyroidism† | 3 (0.38) | 2 (0.13) | 1.0 |
| Sleep apnea† | 7 (43.8) | 4 (0.25) | 0.46 |
| Gastrointestinal reflux† | 1 (6.3) | 4 (0.25) | 0.33 |
*Data are mean (SD).
†Data are n (%).
‡Subjects were sex-matched.
§Mann-Whitney U statistic.
‖Diabetes in remission after RYGB.
Absorption and bioavailability
Urine excretion was available in 15 gastric bypass patients and 16 control subjects because one urine sample was accidently discarded prior to analysis.
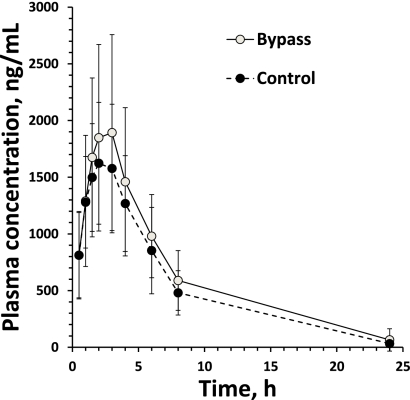
Compared with control subjects, gastric bypass subjects exhibited nonsignificant 21% increases in the AUC0–∞ compared with control subjects (13.7 vs. 11.4 μg/mL/h; mean difference 2.3 [95% CI −1.3 to 5.9]) (Fig. 1 and Table 2). However, gastric bypass subjects demonstrated 50% higher bioavailability compared with control subjects (41.8 vs. 27.8%; 14.0 [4.1–23.9]). These differences remained statistically significant after correction for baseline body weight (Table 2). There was little correlation between AUC0–∞ metformin levels and body weight (Pearson r = −0.3; P = 0.1). Ninety-eight percent of renal excretion took place within the first 24 h.
Figure 1.
Plasma concentration time curve.
Table 2.
Pharmacokinetic and pharmacodynamic outcomes
| Variable | Gastric bypass subjects | Control subjects | Mean difference (95% CI) | P |
|---|---|---|---|---|
| n | 16 | 16 | ||
| Pharmacokinetic outcomes | ||||
| AUC0–∞ (µg/h/mL) | 13.7 (6.0) | 11.4 (3.6) | 2.3 (−1.3 to 5.9) | 0.20 |
| Bioavailability (%) | 41.8 (16.2)* | 27.8 (10.4) | 14.0 (4.1–23.9) | 0.007 |
| AUC0–24 h (µg/h/mL) | 13.4 (5.7) | 11.1 (3.6) | 2.2 (−1.3 to 5.6) | 0.20 |
| Cmax (µg/mL) | 2.0 (0.86) | 1.8 (0.61) | 0.2 (−0.3 to 0.8) | 0.32 |
| Tmax (h) | 3.0 (1.5–3.0)† | 3.0 (1.5–3.0)† | 0 (0)† | 0.89† |
| Half-life (h) | 3.9 (0.74) | 4.0 (0.87) | −0.1 (−0.7 to 0.5) | 0.66 |
| Urinary recovery (0–24 h) (mg) | 326 (126) | 217 (81) | 140 (41–239) | 0.007 |
| Weight-normalized urinary recovery (0–24 h; mg/kg) | 3.1 (1.2)* | 2.0 (0.78) | 1.5 (0.5–2.5) | 0.003 |
| Renal clearance (mL/min) | 461 (199)* | 337 (131) | 125 (1.5–248) | 0.047 |
| Weight-normalized renal clearance (mL/min/kg) | 4.3 (1.6)* | 3.0 (1.0) | 1.3 (0.3–2.3) | 0.009 |
| Weight-normalized volume of distribution (L/kg) | 1.4 (0.4) | 1.0 (0.4) | 0.4 (0.1–0.7) | 0.02 |
| Pharmacodynamic outcome | ||||
| AUC glucose0–8 h (mmol/mL/h) | 35.8 (3.7) | 41.7 (4.1) | 5.9 (3.1–8.8) | 0.0002 |
Data are means (SD), unless otherwise indicated.
*Sample size was 15. One sample was lost.
†Numbers are median (range). Mann-Whitney U statistic used.
Other pharmacokinetic parameters
Weight-normalized volume of distribution and weight-normalized renal clearance were 40 and 43% higher (P < 0.05) in gastric bypass subjects than control subjects, respectively (Table 2). No differences were found between groups in Cmax, Tmax, or half-life (Table 2).
