Abstract
OBJECTIVE
Coronary heart disease (CHD) is a major cause of mortality among people with diabetes. The objective of this study was to examine the trend in an estimated 10-year risk for developing CHD among adults with diagnosed diabetes in the U.S.
RESEARCH DESIGN AND METHODS
Data from 1,977 adults, aged 30–79 years, with diagnosed diabetes who participated in the National Health and Nutrition Examination Survey from 1999–2000 to 2007–2008 were used. Estimated risk was calculated using risk prediction algorithms from the UK Prospective Diabetes Study (UKPDS), the Atherosclerosis Risk in Communities study, and the Framingham Heart Study.
RESULTS
Significant improvements in mean HbA1c concentrations, systolic blood pressure, and the ratio of total cholesterol to HDL cholesterol occurred. No significant linear trend for current smoking status was observed. The estimated UKPDS 10-year risk for CHD was 21.1% in 1999–2000 and 16.4% in 2007–2008 (Plinear trend < 0.001). The risk decreased significantly among men, women, whites, African Americans, and Mexican Americans.
CONCLUSIONS
The estimated 10-year risk for CHD among adults with diabetes has improved significantly from 1999–2000 to 2007–2008. Sustained efforts in improving risk factors should further benefit the cardiovascular health of people with diabetes.
Many people with diabetes will die from cardiovascular disease. Therefore, controlling the risk factors for cardiovascular disease is of the utmost importance in reducing the risk for developing cardiovascular disease in the diabetic population. In the U.S., important strides have been made in reducing the impact of several key risk factors for cardiovascular disease. Thus, the prevalence of smoking has fallen substantially, and concentrations of total cholesterol (TC) have decreased, but blood pressure levels have moved less consistently (1–3). Some data suggest that these trends in the general population also have played out in the diabetic population (4). If so, the risk for developing coronary heart disease (CHD) should have decreased among people with diabetes. Therefore, the objective of this study was to examine the trends in the 10-year risk for CHD among adults with diagnosed diabetes in the U.S. from 1999 to 2008.
RESEARCH DESIGN AND METHODS
This study included data from the National Health and Nutrition Examination Survey (NHANES) 1999–2008 (5). During each consecutive 2-year cycle, a national sample was recruited using a multistage, stratified sampling design. The surveys were designed to produce results representative of the civilian, noninstitutionalized U.S. population. Participants were interviewed at home and were invited to attend a mobile examination center, where they were asked to complete additional questionnaires, to undergo various examinations, and to provide a blood sample. The study received approval from the National Center for Health Statistics Research Ethics Review Board, and participants were asked to sign an informed consent form. Details about the survey can be found elsewhere (5).
Participants who responded affirmatively to the question, “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” were considered to have been diagnosed with diabetes. The UK Prospective Diabetes Study (UKPDS) Risk Engine incorporates the following variables: age at diagnosis of diabetes; sex; race or ethnicity; smoking status; concentration of HbA1c; systolic blood pressure; and the ratio of the concentrations of TC-to-HDL cholesterol (HDLC) (6). The 10-year probability of developing CHD also was calculated using risk equations generated from the Framingham Heart Study (FHS) (7) and the Atherosclerosis Risk in Communities (ARIC) study (8). The FHS risk equations use age, age2, categories of concentrations of TC, categories of concentrations of HDLC, categories of blood pressure, diabetes status, and smoking status. The ARIC study risk equations incorporate age, age2, race, categories of concentrations of TC, categories of concentrations of HDLC, systolic blood pressure, use of antihypertensive medications, and current smoking status.
Participants with diagnosed diabetes were asked about their age when they were first told by a doctor or other health professional that they had diabetes. Participants who had smoked at least 100 cigarettes during their lifetime and currently were smoking were designated as current smokers. Concentrations of HbA1c were measured using Primus Automated HPLC Systems (models CLC330 and CLC385; Primus, Kansas City, MO) during 1999–2004 at the University of Missouri (Columbia, MO) and using an A1C 2.2 Plus Glycohemoglobin Analyzer or an A1C G7 HPLC Glycohemoglobin Analyzer during 2005–2008 (Tosoh Medics, South San Francisco, CA) at the Fairview University Medical Center. The average of the last two measurements of blood pressure for participants who had three or four measurements, the last measurement for participants with only two measurements, and the only measurement for participants who had one measurement were used. Current use of antihypertensive medications was based on self-report. From 1999 to 2006, serum TC and HDLC were measured enzymatically using a Hitachi 704, 717, or 912 Analyzer (Roche Diagnostics, Indianapolis, IN) at Johns Hopkins University. During 2007–2008, serum cholesterol and HDLC were measured enzymatically on a Roche Modular P Chemistry Analyzer (Roche Diagnostics) at the University of Minnesota. Because changes in the methodology to measure HDLC were enacted during the study period, studies were conducted to measure the effect of those changes in methods on concentrations, and corrections were made as necessary. Corrected concentrations were used in this study.
