Abstract
OBJECTIVE
We prospectively investigated in hypertensive patients with type 2 diabetes if bedtime treatment with ≥1 hypertension medications exerts better blood pressure control and cardiovascular risk reduction than conventional therapy, in which all medications are ingested in the morning.
RESEARCH DESIGN AND METHODS
We conducted a prospective, randomized, open-label, blinded end point trial on 448 hypertensive patients with type 2 diabetes, 255 men/193 women, mean ± SD age 62.5 ± 10.8 years, randomized to ingest all their prescribed hypertension medications upon awakening or ≥1 of them at bedtime. Ambulatory blood pressure was measured for 48 h at baseline and again annually or even more frequently (quarterly) after adjustments in treatment.
RESULTS
After a median follow-up of 5.4 years, patients ingesting ≥1 hypertension medications at bedtime showed a significantly lower cardiovascular risk (adjusted by age and sex) than subjects ingesting all medications upon awakening (hazard ratio 0.33 [95% CI 0.21–0.54]; P < 0.001). The difference between groups in the adjusted risk of major events (cardiovascular death, myocardial infarction, and stroke) was also statistically significant (0.25 [0.10–0.61]; P = 0.003). Patients treated at bedtime showed significantly lower sleep time blood pressure mean and higher prevalence of controlled ambulatory blood pressure (62.5 vs. 50.9%; P = 0.013). There was a significant 12% cardiovascular risk reduction per each 5 mmHg decrease in asleep systolic blood pressure during follow-up (P < 0.001).
CONCLUSIONS
Among patients with diabetes, treatment with ≥1 hypertension medications at bedtime, compared with all medications upon waking, resulted in improved ambulatory blood pressure control and significantly reduced cardiovascular morbidity and mortality.
A number of published prospective trials reviewed elsewhere (1) have reported clinically meaningful morning/evening treatment time differences in blood pressure lowering efficacy, duration of action, safety profile, and/or effects on the circadian blood pressure pattern for different classes of hypertension medications. For instance, a once-daily evening, in comparison with morning, ingestion schedule of angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) results in greater therapeutic effect on asleep blood pressure, independent of the terminal half-life of each individual medication (1).
The impact of bedtime chronotherapy on sleep time blood pressure regulation might be of clinical importance. This perspective is based on the growing number of studies, all concerning ambulatory blood pressure monitoring (ABPM), that have consistently shown an association between blunted sleep time blood pressure decline and increased incidence of cardiovascular disease (CVD) events, both in subjects without (2–4) as well as with diabetes (5–7). Independent prospective studies have also found that the sleep time blood pressure mean is a better predictor of CVD risk than the daytime or 24-h blood pressure mean (3,8–12), a relevant finding also documented for patients with diabetes (13–15). Nocturnal hypertension is not only frequent but also highly predominant in patients with diabetes (6,7,13–15). A limitation of all of these previous studies on the prognostic value of nighttime blood pressure is their reliance on a single baseline ABPM profile from each participant at the time of inclusion, without accounting for changes in the blood pressure pattern or level during the years of follow-up. Thus, the potential reduction in CVD risk associated with specifically reducing sleep time blood pressure, which has been found to be much more feasible by bedtime than by upon waking dosing of conventional hypertension medications (1), is still a matter of debate.
The MAPEC (Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares [Ambulatory Blood Pressure Monitoring for Prediction of Cardiovascular Events]) study was specifically designed to investigate prospectively whether bedtime treatment with ≥1 hypertension medications exerts significantly better blood pressure control and CVD risk reduction than conventional therapy, in which all medications are ingested upon waking (16,17). We here report results on the differential effect of blood pressure–lowering chronotherapy on CVD risk in hypertensive patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Inclusion and exclusion criteria
An extended version of the methods is available in the Supplementary Data. In summary, the sample represents a population of Spanish hypertensive patients with type 2 diabetes of both sexes and ≥18 years of age. Exclusion criteria were pregnancy, history of drug/alcohol abuse, night/shift work employment, diagnosis of AIDS, type 1 diabetes, secondary hypertension, CVD disorders (unstable angina pectoris, heart failure, life-threatening arrhythmia, nephropathy, and grade III-IV retinopathy), intolerance to ABPM, and inability to communicate and comply with all study requirements. This prospective single-center study (registered at www.clinicaltrials.gov, NCT00295542) was approved by the state Ethics Committee of Clinical Research. All patients gave written informed consent.