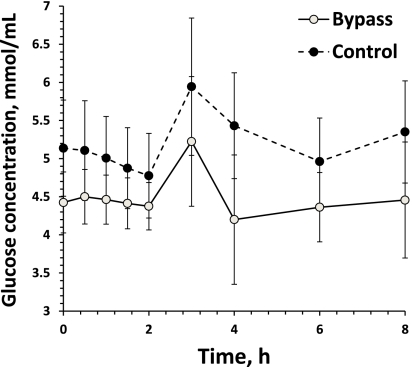
AUC0–8 glucose concentrations
The AUC0–8 glucose concentrations in the gastric bypass patients were 14% lower compared with control subjects (P < 0.05) (Table 2 and Fig. 2). However, AUC0–∞ metformin levels were not correlated with the AUC0–8 glucose levels (r = −0.02; P = 0.91). In multivariable linear regression modeling, baseline fasting glucose (parameter estimate 6.6; P < 0.0001) was a significant predictor of AUC0–8 glucose, whereas weight was of borderline significance (0.03; P = 0.08) and AUC0–∞ metformin levels were not a significant predictor (−0.00005; P = 0.5) (overall model-adjusted R2 = 0.8).
Figure 2.
Glucose concentration time curve.
CONCLUSIONS
The rate and extent of absorption of metformin between post-RYGB subjects and matched control subjects was compared. Contrary to our hypothesis, the metformin bioavailability in RYGB subjects was significantly increased. Although the AUC0–8 h glucose level was significantly lower in bypass subjects compared with control subjects, this finding was likely explained by baseline differences in glucose levels and not differences in metformin absorption.
RYGB is considered by some to be procedure of choice in patients with type 2 diabetes because remission rates of type 2 diabetes are higher after RYGB compared with gastric banding (the other most commonly performed bariatric procedure). RYGB reduces average weight to a greater extent than gastric banding (43 vs. 29 kg) (13) and leads to favorable neuroendocrine hormone-level alterations that correlate with improved glycemic control (14). Currently, ~15% of patients undergoing bariatric surgery have type 2 diabetes, and one-quarter of these patients still require diabetes treatment immediately after surgery (13). Nearly all patients remain clinically obese after surgery, and weight often is slowly regained postoperatively (15). After 5 years, approximately 43% of diabetic patients in postoperative remission develop recurrence (10). Therefore, assessing metformin absorption after bariatric surgery is of high clinical relevance because many patients will require continuation or reinitiation of metformin treatment postoperatively.
Metformin absorption is of clinical relevance because relationships have been observed between its plasma concentration and its glucose-lowering and glucose-regulating effects (9). With respect to its biodisposition, metformin displays low and variable bioavailability, is not metabolized or excreted in bile, is 100% excreted in the urine, and has a short terminal half-life (4 h), meaning that nearly all of the bioavailable dose is eliminated within 24 h (7–9). Therefore, unlike most drugs, 24-h urinary excretion can reliably estimate bioavailability without requiring intravenous administration. Based on its intrinsically low bioavailability and its apparent primary site for maximal absorption in the proximal small intestine, which possesses the largest overall surface area per unit length of the entire gut (16), we theorized that absorption would be diminished after RYGB, a procedure that bypasses the proximal small intestine. The results provided several unexpected outcomes, including not only increased bioavailability but also increases in volume of distribution and renal clearance.
For the increase in the extent of absorption, several potential mechanisms may explain the results, including the following:
RYGB performed with pouch volumes of 60–80 mL seems to delay gastric emptying for solid foods (17). In addition, RYGB increases intestinal transit time (18,19). Although data examining more contemporary pouch sizes (30 mL) and pills rather than food are lacking, we speculate that these gastrointestinal alterations may increase the overall absorption of metformin by increasing the duration of exposure of the drug to small-intestinal mucosa. The absorption of metformin is permeability rate limited, and the drug is almost exclusively absorbed in the small intestine (6,7,9). The drug that reaches the colon is not absorbed and is fecally excreted, with ~30% of the drug being eliminated in this manner (6). Because metformin has a limited window for absorption and absorption is incomplete, prolonging the intestinal transit time increases absorption (20). Similarly, delaying gastric emptying may decrease the rate at which the drug enters the small bowel, thus preventing saturation of absorptive mechanisms and increasing overall absorption (18). Slow-release formulations of metformin act in an analogous manner; the drug is physically retained in the stomach and released gradually into the upper small bowel, resulting in sustained and prolonged steady-state metformin levels (21).