Risk estimates were calculated for participants aged 30–79 years with diagnosed diabetes for the UKPDS risk engine, for participants aged 45–65 years for the ARIC study risk engine, and for participants aged 30–74 years for the FHS risk engine. Tests for linear trend were conducted by using regression analyses, in which survey cycle was used as a continuous variable. For race or ethnic-specific estimates, only results for the three major groups (whites, African Americans, and Mexican Americans) are shown. Hypothetical estimates of 10-year UKPDS risk for diabetic adults were calculated under the following scenarios: 1) eliminate all current smoking; 2) reduce the concentration of HbA1c to 6.9% for those who had a concentration of ≥7%; 3) reduce systolic blood pressure to 129 mmHg for those with a systolic blood pressure ≥130 mmHg; 4) reduce TC to 199 mg/dL for those with a concentration of TC ≥200 mg/dL and increase concentrations of HDLC to 40 mg/dL for diabetic men with a concentration <40 mg/dL and to 50 mg/dL for diabetic women with a concentration <50 mg/dL; and 5) all of the above. SUDAAN (software for the statistical analysis of correlated data; Research Triangle Institute, Research Triangle Park, NC) was used for the analyses to account for the complex sampling design, and sampling weights were used to generate estimates.
RESULTS
A total of 2,296 participants, aged 30–79 years, diagnosed with diabetes had a medical examination. After excluding participants with missing data for the variables needed to calculate the estimated risk, 1,977 participants were included in the analyses (1,021 men and 956 women; 715 whites, 531 African Americans, 527 Mexican Americans, and 204 of another race or ethnicity). No significant linear trend in age was noted (P = 0.250).
In the entire sample, there were no significant linear trends with respect to the percentage of women, the percentage of African Americans, and the percentage of current smokers (Table 1). However, declining trends were observed for concentrations of HbA1c, mean systolic blood pressure, TC, and the ratio of TC/HDLC. With the exception of the percentage of current smokers and mean systolic blood pressure in men, the direction or significance of the trends of the UKPDS risk engine variables among men and women were similar.
Table 1.
Characteristics of variables used to calculate the estimated 10-year risk for CHD and estimated 10-year risk for CHD among adults with diagnosed diabetes, aged ≥30–79 years, in the U.S. by study period, NHANES 1999–2008
| 1999–2000 |
2001–2002 |
2003–2004 |
2005–2006 |
2007–2008 |
P |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
n* |
Percentage or mean (SE)† | n* | Percentage or mean (SE)† | n* | Percentage or mean (SE)† | n* | Percentage or mean (SE)† | n* | Percentage or mean (SE)† | ||
