Abstract
Type 2 diabetes is a global public health crisis that threatens the economies of all nations, particularly developing countries. Fueled by rapid urbanization, nutrition transition, and increasingly sedentary lifestyles, the epidemic has grown in parallel with the worldwide rise in obesity. Asia's large population and rapid economic development have made it an epicenter of the epidemic. Asian populations tend to develop diabetes at younger ages and lower BMI levels than Caucasians. Several factors contribute to accelerated diabetes epidemic in Asians, including the “normal-weight metabolically obese” phenotype; high prevalence of smoking and heavy alcohol use; high intake of refined carbohydrates (e.g., white rice); and dramatically decreased physical activity levels. Poor nutrition in utero and in early life combined with overnutrition in later life may also play a role in Asia's diabetes epidemic. Recent advances in genome-wide association studies have contributed substantially to our understanding of diabetes pathophysiology, but currently identified genetic loci are insufficient to explain ethnic differences in diabetes risk. Nonetheless, interactions between Westernized diet and lifestyle and genetic background may accelerate the growth of diabetes in the context of rapid nutrition transition. Epidemiologic studies and randomized clinical trials show that type 2 diabetes is largely preventable through diet and lifestyle modifications. Translating these findings into practice, however, requires fundamental changes in public policies, the food and built environments, and health systems. To curb the escalating diabetes epidemic, primary prevention through promotion of a healthy diet and lifestyle should be a global public policy priority.
THE GLOBAL BURDEN OF TYPE 2 DIABETES
The dynamics of the diabetes epidemic are changing rapidly. Once a disease of the West, type 2 diabetes has now spread to every country in the world. Once “a disease of affluence,” it is now increasingly common among the poor. Once an adult-onset disease almost unheard of in children, rising rates of childhood obesity have rendered it more common in the pediatric population, especially in certain ethnic groups. According to the International Diabetes Federation (1), diabetes affects at least 285 million people worldwide, and that number is expected to reach 438 million by the year 2030, with two-thirds of all diabetes cases occurring in low- to middle-income countries. The number of adults with impaired glucose tolerance will rise from 344 million in 2010 to an estimated 472 million by 2030.
Globally, it was estimated that diabetes accounted for 12% of health expenditures in 2010, or at least $376 billion—a figure expected to hit $490 billion in 2030 (2). Its increasing prevalence and associated health complications threaten to reverse economic gains in developing countries. With limited infrastructures for diabetes care, many countries are ill-equipped to manage this epidemic.
EPICENTERS OF DIABETES
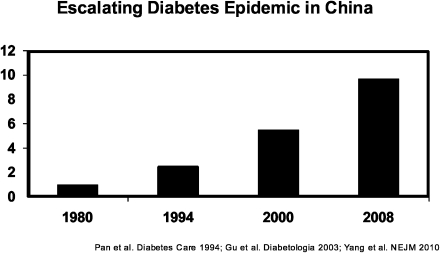
Asia accounts for 60% of the world's diabetic population. In recent decades, Asia has undergone rapid economic development, urbanization, and transitions in nutritional status (3). These have led to an explosive increase in diabetes prevalence within a relatively short time. In 1980, less than 1% of Chinese adults had the disease. By 2008, the prevalence had reached nearly 10% (Fig. 1) (4). It was estimated that more than 92 million Chinese adults had diabetes, and another 148 million were prediabetic. These numbers suggest that China has overtaken India as the global epicenter of the diabetes epidemic. However, in urban areas of south India, the prevalence of diabetes has reached nearly 20% (5).
Figure 1.
Time trends of diabetes prevalence in Chinese adults. Data are based on population-based cross-sectional surveys conducted in China (Chan et al. [3] and Yang et al. [4]).
Compared with Western populations, Asians develop diabetes at younger ages, at lower degrees of obesity, and at much higher rates given the same amount of weight gain (3). Asian women are also at greater risk of gestational diabetes, thereby putting their children at risk for type 2 diabetes later in life.
CONTRIBUTING FACTORS
Obesity and fat distribution
Overweight and obesity are driving the global diabetes epidemic. They affect the majority of adults in most developed countries and are increasing rapidly in developing countries. If current worldwide trends continue, the number of overweight people (BMI ≥25 kg/m2) is projected to increase from 1.3 billion in 2005 to nearly 2.0 billion by 2030 (6).
Compared with Western populations, the prevalence of overweight and obesity in Asia is relatively low, but it is increasing precipitously in parallel with economic development and rapid urbanization. In the past several decades, China's gross domestic product per capita has grown at an average annual rate of nearly 10% (7). Meanwhile, the prevalence of overweight and obesity in Chinese adults increased from 20% in 1992 to 29.9% in 2002 (8).
In Asia, obesity rates do not directly correspond with diabetes rates. India, for example, has a very low prevalence of obesity, but notably high rates of type 2 diabetes (9). A number of Asian countries also have substantially lower rates of overweight and obesity than the U.S., but similar or higher rates of diabetes. In Asian populations, increased risk of diabetes starts at a lower BMI than in Europeans (9). In addition, even a modest amount of weight gain during adulthood substantially increases the risk of diabetes in Asians.