Subjects and diagnostic criteria
We assessed 480 patients fulfilling the inclusion/exclusion criteria. Among these, 448 (255 men/193 women, mean ± SD 62.5 ± 10.8 years of age) provided all required information for the study. We established a priori a minimum time of follow-up of ≥6 months for each patient and a minimum median follow-up of 5 years (16). A total of 32 patients evaluated by ABPM for potential inclusion were not randomized as a result of their lack of consent for additional ABPM evaluations. Diagnosis of hypertension was based on accepted ABPM criteria—an awake blood pressure mean of ≥135/85 mmHg for systolic (SBP)/diastolic blood pressure (DBP), or an asleep blood pressure mean ≥120/70 mmHg (18).
Study design
This was a prospective, randomized, open-label, blinded end point trial. Participants were randomized to ingest all their prescribed blood pressure–lowering medications upon awakening (232 patients) or ≥1 of them at bedtime (216 patients). Blood samples were obtained between 08:00 and 09:00 h, after overnight fasting, the same week when each 48-h ABPM session was initiated. Just before commencing ABPM, six clinic blood pressure measurements were obtained with a validated automatic oscillometric device (HEM-705IT; Omron Health Care, Vernon Hills, IL) after the patient had rested in a seated position for ≥10 min.
ABPM assessment
At inclusion, as well as at each scheduled visit for ABPM during follow-up (see below), the SBP and DBP of each patient were automatically measured every 20 min between 07:00 and 23:00 h and every 30 min during the night for 48 consecutive hours with a calibrated SpaceLabs 90207 ABPM monitor (SpaceLabs, Issaquah, WA). Blood pressure series were considered invalid for analysis if ≥30% of the measurements were missing, if data were lacking for an interval of >2 h, if data were obtained while patients had an irregular rest-activity schedule during the two days of monitoring, or if the nighttime sleep period was <6 or >12 h during ABPM.
Actigraphy
All patients wore an actigraph (Mini-Motion-Logger; Ambulatory Monitoring, Ardsley, NY) on the dominant wrist to monitor physical activity every minute during ABPM. The actigraphy data, combined with patient diaries, were used to corroborate the absence of daytime napping and to define the commencement and termination of the daytime awake and nocturnal asleep spans so the respective blood pressure means for each subject could be accurately determined.
Follow-up
The same evaluation procedure described above, including conventional clinic blood pressure measurement, 48-h ABPM and wrist activity monitoring, and blood sampling, was scheduled annually or more frequently (after 3 months of any change in treatment) if the therapeutic scheme was modified to improve ambulatory blood pressure control. Investigators blinded to the timed-treatment scheme of each participant (thus excluding those performing clinic evaluation at each visit to the hospital, clinic and ambulatory blood pressure measurement, and/or statistical analyses) reviewed at least annually the complete clinical records of all enrolled patients to assess CVD morbidity and mortality. Registered events included death from all causes, myocardial infarction, angina pectoris, coronary revascularization, heart failure, acute arterial occlusion of lower extremities, thrombotic occlusion of the retinal artery, hemorrhagic stroke, ischemic stroke, and transient ischemic attack.
Statistical methods
To correct for measurement errors and outliers, ABPM profiles were edited according to conventional criteria. Thus, SBP readings >250 or <70 mmHg, DBP >150 or <40 mmHg, and pulse pressure (difference between SBP and DBP) >150 or <20 mmHg were automatically discarded. The primary outcomes study end points were total CVD morbidity and mortality, which included all the events listed above, and major CVD events, i.e., a composite of CVD deaths, myocardial infarction, and stroke. Demographic and clinical characteristics were compared on an intention-to-treat basis among groups of subjects randomized to the two treatment time groups by t test (quantitative variables) or nonparametric χ2 test (proportions). The Cox proportional-hazard model was used to estimate hazard ratios (95% CIs) for events associated with time of treatment, with adjustment for significant confounding variables. Event rates for fatal and nonfatal CVD events during follow-up were also expressed as the number/1,000 patient-years, i.e., ratio of the observed number of events to the total number of patient-years of exposure. Survival curves were generated using the Kaplan-Meier product-limit method and compared by the Mantel log-rank test.
RESULTS
Demographic characteristics, laboratory variables, and ambulatory blood pressure
At baseline, the two treatment time groups were comparable for the prevalence of obstructive sleep apnea, metabolic syndrome, microalbuminuria, and obesity, plus all anthropometric variables and clinical laboratory test values (Table 1). The clinic blood pressure, mean ambulatory blood pressure values, and prevalence of nondipping at baseline were also comparable between groups (Table 1).