| Total | |||||||||||
| Dichotomous variables | |||||||||||
| Women (%) | 335 | 46.7 (3.9) | 338 | 46.6 (2.2) | 363 | 48.6 (2.3) | 374 | 52.4 (3.7) | 567 | 49.2 (3.9) | 0.388 |
| African American (%) | 335 | 16.3 (4.3) | 338 | 14.7 (3.2) | 363 | 13.6 (3.1) | 374 | 16.6 (3.0) | 567 | 17.9 (4.0) | 0.608 |
| Current smoker (%) | 335 | 17.3 (2.7) | 338 | 24.2 (2.5) | 363 | 22.0 (2.4) | 374 | 14.8 (2.3) | 567 | 16.8 (1.8) | 0.091 |
| Continuous variables | |||||||||||
| Age (years) | 335 | 57.5 (0.8) | 338 | 56.3 (1.0) | 363 | 59.0 (1.0) | 374 | 57.3 (1.0) | 567 | 58.4 (0.6) | 0.250 |
| Age of diabetes diagnosis (years) | 335 | 46.0 (0.9) | 338 | 45.9 (1.0) | 363 | 46.7 (1.4) | 374 | 47.5 (0.8) | 567 | 47.5 (0.9) | 0.101 |
| HbA1c (%) | 335 | 7.9 (0.2) | 338 | 7.6 (0.2) | 363 | 7.3 (0.1) | 374 | 7.1 (0.1) | 567 | 7.2 (0.1) | 0.001 |
| Systolic blood pressure (mmHg) | 335 | 133.7 (1.5) | 338 | 129.7 (1.4) | 363 | 130.3 (1.4) | 374 | 130.3 (1.3) | 567 | 128.9 (0.9) | 0.026 |
| Diastolic blood pressure (mmHg) | 335 | 71.4 (1.5) | 338 | 70.7 (1.2) | 363 | 68.3 (1.3) | 374 | 70.3 (1.0) | 567 | 69.6 (0.9) | 0.346 |
| TC (mmol/L) | 335 | 5.4 (0.1) | 338 | 5.4 (0.2) | 363 | 5.3 (0.1) | 374 | 4.9 (0.1) | 567 | 4.7 (0.1) | <0.001 |
| HDLC (mmol/L) | 335 | 1.1 (<0.1) | 338 | 1.2 (<0.1) | 363 | 1.3 (<0.1) | 374 | 1.3 (<0.1) | 567 | 1.2 (<0.1) | 0.093 |
| Ratio of TC/HDLC | 335 | 5.0 (0.1) | 338 | 4.7 (0.1) | 363 | 4.5 (0.1) | 374 | 4.0 (0.1) | 567 | 4.3 (0.1) | <0.001 |
| UKPDS 10-year risk | 335 | 21.1 (1.4) | 338 | 17.6 (0.7) | 363 | 19.0 (1.1) | 374 | 13.8 (0.9) | 567 | 16.4 (0.8) | <0.001 |
| ARIC study 10-year risk‡ | 165 | 18.7 (0.8) | 178 | 19.6 (1.2) | 163 | 18.9 (0.9) | 195 | 14.9 (0.5) | 300 | 15.9 (0.7) | <0.001 |
| FHS 10-year risk§ | 296 | 18.6 (0.7) | 311 | 15.6 (0.8) | 319 | 16.5 (0.7) | 340 | 13.0 (0.6) | 509 | 14.6 (0.7) | <0.001 |
| Men | |||||||||||
| Dichotomous variables | |||||||||||
| African American (%) | 170 | 12.7 (4.0) | 180 | 11.2 (3.0) | 190 | 11.3 (1.8) | 190 | 16.1 (3.1) | 291 | 14.9 (3.7) | 0.402 |
| Current smoker (%) | 170 | 20.1 (5.0) | 180 | 27.8 (4.9) | 190 | 26.6 (4.8) | 190 | 15.1 (2.6) | 291 | 15.7 (2.0) | 0.033 |
| Continuous variables | |||||||||||
| Age (years) | 170 | 56.8 (1.1) | 180 | 55.8 (0.9) | 190 | 58.7 (1.1) | 190 | 56.7 (1.3) | 291 | 58.6 (0.9) | 0.153 |
| Age of diabetes diagnosis (years) | 170 | 45.5 (1.7) | 180 | 47.2 (1.2) | 190 | 46.6 (1.6) | 190 | 47.7 (1.1) | 291 | 48.0 (1.2) | 0.211 |
| HbA1c (%) | 170 | 7.7 (0.2) | 180 | 7.6 (0.2) | 190 | 7.4 (0.2) | 190 | 7.1 (0.1) | 291 | 7.3 (0.1) | 0.008 |
| Systolic blood pressure (mmHg) | 170 | 130.5 (2.1) | 180 | 127.0 (1.3) | 190 | 126.4 (1.6) | 190 | 128.9 (1.6) | 291 | 128.7 (1.4) | 0.877 |