After RYGB, the newly created 20- to 30-mL gastric pouch is largely devoid of acid-producing cells, and acid secretion is virtually absent (22). Although this more alkaline environment may potentially enhance the solubility of an acidic drug, metformin is a base, with a pKa of 12.4–13.8 and is almost completely ionized at all ranges of intestinal pH (23). Therefore, increases in intestinal pH are unlikely to play a major role in increasing metformin absorption after bypass.
Metformin is a substrate for organic cation transporters (OCTs), examples of which include human OCTs 1 and 2 (hOCT1 and -2), which are primarily found in the liver and kidney, and plasma membrane monoamine transporter in the intestine (23). Absorption in the intestinal tract appears to occur both transcellularly, which appears dependent upon OCTs (23), and paracellularly, which occurs via facilitated diffusion and may account for up to 90% of absorption (24). We speculate that alterations in these transport mechanisms, such as transporter upregulation, may be occurring after surgery, which may explain our findings.
Small-intestinal adaptation resulting from villous hyperplasia and possibly related to increased luminal nutrient exposure is a phenomenon that has been described after bowel resection (24). In theory, a similar mechanism may occur after gastric bypass, although this has not been previously described in this patient population.
We documented a 50% increase in the bioavailability of metformin, which is large enough to be potentially clinically relevant, particularly if sustained with chronic dosing and especially in the presence of renal dysfunction. We did not find a relationship between increased absorption and reduced AUC glucose levels, but this was not unexpected. Previous studies have demonstrated that metformin has little effect on glucose levels in nondiabetic patients unless toxic levels of the drug are administered (7). Furthermore, even in diabetic subjects, single doses of metformin have no effect on preprandial glucose and doses >1,700 mg are required to affect postprandial glucose levels (9). Therefore, a notable effect on glucose levels would not be expected after administration of a single dose to nondiabetic subjects even if bioavailability increased by >50%.
In addition to the increase in oral bioavailability, higher metformin volume of distribution and renal clearance values in the surgical patients were found. The current study was not designed to provide insight into mechanisms underlying these interesting observations. It was apparent that the creatinine plasma concentrations and clearances were highly similar in the two groups (Table 1). Because metformin has low plasma protein binding and is efficiently renally secreted, the difference in renal clearance in the surgical patients may be a consequence of more efficient secretion, perhaps mediated by OCT2.
A limitation of our study was that a single dose was administered to fasting subjects. Fasting subjects were studied to minimize the effect of food on metformin absorption (food decreases absorption by ~25%) (25). Although single-dose studies are easier to perform and are typically used to gain initial insight into an area, they have the drawback of not being able to assess steady-state levels. In clinical practice, multiple doses of metformin typically are administered with food. Thus, additional study examining steady-state levels in nonfasting subjects would be required to more closely mimic clinical practice. An additional limitation to our study was that nonsignificant differences in body weight and significant differences in metabolic parameters, including fasting glucose levels, were present at baseline. We also cannot rule out the possibility that secondary metabolic changes in unmeasured parameters occurring after gastric bypass (e.g., thyroid hormone alterations) influenced our results. In our analysis, adjustment for body weight was performed for relevant pharmacokinetic parameters and results were unchanged. Because metformin absorption does not vary according to diabetes status (7), the baseline between-group differences in glucose levels are unlikely to explain our findings regarding metformin bioavailability. A post hoc analysis examining between-group differences in bioavailability corrected for baseline glucose levels did not change the results (9.6 vs. 5.4% · L/mmol; mean difference −4.2 [95% CI −2.0 to −6.5]).
In conclusion, metformin absorption and bioavailability were unexpectedly increased in gastric bypass subjects compared with control subjects. Therefore, factors other than diminished small-bowel length influence overall metformin absorption. This has potential implications for metformin dosing after bypass and for medications with similar pharmacological properties. Studies are needed to confirm these findings and delineate potential mechanisms.
Acknowledgments
The University of Alberta Hospital Foundation funded this study. R.Q.G. is a recipient of a studentship award from the government of Egypt.
The study sponsor had no input into the study design, conduct, or reporting.
R.S.P. received research funding from Covidien, the maker of bariatric surgery equipment. A.M.S. has received consulting and speaking honoraria from Allergan Canada Inc., and Johnson & Johnson Medical Products. D.W.B. has been an advisor and has received speaking honoraria and research funding from Johnson & Johnson Medical Products and Ethicon Endo-Surgery. No other potential conflicts of interest relevant to this study were reported.