Table 1.
Baseline characteristics of patients investigated according to treatment time (either all hypertension medications upon awakening or ≥1 medications at bedtime)
| Awakening | Bedtime | P between groups | |
|---|---|---|---|
| Demographic characteristics | |||
| n | 232 | 216 | |
| Sex (% men) | 59.1 | 54.6 | 0.345 |
| Obstructive sleep apnea (%) | 13.4 | 11.6 | 0.568 |
| Metabolic syndrome (%) | 86.6 | 86.6 | 0.984 |
| Cigarette smoking (%) | 8.6 | 7.9 | 0.773 |
| Obesity (%) | 66.8 | 64.8 | 0.656 |
| Microalbuminuria (%) | 28.0 | 26.9 | 0.782 |
| Previous CVD events (%) | 8.2 | 9.3 | 0.688 |
| Duration of known diabetes (years) | 8.9 ± 8.4 | 8.7 ± 8.0 | 0.804 |
| Duration of known hypertension (years) | 7.4 ± 8.2 | 7.6 ± 8.7 | 0.674 |
| Anthropometric variables and clinic blood pressure | |||
| Age (years) | 62.5 ± 10.9 | 62.5 ± 10.7 | 0.935 |
| Height (cm) | 160.6 ± 9.2 | 159.3 ± 9.0 | 0.117 |
| Weight (kg) | 83.5 ± 16.9 | 81.1 ± 16.0 | 0.124 |
| BMI (kg/m2) | 32.1 ± 5.5 | 31.9 ± 5.2 | 0.631 |
| Waist circumference (cm) | 102.9 ± 13.9 | 102.0 ± 11.6 | 0.442 |
| Clinic SBP (mmHg)† | 158.1 ± 24.7 | 161.9 ± 22.2 | 0.085 |
| Clinic DBP (mmHg)† | 85.7 ± 13.6 | 87.0 ± 12.3 | 0.303 |
| Clinic PP (mmHg)† | 72.4 ± 16.9 | 74.9 ± 17.8 | 0.117 |
| Clinic HR (bpm)† | 75.6 ± 10.5 | 76.6 ± 13.7 | 0.391 |
| Clinical laboratory test values | |||
| HbA1c (%) | 6.9 ± 1.7 | 6.8 ± 1.7 | 0.627 |
| Glucose (mg/dL) | 157.2 ± 49.1 | 150.8 ± 51.2 | 0.178 |
| Creatinine (mg/dL) | 1.03 ± 0.25 | 1.01 ± 0.27 | 0.393 |
| Uric acid (mg/dL) | 6.1 ± 2.0 | 6.0 ± 1.6 | 0.271 |
| Total cholesterol (mg/dL) | 203.3 ± 45.7 | 202.7 ± 42.0 | 0.886 |
| Triglycerides (mg/dL) | 126.9 ± 63.2 | 120.6 ± 68.3 | 0.321 |
| HDL cholesterol (mg/dL) | 44.3 ± 13.6 | 46.6 ± 15.2 | 0.092 |
| LDL cholesterol (mg/dL) | 134.9 ± 38.1 | 131.7 ± 35.5 | 0.378 |
| Fibrinogen (mg/dL) | 357.7 ± 78.1 | 346.8 ± 88.3 | 0.265 |
| Erythrocyte sedimentation rate (mm) | 19.1 ± 16.8 | 17.6 ± 15.4 | 0.349 |
| Glomerular filtration rate‡ | 71.0 ± 18.2 | 72.7 ± 17.1 | 0.206 |
| Ambulatory blood pressure | |||
| Duration of nocturnal rest (h) | 9.2 ± 1.3 | 9.2 ± 1.2 | 0.902 |
| Awake SBP mean (mmHg) | 135.4 ± 17.9 | 135.9 ± 15.8 | 0.762 |
| Asleep SBP mean (mmHg) | 128.5 ± 21.7 | 129.2 ± 20.2 | 0.702 |
| 48-h SBP mean (mmHg) | 133.2 ± 18.6 | 133.5 ± 16.5 | 0.837 |
| Sleep time relative SBP decline (%) | 5.2 ± 8.3 | 5.0 ± 8.7 | 0.779 |
| Awake DBP mean (mmHg) | 76.7 ± 11.0 | 77.3 ± 11.0 | 0.553 |
| Asleep DBP mean (mmHg) | 68.3 ± 11.4 | 69.3 ± 10.9 | 0.346 |
| 48-h DBP mean (mmHg) | 73.9 ± 10.8 | 74.6 ± 10.6 | 0.538 |
| Sleep time relative DBP decline (%) | 10.7 ± 8.7 | 10.1 ± 8.9 | 0.432 |
| Nondipper (%) | 72.4 | 70.0 | 0.558 |
Data are means ± SD unless otherwise indicated. Metabolic syndrome is determined by the National Cholesterol Education Program Adult Treatment Panel III (ATP-III) revised definition (19). The sleep time relative blood pressure decline, an index of blood pressure dipping, is defined as the percent decline in mean blood pressure during nocturnal sleep relative to the mean blood pressure during daytime activity, and calculated as [(awake blood pressure mean – asleep blood pressure mean)/awake blood pressure mean] × 100. HR, heart rate; Nondipper, patients with sleep time relative SBP decline <10% using data sampled by ABPM for 48 consecutive hours; obesity, BMI ≥30 kg/m2; PP, pulse pressure.