| Diastolic blood pressure (mmHg) | 170 | 72.9 (1.3) | 180 | 72.4 (1.5) | 190 | 70.2 (1.2) | 190 | 71.6 (1.3) | 291 | 70.8 (1.5) | 0.303 |
| TC (mmol/L) | 170 | 5.3 (0.1) | 180 | 5.4 (0.3) | 190 | 5.2 (0.1) | 190 | 4.7 (0.1) | 291 | 4.6 (0.1) | <0.001 |
| HDLC (mmol/L) | 170 | 1.1 (<0.1) | 180 | 1.1 (<0.1) | 190 | 1.1 (<0.1) | 190 | 1.2 (<0.1) | 291 | 1.1 (<0.1) | 0.139 |
| Ratio of TC/HDLC | 170 | 5.2 (0.2) | 180 | 5.1 (0.2) | 190 | 4.8 (0.1) | 190 | 4.2 (0.2) | 291 | 4.4 (0.2) | <0.001 |
| UKPDS 10-year risk | 170 | 25.8 (1.7) | 180 | 21.7 (1.1) | 190 | 24.0 (1.5) | 190 | 17.4 (1.5) | 291 | 21.1 (1.0) | 0.005 |
| ARIC study 10-year risk‡ | 84 | 23.1 (1.1) | 102 | 21.9 (0.9) | 81 | 22.7 (1.5) | 95 | 18.5 (1.0) | 146 | 19.8 (0.7) | 0.002 |
| FHS 10-year risk§ | 155 | 20.9 (0.8) | 165 | 17.8 (0.7) | 171 | 19.5 (0.9) | 170 | 15.0 (0.9) | 257 | 16.6 (0.9) | <0.001 |
| Women | |||||||||||
| Dichotomous variables | |||||||||||
| African American (%) | 165 | 20.3 (5.6) | 158 | 18.7 (4.2) | 173 | 15.9 (4.8) | 184 | 17.1 (3.3) | 276 | 21.0 (5.1) | 0.920 |
| Current smoker (%) | 165 | 14.0 (3.7) | 158 | 20.0 (2.9) | 173 | 17.2 (3.5) | 184 | 14.5 (3.4) | 276 | 17.9 (3.1) | 0.863 |
| Continuous variables | |||||||||||
| Age (years) | 165 | 58.3 (1.0) | 158 | 56.7 (1.7) | 173 | 59.4 (1.2) | 184 | 57.9 (1.5) | 276 | 58.1 (1.0) | 0.842 |
| Age of diabetes diagnosis (years) | 165 | 46.5 (1.1) | 158 | 44.3 (1.9) | 173 | 46.7 (2.0) | 184 | 47.3 (1.4) | 276 | 47.0 (1.0) | 0.309 |
| HbA1c (%) | 165 | 8.0 (0.3) | 158 | 7.5 (0.2) | 173 | 7.1 (0.1) | 184 | 7.1 (0.2) | 276 | 7.2 (0.1) | 0.009 |
| Systolic blood pressure (mmHg) | 165 | 137.4 (2.6) | 158 | 132.9 (2.7) | 173 | 134.5 (2.1) | 184 | 131.5 (2.2) | 276 | 129.1 (1.1) | 0.006 |
| Diastolic blood pressure (mmHg) | 165 | 69.7 (2.2) | 158 | 68.8 (1.8) | 173 | 66.4 (1.8) | 184 | 69.1 (1.3) | 276 | 68.3 (0.8) | 0.733 |
| TC (mmol/L) | 165 | 5.6 (0.1) | 158 | 5.4 (0.1) | 173 | 5.4 (0.1) | 184 | 5.1 (0.1) | 276 | 4.9 (0.1) | <0.001 |
| HDLC (mmol/L) | 165 | 1.2 (<0.1) | 158 | 1.3 (<0.1) | 173 | 1.4 (<0.1) | 184 | 1.5 (<0.1) | 276 | 1.2 (<0.1) | 0.454 |
| Ratio of TC/HDLC | 165 | 4.8 (0.1) | 158 | 4.2 (0.1) | 173 | 4.2 (0.1) | 184 | 3.7 (0.1) | 276 | 4.3 (0.1) | 0.007 |
| UKPDS 10-year risk | 165 | 15.7 (1.7) | 158 | 12.9 (1.2) | 173 | 13.7 (1.3) | 184 | 10.5 (1.0) | 276 | 11.6 (1.3) | 0.028 |
| ARIC study 10-year risk‡ | 81 | 13.9 (1.6) | 76 | 16.3 (2.1) | 82 | 15.1 (1.3) | 100 | 11.3 (1.0) | 154 | 11.9 (0.9) | 0.026 |
| FHS 10-year risk§ | 141 | 15.9 (0.9) | 146 | 12.9 (1.1) | 148 | 13.3 (0.9) | 170 | 11.1 (0.9) | 252 | 12.6 (0.7) | 0.007 |
*Unweighted sample size.
†Estimates calculated using sampling weights.
‡Estimates are for ages 45–65 years.
§Estimates are for ages 30–74 years.