R.S.P. developed the initial study concept; drafted the initial manuscript, which was revised critically by the other authors; and had full access to data and takes responsibility for the study’s integrity. R.Q.G. performed drug assays and pharmacokinetic data analyses. L.-A.L. coordinated the study. D.W.B. and S.K. performed the surgeries. D.R.B. performed drug assays and pharmacokinetic data analyses. A.M.S. had input into all aspects of the study, including study design, analysis, and manuscript preparation. All authors approved the final version.
Parts of this study were submitted for presentation at the 2nd National Obesity Summit, Montreal, Canada, 28 April to 1 May 2011.
Footnotes
Clinical trial reg. no. NC01013051, clinicaltrials.gov.
References
- 1.Lau DCW, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 2007;176(Suppl. 8):S1–S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flum DR, Khan TV, Dellinger EP. Toward the rational and equitable use of bariatric surgery. JAMA 2007;298:1442–1444 [DOI] [PubMed] [Google Scholar]
- 3.Padwal RS, Sharma AM. Treating severe obesity: morbid weights and morbid waits. CMAJ 2009;181:777–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg 2009;19:1605–1611 [DOI] [PubMed] [Google Scholar]
- 5.Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev 2010;11:41–50 [DOI] [PubMed] [Google Scholar]
- 6.Vidon N, Chaussade S, Noel M, Franchisseur C, Huchet B, Bernier JJ. Metformin in the digestive tract. Diabetes Res Clin Pract 1988;4:223–229 [DOI] [PubMed] [Google Scholar]
- 7.Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol 1981;12:235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pentikäinen PJ, Neuvonen PJ, Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol 1979;16:195–202 [DOI] [PubMed] [Google Scholar]
- 9.Sambol NC, Chiang J, O’Conner M, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol 1996;36:1012–1021 [DOI] [PubMed] [Google Scholar]
- 10.Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2010;6:254–259 [DOI] [PubMed] [Google Scholar]
- 11.Cullen E, Liao J, Lukacsko P, Niecestro R, Friedhoff L. Pharmacokinetics and dose proportionality of extended-release metformin following administration of 1000, 1500, 2000 and 2500 mg in healthy volunteers. Biopharm Drug Dispos 2004;25:261–263 [DOI] [PubMed] [Google Scholar]
- 12.Gabr RQ, Padwal RS, Brocks DR. Determination of metformin in human plasma and urine by high-performance liquid chromatography using small sample volume and conventional octadecyl silane column. J Pharm Pharm Sci 2010;13:486–494 [DOI] [PubMed] [Google Scholar]
- 13.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 14.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 2004;89:2608–2615 [DOI] [PubMed] [Google Scholar]
- 15.Sjöström L, Narbro K, Sjöström CD, et al. ; Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 16.Gubbins PO, Bertch KE. Drug absorption in gastrointestinal disease and surgery: clinical pharmacokinetic and therapeutic implications. Clin Pharmacokinet 1991;21:431–447 [DOI] [PubMed] [Google Scholar]
- 17.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos 2007;35:1956–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horowitz M, Cook DJ, Collins PJ, et al. Measurement of gastric emptying after gastric bypass surgery using radionuclides. Br J Surg 1982;69:655–657 [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 2005;138:283–290 [DOI] [PubMed] [Google Scholar]
- 20.Marathe PH, Wen Y, Norton J, Greene DS, Barbhaiya RH, Wilding IR. Effect of altered gastric emptying and gastrointestinal motility on metformin absorption. Br J Clin Pharmacol 2000;50:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmins P, Donahue S, Meeker J, Marathe P. Steady-state pharmacokinetics of a novel extended-release metformin formulation. Clin Pharmacokinet 2005;44:721–729 [DOI] [PubMed] [Google Scholar]
- 22.Smith CD, Herkes SB, Behrns KE, Fairbanks VF, Kelly KA, Sarr MG. Gastric acid secretion and vitamin B12 absorption after vertical Roux-en-Y gastric bypass for morbid obesity. Ann Surg 1993;218:91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proctor WR, Bourdet DL, Thakker DR. Mechanisms underlying saturable intestinal absorption of metformin. Drug Metab Dispos 2008;36:1650–1658 [DOI] [PubMed] [Google Scholar]
- 24.Dowling RH. Intestinal adaptation. N Engl J Med 1973;288:520–521 [DOI] [PubMed] [Google Scholar]
- 25.Sambol NC, Brookes LG, Chiang J, et al. Food intake and dosage level, but not tablet vs solution dosage form, affect the absorption of metformin HCl in man. Br J Clin Pharmacol 1996;42:510–512 [DOI] [PubMed] [Google Scholar]