†Values correspond to the average of six conventional blood pressure measurements obtained for each subject at the clinic before starting ABPM.
‡Glomerular filtration rate (mL/min/1.73 m2) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (20).
There were no differences in the classes and number of hypertension medications used for therapy between the two treatment groups (Table 2). The percentage of patients treated with statins (40.5% in the morning treatment group, 46.3% in the evening treatment group; P = 0.217 between groups) or low-dose (100 mg/day) aspirin (22.4 vs. 22.7%; P = 0.945) were also similar in both treatment time groups.
Table 2.
Final characteristics of patients investigated according to treatment time (either all hypertension medications upon awakening or ≥1 medications at bedtime)
| Awakening | Bedtime | P between groups | |
|---|---|---|---|
| n | 232 | 216 | |
| Primary end points* | |||
| Total events | 54.24 (68) | 19.80 (23) | <0.001 |
| Major events | 17.55 (22) | 5.16 (6) | <0.001 |
| Secondary end points* | |||
| Total death | 6.38 (8) | 2.58 (3) | 0.097 |
| Cardiovascular death | 4.79 (6) | 0.86 (1) | 0.038 |
| Other cause | 1.60 (2) | 1.72 (2) | 0.968 |
| Cardiovascular events | 15.95 (20) | 6.89 (8) | 0.008 |
| Cerebrovascular events | 6.38 (8) | 0.86 (1) | 0.010 |
| Heart failure | 13.56 (17) | 6.02 (7) | 0.020 |
| Other events | 11.96 (15) | 3.44 (4) | 0.005 |
| Hypertension treatment | |||
| Number of medications | 2.6 ± 1.1 | 2.4 ± 1.2 | 0.145 |
| 1 Medication (%) | 23.7 | 28.7 | 0.229 |
| 2 Medications (%) | 15.9 | 19.4 | 0.332 |
| ≥3 Medications (%) | 60.3 | 51.9 | 0.070 |
| ARB (%) | 63.4 | 67.1 | 0.403 |
| ACEI (%) | 27.2 | 20.4 | 0.159 |
| Calcium channel blocker (%) | 50.0 | 49.1 | 0.845 |
| α-Blocker (%) | 29.7 | 28.7 | 0.809 |
| β-Blocker (%) | 21.1 | 22.2 | 0.777 |
| Diuretic (%) | 63.4 | 56.5 | 0.137 |
| Clinic and ambulatory blood pressure | |||
| Clinic SBP (mmHg)† | 150.3 ± 28.6 | 147.9 ± 21.3 | 0.309 |
| Clinic DBP (mmHg)† | 80.5 ± 16.3 | 78.6 ± 14.3 | 0.187 |
| Clinic PP (mmHg)† | 69.8 ± 18.2 | 69.3 ± 14.7 | 0.742 |
| Clinic HR (bpm)† | 73.6 ± 13.8 | 74.6 ± 14.2 | 0.453 |
| Awake SBP mean (mmHg) | 127.1 ± 17.8 | 126.8 ± 14.6 | 0.861 |
| Asleep SBP mean (mmHg) | 122.4 ± 21.8 | 115.0 ± 17.1 | <0.001 |
| 48-h SBP mean (mmHg) | 125.5 ± 18.3 | 122.8 ± 15.0 | 0.097 |
| Sleep time relative SBP decline (%) | 3.7 ± 10.3 | 9.4 ± 7.8 | <0.001 |
| Awake DBP mean (mmHg) | 70.5 ± 10.8 | 71.0 ± 10.7 | 0.621 |
| Asleep DBP mean (mmHg) | 63.7 ± 11.3 | 60.2 ± 10.1 | <0.001 |
| 48-h DBP mean (mmHg) | 68.2 ± 10.4 | 67.4 ± 10.1 | 0.406 |
| Sleep time relative DBP decline (%) | 9.3 ± 11.4 | 14.9 ± 9.2 | <0.001 |
| Nondipper (%) | 76.3 | 49.5 | <0.001 |
| Controlled ambulatory blood pressure (%) | 50.9 | 62.5 | 0.013 |
| Controlled awake blood pressure (%) | 75.4 | 72.2 | 0.439 |
| Controlled asleep blood pressure (%) | 54.7 | 70.8 | <0.001 |
Data are means ± SD. Event rates (95% CIs) are expressed as the number/1,000 patient-years, i.e., ratio of the observed number of events to the total number of patient-years of exposure. Total events include death (from all causes), cardiovascular events (myocardial infarction, angina pectoris, and coronary revascularization), cerebrovascular events (stroke and transient ischemic attack), heart failure, and other events (acute arterial occlusion of lower extremities and thrombotic occlusion of the retinal artery). Major events