Abstract
OBJECTIVE
To compare the pharmacokinetics and pharmacodynamics of NPH, glargine, and detemir insulins in type 2 diabetic subjects.
RESEARCH DESIGN AND METHODS
This study used a single-blind, three-way, cross-over design. A total of 18 type 2 diabetic subjects underwent a euglycemic clamp for 32 h after a subcutaneous injection of 0.4 units/kg at 2200 h of either NPH, glargine, or detemir after 1 week of bedtime treatment with each insulin.
RESULTS
The glucose infusion rate area under the curve0–32 h was greater for glargine than for detemir and NPH (1,538 ± 688; 1,081 ± 785; and 1,170 ± 703 mg/kg, respectively; P < 0.05). Glargine suppressed endogenous glucose production more than detemir (P < 0.05) and similarly to NPH (P = 0.16). Glucagon, C-peptide, free fatty acids, and β-hydroxy-butyrate were more suppressed with glargine than detemir. All 18 subjects completed the glargine study, but two subjects on NPH and three on detemir interrupted the study because of plasma glucose >150 mg/dL.
CONCLUSIONS
Compared with NPH and detemir, glargine provided greater metabolic activity and superior glucose control for up to 32 h.
Very few studies have investigated the pharmacokinetics and pharmacodynamics of NPH insulin in type 2 diabetes (1,2). These studies have provided valuable information that is limited, however, by a short period of observation (12–16 h) and by the comparison of NPH with only one long-acting insulin analog, either glargine or detemir. The present randomized cross-over study was undertaken to establish the pharmacokinetics and pharmacodynamics of the basal insulins NPH, glargine, and detemir in subjects with type 2 diabetes who need insulin.
RESEARCH DESIGN AND METHODS
After approval by the local ethical committee, and after receiving informed, written consent, 18 type 2 diabetic subjects on insulin (NPH as basal) and/or oral hypoglycemic agents were recruited (Supplementary Table 1) and studied according to the Helsinki Declaration and GCP requirements. This was a randomized, single-dose, single-blind, three-way, cross-over study that used the previously described euglycemic glucose clamp technique (3). Each subject received the three insulins by assignment to one of three sequences, as directed by a Latin-square design (ABC/BCA/CAB). After a 2-week run-in period, during which the previous treatment was continued, subjects were randomly assigned to a once-daily dose of either NPH (Humulin I; Lilly and Co., Indianapolis, IN), detemir (Levemir; Novo Nordisk, Bagsværd, Denmark), or glargine (Lantus; Aventis Pharma, Frankfurt, Germany) at 2200 h for a period of 1 week, during which titration of the dose of basal insulin was continued. After the 7-day treatment, all subjects underwent a euglycemic clamp for 32 h after a 0.4 units/kg subcutaneous injection at 2200 h of the basal insulin they were on (Supplementary Study Design). On the day of the study, subjects received the last subcutaneous insulin (rapid-acting analog) with the 1200-h meal and fasted afterward. Between 1600 and 2200 h, they received intravenous insulin to normalize plasma glucose, as previously described (3). Then, subjects had a 2-week wash-out period, during which they resumed the basal insulin regimen of the run-in period (NPH). The subjects were then crossed over to the second basal insulin according to the randomization schedule and studied as above and finally moved to the third basal insulin and studied for the third and last time.
The primary end point was the glucose infusion rate (GIR) over 0–32 h (area under the curve [AUC]0–32 h). Secondary outcomes are shown in the Supplementary Data. Duration of action was defined as the minimal duration of action and end of insulin action (calculated as the time at which plasma glucose was >118 and 136 mg/dL, respectively). The study ended at the time at which plasma glucose was, for at least 30 min, >150 mg/dL (all definitions in the absence of glucose infusion). Plasma glucose, C-peptide, insulin, glucagon, and nonglucose substrates (free fatty acids, β-hydroxy-butyrate, and glycerol), as well as glucose fluxes, were measured as previously described (4) (Supplementary Analytical Methods). During the clamp, glucose flux calculations were based on a non–steady-state assumption (5).
Data analysis was carried out by using ANOVA for cross-over design (6) and is described in the Supplementary Statistical Analysis.
RESULTS
All 18 subjects performed the three clamp studies. However, two and three of the 18 subjects treated with NPH and detemir, respectively, discontinued the clamp study earlier than 32 h because of plasma glucose >150 mg/dL. Glycemic control and insulin doses in the week prior to the studies, as well as plasma glucose concentration and rates of intravenous insulin infusion prior to the subcutaneous insulin injection at the beginning of the clamp study, are given online (Supplementary Results), likewise for plasma insulin, plasma C-peptide, glucagon, and plasma glycerol (Supplementary Fig. 1 and Supplementary Tables 2 and 3).
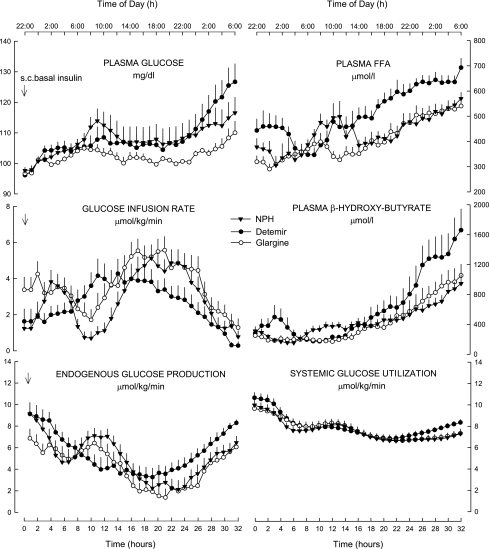
The mean GIR for the 32-h study period (AUC0–32 h) was greater with glargine than NPH by 31% and detemir by 42% (Fig. 1; Supplementary Table 2). Endogenous glucose production (EGP) decreased after the subcutaneous insulin injection in all three treatments but to a greater extent with glargine (Fig. 1). Glucose utilization decreased in all treatments and overall was no different among insulin treatments (P = 0.268) (Fig. 1). Plasma free fatty acid concentrations were higher with detemir compared with NPH (+15%, P = 0.003) and glargine (+26%, P < 0.001) throughout the study, as was plasma β-hydroxy-butyrate (Fig. 1).