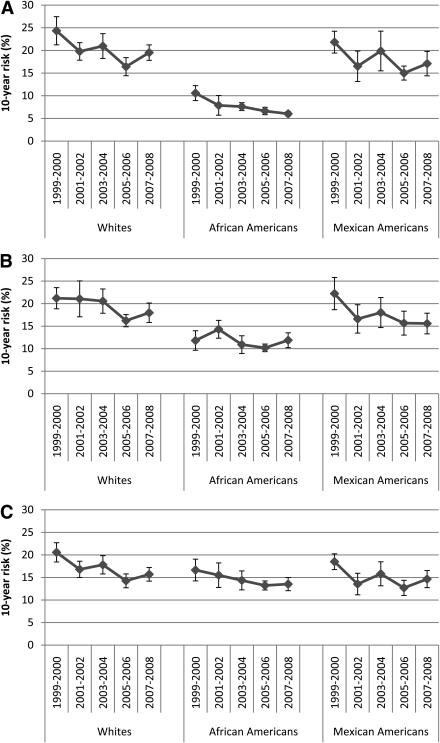
The estimated UKPDS 10-year risk for developing CHD decreased significantly in the total sample (regression coefficient = −0.63% per year, SE = 0.17, Plinear trend < 0.001) as well as in men (regression coefficient = −0.65% per year, SE = 0.22, Plinear trend = 0.005) and women (regression coefficient = −0.51% per year, SE = 0.23, Plinear trend = 0.028) (Table 1). The test for an interaction between sex and study cycle was not significant (P = 0.652). Significant decreases in the risk for CHD were noted among whites (regression coefficient = −0.60% per year, SE = 0.19, Plinear trend 0.002), African Americans (regression coefficient = −0.50% per year, SE = 0.10, Plinear trend <0.001), and Mexican Americans (regression coefficient = −0.50% per year, SE = 0.23, Plinear trend = 0.031) (Fig. 1). The regression coefficients of the three groups did not differ statistically (P = 0.915).
Figure 1.
Estimated 10-year risk (95% CI) for developing CHD among adults with diagnosed diabetes in the U.S. by race or ethnicity (NHANES). A: UKPDS risk estimates among participants aged 30–79 years. B: ARIC study risk estimates among participants aged 45–65 years. C: FHS risk estimates among participants aged 30–74 years.
Although the 10-year risk for CHD, calculated using the ARIC study and FHS risk equations, also decreased significantly among all participants, there was some inconsistency among the risk equations regarding the significance of trends stratified by race or ethnicity (Fig. 1). The UKPDS risk estimates showed significant decreases among all three major racial or ethnic groups (Plinear trend: whites = 0.002; African Americans <0.001; and Mexican Americans = 0.031), the ARIC study risk estimates indicated significant reductions among whites and Mexican Americans but not among African Americans (Plinear trend: whites = 0.006; African Americans = 0.274; and Mexican Americans = 0.014), and the FHS risk estimates showed significant decreases among whites and African Americans (Plinear trend: whites = 0.001; African Americans = 0.033; and Mexican Americans = 0.065).
Calculations of hypothetical reductions in 10-year UKPDS risk suggested that improving the ratio of TC/HDLC could result in the largest reduction in risk for CHD (Table 2).The application of simultaneous modifications of all four risk factors to the 2007–2008 data suggested that the estimated risk for CHD (11.4%) could have been ~30% lower than the actual estimates (16.4%).
Table 2.
Estimated UKPDS 10-year risk for CHD among adults with diagnosed diabetes, aged 30–79 years, in the U.S. under different scenarios by study period, NHANES 1999–2008
| Reduction scenario | 1999–2000 |
2001–2002 |
2003–2004 |
2005–2006 |
2007–2008 |
|---|---|---|---|---|---|
| Percentage or mean (SE)† | Percentage or mean (SE)† | Percentage or mean (SE)† | Percentage or mean (SE)† | Percentage or mean (SE)† | |
| n* | 335 | 338 | 363 | 374 | 567 |
| UKPDS 10-year risk | 21.1 (1.4) | 17.6 (0.7) | 19.0 (1.1) | 13.8 (0.9) | 16.4 (0.8) |
| UKPDS 10-year risk, eliminate smoking | 20.3 (1.3) | 16.9 (0.8) | 18.0 (1.1) | 13.3 (0.9) | 15.8 (0.8) |
| UKPDS 10-year risk, HbA1c <7% | 18.5 (1.3) | 15.5 (0.7) | 17.3 (1.0) | 12.8 (0.9) | 15.0 (0.7) |
| UKPDS 10-year risk, systolic blood pressure <130 mmHg | 19.7 (1.3) | 16.7 (0.7) | 17.9 (1.1) | 12.9 (0.8) | 15.5 (0.7) |
| UKPDS 10-year risk, decrease ratio of TC/HDLC | 17.2 (1.2) | 14.4 (0.5) | 16.0 (0.9) | 11.9 (0.8) | 13.8 (0.7) |
| UKPDS 10-year risk, implement all | 13.4 (1.0) | 11.4 (0.6) | 13.2 (0.8) | 10.0 (0.7) | 11.4 (0.6) |
*Unweighted sample size.