include cardiovascular deaths, myocardial infarction, ischemic stroke, and hemorrhagic stroke. Comparison of event rates between treatment time groups was done by the Mantel log-rank test. The sleep time relative blood pressure decline, an index of blood pressure dipping, is defined as the percent decline in mean blood pressure during nocturnal sleep relative to the mean blood pressure during daytime activity, and calculated as: [(awake blood pressure mean – asleep blood pressure mean)/awake blood pressure mean] x 100. HR, heart rate; Nondipper, patients with sleep time relative SBP decline <10% using data sampled by ABPM for 48 consecutive hours; PP, pulse pressure.
*Number of events is shown in parentheses.
†Values correspond to the average of six conventional blood pressure measurements obtained for each subject at the clinic before starting ABPM.
The data of the last evaluation revealed differences between the two treatment time groups, significant even after correction for multiple testing. The group of patients ingesting ≥1 medications at bedtime showed significantly lower mean asleep blood pressure than the group of patients ingesting all their medications upon awakening (P < 0.001; Table 2). Differences between groups in clinic and awake blood pressure were small and nonsignificant. The sleep time relative blood pressure decline was significantly greater among patients ingesting ≥1 medications at bedtime; accordingly, the proportion of patients in this treatment time group with a nondipper blood pressure profile (sleep time SBP decline <10%) was significantly lower than that in the upon-awakening treatment time group (49.5 vs. 76.3%; P < 0.001). Finally, the proportion of participants with controlled blood pressure, with reference to established ABPM criteria for both awake and asleep blood pressure mean, was significantly greater among patients ingesting ≥1 medications at bedtime than in those ingesting all medications upon awakening (62.5 vs. 50.9%; P = 0.013; Table 2). This difference in ambulatory blood pressure control was mainly due to improved sleep time blood pressure control in subjects ingesting medication at bedtime. Thus, although the percentage of subjects with controlled awake blood pressure mean was similar for both treatment time groups (P = 0.439), asleep blood pressure mean was controlled in a significantly larger percentage of patients treated at bedtime (70.8 vs. 54.7%; P < 0.001, Table 2).
CVD risk according to time of day of hypertension treatment
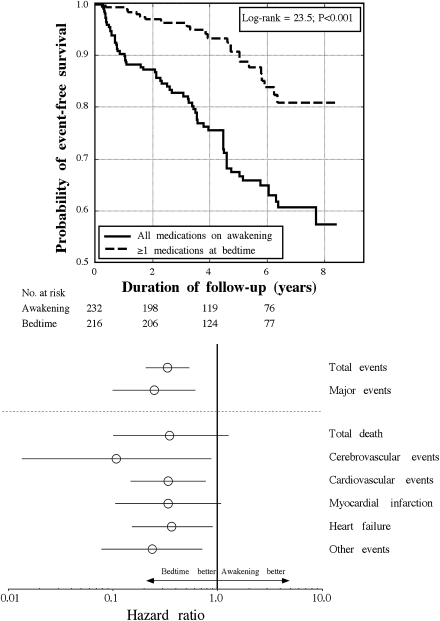
During the median follow-up period of 5.4 years (range 0.5–8.4), we documented 91 events (11 deaths, 14 myocardial infarctions, 8 angina pectoris, 6 coronary revascularizations, 9 cerebrovascular events, 24 heart failures, 7 cases of aortoiliac occlusive disease, and 12 thrombotic occlusions of the retinal artery; Table 2). Figure 1 (top) presents, for the total of all the documented events, the Kaplan-Meier survival curves for the subjects of the two treatment time groups; a highly significant difference was detected in event-free survival (log-rank 23.5; P < 0.001). Table 2 provides further information on the distribution of the CVD events in both treatment time groups. The bedtime group showed a significantly lower incidence of total and major CVD events. CVD mortality was more prevalent among patients who ingested all their hypertension medications upon awakening (P = 0.038).