Figure 1.
Plasma glucose, GIR, rates of EGP and glucose utilization, and plasma concentrations of free fatty acids (FFAs) and β-hydroxy-butyrate after a subcutaneous (s.c.) injection of NPH, detemir, and glargine insulin in type 2 diabetic subjects.
The median time of minimal duration of action (plasma glucose >118 mg/dL) and duration of action (plasma glucose >136 mg/dL) differed among the three treatments. Glargine exhibited a smaller interquartile range in time of duration of action than NPH and detemir, although the statistical significance was achieved only versus detemir (Supplementary Table 2).
Results of the 0- to 32-h period and of the 0- to 16-h and 16- to 32-h periods are given online (Supplementary Tables 2 and 3, respectively).
CONCLUSIONS
The results of the current study indicate that the main outcome pharmacodynamic parameter GIR0–32 h, taken as a surrogate measure of insulin metabolic activity, was greater for glargine compared with NPH and detemir. GIR with NPH increased shortly after the subcutaneous injection at 0400 h, as a result of a rapid suppression of EGP by ~50%, followed by a sensible reduction after 10–11 h because of increased EGP, which resulted in a significant increase in plasma glucose. This most likely accounts for the greater risk for nocturnal hypoglycemia reported in clinical studies with NPH versus glargine and detemir (7), as well as for the abnormal early-morning increase in blood glucose known as the “dawn phenomenon” (8). In the second half of the study, from 1400 h, GIR requirements for NPH and glargine were greater than those for detemir up to the end of the study. Likely, this was the result of the interplay of greater activity of NPH and glargine compared with detemir and the afternoon increased insulin sensitivity in type 2 diabetes (9). This finding is in line with the observation of more frequent predinner hyperglycemia and/or need for twice-daily detemir dosing in clinical studies compared with glargine (10). Finally, glargine had greater effects on suppression of lipolysis than NPH and detemir. Compared with detemir only, glargine suppressed more C-peptide and glucagon concentrations. In addition, it had a longer duration of action with lower variability across subjects, as suggested by the smaller interquartile range.
Supplementary Material
Acknowledgments
This was an independent, investigator-designed project, which was neither shared with nor supported by any pharmaceutical company. The study is registered as EudraCT 2007-004571-18.
G.B.B. has received honoraria for scientific advising and consulting from sanofi-aventis, MannKind, and Eli Lilly. No other potential conflicts of interest relevant to this article were reported.
P.L. performed clamps and glucose turnover measurements, analyzed data, and reviewed and edited the manuscript. F.P. wrote the study protocol, performed clamps, researched data, and reviewed and edited the manuscript. P.R. enrolled patients, performed clamps, researched data, and reviewed and edited the manuscript. P.Ca. performed clamps and laboratory assays. P.Ci., S.M., A.M.A., and R.F. performed clamps and reviewed and edited the manuscript. G.B.B. provided the study concept and design, supervised the protocol development and the research, contributed to discussion, and reviewed and edited the manuscript. C.G.F. performed clamps, analyzed data, performed statistical analysis, and wrote the manuscript.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
This study is dedicated to the people with type 2 diabetes who volunteered in the study.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1911/-/DC1.
References
- 1.Hompesch M, Troupin B, Heise T, et al. Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab 2006;8:568–573 [DOI] [PubMed] [Google Scholar]
- 2.Linn T, Fischer B, Soydan N, et al. Nocturnal glucose metabolism after bedtime injection of insulin glargine or neutral protamine hagedorn insulin in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:3839–3846 [DOI] [PubMed] [Google Scholar]
- 3.Porcellati F, Rossetti P, Busciantella NR, et al. Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes: a double-blind, randomized, crossover study. Diabetes Care 2007;30:2447–2452 [DOI] [PubMed] [Google Scholar]
- 4.Lucidi P, Rossetti P, Porcellati F, et al. Mechanisms of insulin resistance after insulin-induced hypoglycemia in humans: the role of lipolysis. Diabetes 2010;59:1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gastaldelli A, Coggan AR, Wolfe RR. Assessment of methods for improving tracer estimation of non-steady-state rate of appearance. J Appl Physiol 1999;87:1813–1822 [DOI] [PubMed] [Google Scholar]
- 6.Senn S. Normal data from designs with three or more treatments. In Cross-Over Trials in Clinical Research. 2nd ed. New York, John Wiley & Sons, 2002, p. 157–182 [Google Scholar]
- 7.Owens DR, Bolli GB. Beyond the era of NPH insulin: long-acting insulin analogs: chemistry, comparative pharmacology, and clinical application. Diabetes Technol Ther 2008;10:333–349 [DOI] [PubMed] [Google Scholar]
- 8.Bolli GB, Gerich JE. The “dawn phenomenon”: a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med 1984;310:746–750 [DOI] [PubMed] [Google Scholar]
- 9.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 1996;45:1044–1050 [DOI] [PubMed] [Google Scholar]
- 10.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.