†Estimates calculated using sampling weights.
CONCLUSIONS
Compared with 1999–2000, the estimated 10-year UKPDS risk for developing CHD among people with diagnosed diabetes was 22% lower by 2007–2008. The estimated risk for CHD decreased significantly among men, women, whites, African Americans, and Mexican Americans. Improvements in concentrations of HbA1c, systolic blood pressure, and the ratio of TC/HDLC mostly accounted for the decreased risk.
The mortality rate of CHD in the U.S. population has decreased tremendously since the 1960s (9). Medical treatments of CHD and favorable trends in key risk factors for CHD have contributed roughly equally to this decline (10). Whether the incidence of CHD also demonstrated favorable secular trends has been less clear. Although trends in the incidence of CHD among people with diabetes remain unknown, people with diabetes have experienced decreases in the mortality rate from cardiovascular disease (11–13). One study (12) showed that the mortality rate from cardiovascular disease decreased substantially among men but decreased little among women with diabetes. In the FHS, however, men and women experienced similar reductions in mortality from cardiovascular disease (13). The results of the current study showed that risk declined significantly to a similar extent among both men and women. Because the UKPDS risk engine used fatal and nonfatal myocardial infarction or sudden death as its end point, it is possible that changes in mortality between men and women could differ even if changes in incidence were similar because of differences in the case-fatality rate. Therefore, surveillance for the incidence of cardiovascular disease and the case-fatality rate among people with diabetes is of critical importance to understanding the dynamics of this diabetes complication.
The difference in estimated UKPDS risk between men and women is partially a function of the UKPDS risk equation because the sex coefficient assigns a lower risk to women. The difference in the estimated risk for CHD among the three major racial or ethnic groups can be attributed to differences in the coefficient for race or ethnicity in the UKPDS risk engine as well as to the more favorable lipid profile in African Americans than the other groups because the UKPDS risk engine assigns a great deal of weight to the coefficient for the ratio of TC/HDLC. The coefficient for the race or ethnicity variable in the ARIC study risk equations also indicates a lower risk for African Americans. Thus, both the UKPDS and ARIC study risk equations predict that the risk for developing CHD is lower among African Americans than whites when values for the risk factors are the same.
The UKPDS and FHS risk estimates suggested that favorable developments in the risk for developing CHD extended to all three major racial or ethnic groups. The estimated UKPDS risk during 2007–2008 was 43% lower among African Americans, 22% lower among Mexican Americans, and 20% lower among whites compared with 1999–2000. However, the ARIC study risk estimates did not show a significant decrease in the risk for CHD among African Americans, and the FHS risk estimates for Mexican Americans showed a reduction in risk over time that was of borderline significance. Because analyses stratified by race or ethnicity failed to demonstrate significant reductions in several risk factors, reducing smoking, HbA1c, and blood pressure among whites; smoking among African Americans; and blood pressure and TC among Mexican Americans is critical to further reducing the future risk for CHD in these groups.
Population studies in the U.S. have shown increased treatment for and control of hypercholesterolemia and hypertension (3,14). Increased uptake of medications to lower blood glucose, systolic blood pressure, and TC by people with diabetes likely contributed to the improvements in these risk factors (4). Less clear is the role of lifestyle behaviors, such as physical activity and diet (saturated fat, polyunsaturated fat, trans fats, dietary cholesterol, salt, and fruits and vegetables), in affecting the observed trends. Although an anthropometric measure is not part of any of the three risk engines used in the current study, excess weight promotes increased blood pressure and dyslipidemias. Thus, weight management is critical to optimizing levels of blood pressure and lipids that are important components of the UKPDS risk engine and to estimating cardiovascular risk.
The present analysis suggests that reducing TC to <200 mg/dL and raising HDLC to at least 40 mg/dL in men and 50 mg/dL in women would result in the largest reduction in risk. In part, the size of the potential risk reduction was governed by the high prevalence of dyslipidemias of 69.5%. Optimizing medical management and promoting behavioral change, such as increasing physical activity (keeping in mind medical limitations), improving dietary quality, and weight management, can contribute to lowering concentrations of HbA1c, blood pressure, and concentrations of lipids. Smoking is an important risk factor for CHD. Therefore, the absence of a meaningful decrease in the prevalence of smoking in diabetic adults during the 10-year study period suggests that this behavior contributed little to the observed trends in the estimated risk for CHD. Had all diabetic participants who were current smokers not smoked, the estimated risk would have been ~4% lower in 1999–2000 and in 2007–2008.