Figure 1.
Top: Kaplan-Meier survival curves as a function of time of day of hypertension treatment, i.e., for patients with type 2 diabetes ingesting either all their blood pressure–lowering medications upon awakening or ≥1 medications at bedtime. Bottom: Hazard ratios (95% CIs) of CVD events (adjusted by age and sex) as a function of time of day of hypertension treatment, i.e., for patients with type 2 diabetes ingesting either all their blood pressure–lowering medications upon awakening or ≥1 medications at bedtime. Total events include death (from all causes), cardiovascular events (myocardial infarction, angina pectoris, and coronary revascularization), cerebrovascular events (stroke and transient ischemic attack), heart failure, and other events (acute arterial occlusion of lower extremities and thrombotic occlusion of the retinal artery). Major events include cardiovascular deaths, myocardial infarction, ischemic stroke, and hemorrhagic stroke.
Figure 1 (bottom) also shows the hazard ratios of CVD events estimated by the Cox proportional-hazard model for the participants of the respective treatment time groups. Adjustments were applied for sex and age in all comparisons, as these influential factors were the only ones among all the demographic and laboratory variables shown in Table 1 that were consistently significant in all tested Cox regression models. Patients ingesting ≥1 blood pressure–lowering medications at bedtime evidenced a significantly lower risk of total events than patients ingesting all of their medications upon awakening (hazard ratio 0.33 [95% CI 0.21–0.54]; P < 0.001). Particularly relevant is the difference between the two treatment time groups in the risk of major CVD events, i.e., CVD deaths, myocardial infarction, and stroke (0.25 [0.10–0.61]; P = 0.003). Results remained unchanged after correction by the use of statins and/or aspirin, as these variables were not significantly associated with event-free survival.
Changes in clinic and ambulatory blood pressure during follow-up as predictors of CVD risk
The results shown in Table 2 indicate that those patients randomized to ingest ≥1 medications at bedtime experienced significantly better nighttime blood pressure control as expressed by the enhanced reduction of the sleep time blood pressure mean and increased sleep time relative blood pressure decline toward a more dipping blood pressure pattern. Moreover, only 23% of the patients who experienced a CVD event had their asleep blood pressure properly controlled (<120/70 for SBP/DBP); awake blood pressure, however, was controlled in 43% of event subjects. Accordingly, we further evaluated the influence on CVD risk of changes in ambulatory blood pressure during follow-up. Cox regression analyses, adjusted for sex, age, and change in 48-h blood pressure between the first and last evaluations of each patients, revealed that the progressive decrease in asleep blood pressure mean during follow-up was most significantly associated with event-free survival (12 and 23% risk reduction for every 5 mmHg decrease in asleep blood pressure mean of SBP and DBP, respectively; P < 0.001).
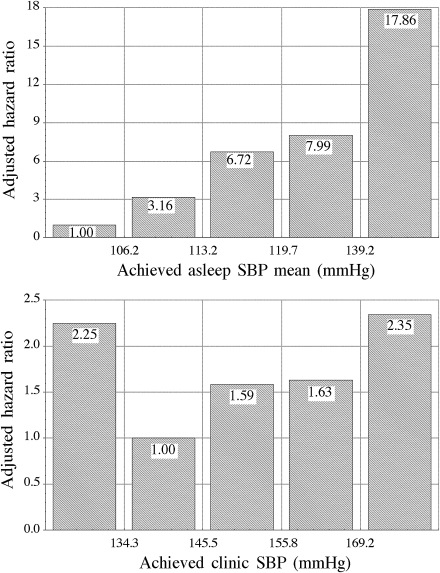
The top panel in Fig. 2 shows, for the studied population divided in quintiles, the significant relationship between progressively lower achieved asleep SBP mean and reduced CVD risk. Adjusted risk was significantly higher (P < 0.001) in the last three quintiles compared with the first two. There was a progressive reduction from 65 to 30% in the percentage of patients treated at bedtime across the quintiles, indicating the association between bedtime treatment, enhanced nighttime blood pressure reduction, and decreased CVD risk. Figure 2 (bottom) also shows a J-shaped relationship between achieved daytime clinic SBP and CVD risk; the adjusted relative CVD risk was significantly higher (P = 0.023) in the first than in the second quintile, and then progressively increased again in the other three (P = 0.028 between the second and the last quintile). The percentage of patients treated with ≥1 hypertension medication at bedtime was lower (41%) in the first than in the second quintile (57%; P = 0.037).
Figure 2.
Hazard ratio of CVD events (adjusted by age, sex, and number of hypertension medications used for treatment) as a function of achieved asleep SBP mean (top) and daytime clinic SBP (bottom) at the time of the last ABPM evaluation. Studied population was divided into five classes of equal size (quintiles).
CONCLUSIONS
This study prospectively investigated in hypertensive patients with type 2 diabetes the hypothesis that bedtime treatment with ≥1 hypertension medications exerts better blood pressure control and CVD risk reduction than conventional therapy, in which all medications are ingested upon waking. The results document, first, greater ambulatory blood pressure control in patients ingesting ≥1 hypertension medications at bedtime than in those ingesting all their medications upon awakening. The main differences between groups in terms of blood pressure control were achievement in patients treated at bedtime of 1) significantly lower asleep blood pressure mean and 2) greater sleep time relative blood pressure decline, without loss of awake blood pressure lowering efficacy (Table 2). These administration-time–dependent effects on sleep time blood pressure control were strongly associated with lower CVD risk and increased event-free survival. Indeed, the progressive reduction in the asleep blood pressure mean from baseline was the most significant predictor of survival. Moreover, lack of sleep time blood pressure control with reference to established ABPM criteria (18) was the most prevalent factor among subjects with documented CVD events. As documented in a series of prospective trials reviewed elsewhere (1), and also corroborated in the long-term evaluation provided here, treatment at bedtime is the most cost-effective and simplest strategy of successfully achieving the therapeutic goals of adequate asleep blood pressure reduction and preserving or reestablishing the normal 24-h blood pressure dipping pattern. One could thus conclude that the increased event-free survival associated with bedtime chronotherapy with ≥1 blood pressure–lowering medications, compared with upon-waking treatment of all medications, is linked to better achievement of these novel hypertension therapeutic goals.
Therapeutic intervention in hypertension consists of adequate control of blood pressure, the goal being to reduce/avert CVD morbidity and mortality. Blood pressure control has been defined so far on the unique basis of lowering blood pressure level (mainly if not exclusively determined conventionally at the clinic), without paying attention to potential alterations in the circadian blood pressure pattern due to treatment. Some of these studies found that too high a reduction in clinic blood pressure might be associated with increased CVD risk, whereas moderate reduction in blood pressure would decrease it; such association is known as the J-shaped or U-shped effect (21). We also found a J-shaped association in the relation between achieved clinic blood pressure and CVD risk (Fig. 2, bottom). However, the relation between achieved asleep blood pressure mean and CVD risk (Fig. 2, top) presented a highly significant lower CVD risk associated with progressive diminished asleep blood pressure mean. Moreover, the amount of the asleep blood pressure reduction during follow-up was significantly correlated with increased number of patients treated at bedtime. One needs to realize that 1) most marketed medictions do not provide homogeneous long-lasting efficacy throughout the entire 24 h, 2) no marketed hypertension medication provides greater reduction of asleep than awake blood pressure when administered in the morning, and 3) increasing the number of hypertension medications administered in the morning may lead to more intensive clinic blood pressure reduction but also to a progressive reduction in the sleep time relative blood pressure decline toward a more nondipper blood pressure pattern as a consequence of the greater reduction in awake than asleep blood pressure (1). We thus conclude that the actual controversy on the possible J-shaped relation with CVD risk, described so far only for clinic blood pressure determined in patients presumably treated in the morning (21,22), might not apply (when avoiding nocturnal hypotension) to asleep blood pressure mean, a more significant predictor of CVD morbidity and mortality that can be cost-effectively modified by proper-timed treatment (1), as also documented here in patients with type 2 diabetes.