Clinical trials, especially the more recent ones, have generated considerable controversy concerning the effect of glycemic control, particularly rigorous glycemic control, on all-cause mortality (15). Therefore, the hypothetical reductions in risk for CHD attributable to glycemic control discussed above should be viewed with considerable caution. Meta-analyses of clinical trials in people with diabetes demonstrated that treating patients with hypertension or elevated concentrations of LDL cholesterol improves cardiovascular outcomes (16,17), and, therefore, the control of these two risk factors is clearly vital in reducing the risk for CHD in people with diabetes. Furthermore, comprehensive management of major cardiovascular risk factors in people with diabetes greatly reduces cardiovascular disease (18).
The number of risk prediction algorithms to calculate the risk for CHD or cardiovascular disease has increased substantially since the emergence of risk prediction for CHD. Because the FHS and other risk prediction algorithms may not adequately assess the risk for CHD among people with diabetes (19), two additional risk prediction algorithms, the UKPDS and ARIC study risk prediction algorithms that were developed using samples of diabetic participants, were used in the current study. The UKPDS risk prediction algorithm, however, was developed using data from a cohort of participants with diabetes who did not have a recent history of myocardial infarction, angina, or heart failure. Thus, this risk prediction algorithm may underestimate the risk for developing CHD in studies, such as the present one that include participants with such histories of myocardial infarction, angina, or heart failure. The predictive ability of several risk prediction algorithms among people with diabetes has been compared with observed outcomes in a number of prospective studies (19–24). The FHS risk prediction algorithm underestimated the observed risk for CHD in some studies (19,21) and overestimated the risk in others (22–24). The UKPDS risk prediction algorithm also overestimated the risk in some studies (21–24). A comparison of risk functions in New Zealand showed that the 5-year UKPDS risk estimates were lower than the FHS risk estimates (25). Thus, considerable uncertainty exists about how best to estimate the risk for cardiovascular disease in people with diabetes.
Several limitations deserve mention. First, diagnosed diabetes was based on self-reported information and is thus subject to some degree of erroneous reporting and misclassification. However, self-reported diabetes generally has good sensitivity and specificity. Second, the number of participants with diagnosed diabetes was too small to calculate reasonably stable estimates of risk for CHD by several levels of stratification of the study variables. Third, estimates of 10-year risk were calculated for the three major racial or ethnic groups. The UKPDS and ARIC study participants included participants of African heritage, and, therefore, the use of these two sets of risk equations among African Americans in the current study appears reasonable, although limited experience exists concerning the use of these risk equations in African American populations. Furthermore, the validity of applying these equations to samples of participants of Hispanic heritage remains to be established. Furthermore, estimates for only three major racial or ethnic groups were calculated because data for other races or ethnicities were not available or had inadequate sample size. Thus, studies focused on other races or ethnicities will be helpful to examine the totality of the dynamics of CHD among people with diabetes. Finally, the laboratories and methods for measuring HbA1c and lipids changed during the 10-year period. Crossover studies were performed to examine the possible effects of these changes, and equations were developed to bridge any differences. The changes in the methodology for measuring concentrations of HDLC may have partially led to the increase in the means of HDLC from 1999 to 2006, particularly after all specimens were analyzed by using the direct method of measuring HDLC starting in 2003. Therefore, it is possible that this artifact may have been responsible for some small proportion of the favorable trend in the risk for CHD that was observed.
In conclusion, recent favorable trends in several major risk factors for CHD, namely HbA1c, systolic blood pressure, and the ratio of TC/HDLC, have resulted in an encouraging reduction in the estimated risk for CHD among adults with diagnosed diabetes in the U.S. The estimates from the current study also suggest that continuing declines in the mortality rate from CHD among people with diabetes can be anticipated going forward. Sustained efforts in targeting these three risk factors as well as an additional focus on reducing smoking in the diabetic population should further benefit the cardiovascular health of people with diabetes. Because the most recent data did not indicate that the decrease in the estimated risk for CHD from 1999–2000 to 2005–2006 continued into 2007–2008, future monitoring will be essential to determine whether this possible interruption in the trend is a temporary phenomenon that may represent sampling variation or represents a real change in the direction of the trend.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
E.S.F. researched the data and wrote the manuscript.