In conclusion, in hypertensive subjects with type 2 diabetes, we recommend taking into account the variable of treatment time with respect to the 24-h rest-activity pattern of each patient. Our findings document that a bedtime schedule with ≥1 blood pressure–lowering medications, in comparison with a schedule in which all such medications are ingested upon awakening, not only improves blood pressure control and decreases the prevalence of nondipping, but it significantly reduces CVD risk. Our results also document for the first time that reducing the asleep blood pressure mean while avoiding nocturnal hypotension, a novel therapeutic target requiring proper patient evaluation by ABPM, significantly decreases CVD morbidity and mortality in patients with type 2 diabetes.
Supplementary Material
Acknowledgments
This independent investigator-promoted research was supported by unrestricted grants from Ministerio de Ciencia e Innovación (SAF2006-6254-FEDER; SAF2009-7028-FEDER); Consellería de Presidencia, Relacións Institucionais e Administración Pública, Secretaría Xeral de Investigación e Desenvolvemento, Xunta de Galicia (PGIDIT03-PXIB-32201PR); Consellería de Economía e Industria, Dirección Xeral de Investigación e Desenvolvemento, Xunta de Galicia (INCITE07-PXI-322003ES; INCITE08-E1R-322063ES; INCITE09-E2R-322099ES; 09CSA018322PR); and Vicerrectorado de Investigación, University of Vigo.
No potential conflicts of interest relevant to this article were reported.
R.C.H. and D.E.A. contributed to every aspect of this article. A.M. and J.R.F. contributed to study design, discussion, research data, statistical analyses, and review of the manuscript.
Footnotes
Clinical trial reg. no. NCT00295542, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0297/-/DC1.
See accompanying editorial, p. 1438.
References
- 1.Smolensky MH, Hermida RC, Ayala DE, Tiseo R, Portaluppi F. Administration-time-dependent effects of blood pressure–lowering medications: basis for the chronotherapy of hypertension. Blood Press Monit 2010;15:173–180 [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002;20:2183–2189 [DOI] [PubMed] [Google Scholar]
- 3.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005;46:156–161 [DOI] [PubMed] [Google Scholar]
- 4.Boggia J, Li Y, Thijs L, et al. ; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007;370:1219–1229 [DOI] [PubMed] [Google Scholar]
- 5.Nakano S, Fukuda M, Hotta F, et al. Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes 1998;47:1501–1506 [DOI] [PubMed] [Google Scholar]
- 6.Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med 2000;17:360–364 [DOI] [PubMed] [Google Scholar]
- 7.Eguchi K, Pickering TG, Hoshide S, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens 2008;21:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension 2005;45:240–245 [DOI] [PubMed] [Google Scholar]
- 9.Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007;49:1235–1241 [DOI] [PubMed] [Google Scholar]
- 10.Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008;51:55–61 [DOI] [PubMed] [Google Scholar]
- 11.Fan HQ, Li Y, Thijs L, et al. ; International Database on Ambulatory Blood Pressure In Relation to Cardiovascular Outcomes Investigators Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens 2010;28:2036–2045 [DOI] [PubMed] [Google Scholar]
- 12.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension 2011;57:3–10 [DOI] [PubMed] [Google Scholar]
- 13.Nakano S, Ito T, Furuya K, et al. Ambulatory blood pressure level rather than dipper/nondipper status predicts vascular events in type 2 diabetic subjects. Hypertens Res 2004;27:647–656 [DOI] [PubMed] [Google Scholar]
- 14.Astrup AS, Nielsen FS, Rossing P, et al. Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens 2007;25:2479–2485 [DOI] [PubMed] [Google Scholar]
- 15.Bouhanick B, Bongard V, Amar J, Bousquel S, Chamontin B. Prognostic value of nocturnal blood pressure and reverse-dipping status on the occurrence of cardiovascular events in hypertensive diabetic patients. Diabetes Metab 2008;34:560–567 [DOI] [PubMed] [Google Scholar]
- 16.Hermida RC. Ambulatory blood pressure monitoring in the prediction of cardiovascular events and effects of chronotherapy: rationale and design of the MAPEC study. Chronobiol Int 2007;24:749–775 [DOI] [PubMed] [Google Scholar]
- 17.Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int 2010;27:1629–1651 [DOI] [PubMed] [Google Scholar]
- 18.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007;25:1105–1187 [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, et al. ; American Heart Association; National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vokó Z, Bots ML, Hofman A, Koudstaal PJ, Witteman JCM, Breteler MMB. J-shaped relation between blood pressure and stroke in treated hypertensives. Hypertension 1999;34:1181–1185 [DOI] [PubMed] [Google Scholar]
- 22.Cushman WC, Evans GW, Byington RP, et al. ; ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.