Footnotes
The findings and conclusions in this article are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention. Trends in current cigarette smoking among high school students and adults, United States, 1965–2007 [article online], 2010. Available at http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm Accessed 18 April 2011
- 2.Carroll MD, Lacher DA, Sorlie PD, et al. Trends in serum lipids and lipoproteins of adults, 1960-2002. JAMA 2005;294:1773–1781 [DOI] [PubMed] [Google Scholar]
- 3.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–2050 [DOI] [PubMed] [Google Scholar]
- 4.Hoerger TJ, Zhang P, Segel JE, Gregg EW, Narayan KM, Hicks KA. Improvements in risk factor control among persons with diabetes in the United States: evidence and implications for remaining life expectancy. Diabetes Res Clin Pract 2009;86:225–232 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey [article online], 2010. Available at http://www.cdc.gov/nchs/nhanes.htm Accessed 18 April 2011
- 6.Stevens RJ, Kothari V, Adler AI, Stratton IM; United Kingdom Prospective Diabetes Study (UKPDS) Group The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–679 [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 8.Folsom AR, Chambless LE, Duncan BB, Gilbert AC, Pankow JS; Atherosclerosis Risk in Communities Study Investigators Prediction of coronary heart disease in middle-aged adults with diabetes. Diabetes Care 2003;26:2777–2784 [DOI] [PubMed] [Google Scholar]
- 9.National Heart, Lung, and Blood Institute Morbidity & Mortality: 2007 Chartbook on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD, U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, 2007 [Google Scholar]
- 10.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med 2007;356:2388–2398 [DOI] [PubMed] [Google Scholar]
- 11.Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004;292:2495–2499 [DOI] [PubMed] [Google Scholar]
- 12.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 2007;147:149–155 [DOI] [PubMed] [Google Scholar]
- 13.Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009;119:1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford ES, Li C, Pearson WS, Zhao G, Mokdad AH. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol 2010;140:226–235 [DOI] [PubMed] [Google Scholar]
- 15.Skyler JS, Bergenstal R, Bonow RO, et al. ; American Diabetes Association; American College of Cardiology Foundation; American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med 2003;138:593–602 [DOI] [PubMed] [Google Scholar]
- 17.Kearney PM, Blackwell L, Collins R, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125 [DOI] [PubMed] [Google Scholar]
- 18.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 19.Coleman RL, Stevens RJ, Retnakaran R, Holman RR. Framingham, SCORE, and DECODE risk equations do not provide reliable cardiovascular risk estimates in type 2 diabetes. Diabetes Care 2007;30:1292–1293 [DOI] [PubMed] [Google Scholar]
- 20.Guzder RN, Gatling W, Mullee MA, Mehta RL, Byrne CD. Prognostic value of the Framingham cardiovascular risk equation and the UKPDS risk engine for coronary heart disease in newly diagnosed type 2 diabetes: results from a United Kingdom study. Diabet Med 2005;22:554–562 [DOI] [PubMed] [Google Scholar]
- 21.Davis WA, Colagiuri S, Davis TM. Comparison of the Framingham and United Kingdom Prospective Diabetes Study cardiovascular risk equations in Australian patients with type 2 diabetes from the Fremantle Diabetes Study. Med J Aust 2009;190:180–184 [DOI] [PubMed] [Google Scholar]
- 22.Simmons RK, Coleman RL, Price HC, et al. Performance of the UK Prospective Diabetes Study Risk Engine and the Framingham Risk Equations in Estimating Cardiovascular Disease in the EPIC-Norfolk Cohort. Diabetes Care 2009;32:708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Heijden AA, Ortegon MM, Niessen LW, Nijpels G, Dekker JM. Prediction of coronary heart disease risk in a general, pre-diabetic, and diabetic population during 10 years of follow-up: accuracy of the Framingham, SCORE, and UKPDS risk functions: the Hoorn Study. Diabetes Care 2009;32:2094–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kengne AP, Patel A, Colagiuri S, et al. ; ADVANCE Collaborative Group The Framingham and UK Prospective Diabetes Study (UKPDS) risk equations do not reliably estimate the probability of cardiovascular events in a large ethnically diverse sample of patients with diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) Study. Diabetologia 2010;53:821–831 [DOI] [PubMed] [Google Scholar]
- 25.Metcalf PA, Wells S, Scragg RK, Jackson R. Comparison of three different methods of assessing cardiovascular disease risk in New Zealanders with type 2 diabetes mellitus. N Z Med J 2008;121:49–57 [PubMed] [Google Scholar]