A tendency toward greater abdominal obesity and less muscle mass among Asians results in an increased propensity for insulin resistance compared with Western populations. This “metabolically obese” phenotype among normal-weight individuals may explain the increased predisposition for diabetes despite a relatively low prevalence of obesity. Computer-based tomography has shown that Asians have more visceral fat than Caucasians with the same waist circumference (10).
Diet
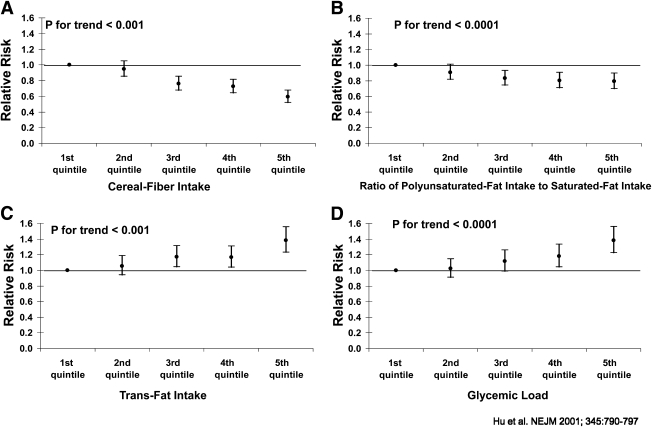
Excessive caloric intake is a major driving force behind escalating obesity and type 2 diabetes epidemics worldwide, but diet quality also has independent effects. In the Nurses’ Health Study (NHS), we found that the quality of fats and carbohydrates play an important role in the development of diabetes, independent of BMI and other risk factors (11). In particular, higher dietary glycemic load (GL) and trans fat are associated with increased diabetes risk, whereas greater consumption of cereal fiber and polyunsaturated fat is associated with decreased risk (Fig. 2). In a meta-analysis, we found that a 2 serving/day increment in whole-grain intake was associated with a 21% lower risk of diabetes (12).
Figure 2.
Multivariate RRs (with 95% CIs) of type 2 diabetes according to ascending quintiles of intake of cereal fiber (A); the ratio of polyunsaturated fat intake to saturated fat intake (B); intake of trans fat (C); and glycemic load (D). Each of the RRs was adjusted for the other 3 dietary variables and for age (in 5-year categories); time (8 periods); the presence or absence of a family history of diabetes; menopausal status and the use or nonuse of postmenopausal hormone therapy; smoking status (never smoked; former smoker; current smoker, 1–14 cigarettes per day or current smoker, ≥15 cigarettes per day); BMI (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥35.0); weekly frequency of moderate-to-vigorous exercise (<0.5 h, 0.5–1.9 h, 2.0–3.9 h, 4.0–6.9 h, or ≥7.0 h); and daily alcohol consumption (0 g, 0.1–5.0 g, 5.1–10.0 g, or ≥10.0 g) (11).
Evidence also indicates that higher consumption of sugar-sweetened beverages (SSBs) increases the risk of type 2 diabetes even after taking into account the effects of body weight. Our recent meta-analysis (13) found that individuals in the highest quantile of SSB intake (most often 1–2 servings/day) had a 26% greater risk of developing the disease than those in the lowest (relative risk [RR] 1.26 [95% CI 1.12–1.41]). In addition to weight gain, several other mechanisms such as increased insulin demand, dyslipidemia, and chronic inflammation may explain the adverse effects of SSBs on cardiometabolic risk. Large quantities of rapidly absorbable carbohydrates (e.g., sucrose) in SSBs result in a high dietary GL that leads to quick increases in blood glucose and insulin levels. A high GL diet, which increases insulin demand and may lead to pancreatic β-cell exhaustion in the long run, has been implicated in increased risk of type 2 diabetes and cardiovascular disease (14). Fructose from high fructose corn syrup or any sugar may also play a role. It is preferentially metabolized to lipid in the liver, leading to increased hepatic de novo lipogenesis, dyslipidemia, and insulin resistance (15). It may also promote visceral adiposity. A recent study that compared the effects of consuming 25% of energy from glucose- or fructose-sweetened beverages showed similar weight gain, but only the fructose group had a significant increase in visceral adiposity (16).
Many developing nations experience rapid economic and social development with concomitant shifts in lifestyle habits and dietary structure. These changes promote overnutrition and positive energy balance. In Asia, traditional dietary patterns are being lost as the population adapts to more industrialized and urban food environments. At the same time, built living environments have become increasingly sedentary. These changes have a significant impact on type 2 diabetes risk by increasing body weight and central adiposity, and decreasing physical activity. With the rapid pace of nutrition transition, many countries are facing coexisting problems of over- and undernutrition, which lead to the double burden of infectious and chronic diseases (17).
Data from the 1992–2002 Chinese National Nutrition Survey show that the proportion of energy from animal foods increased from 9.3 to 13.7%, while the proportion from fat rose from 22 to 29.8% (8). In India, the percentage of energy from animal products increased substantially between 1975 and 1995, mostly among urban residents who consumed 32% of energy from fat compared with 17% of those in rural areas (18).
Both vegetable and animal ghee, which are used for cooking in India and other South Asian countries, have an extremely high trans fatty acid content. Dalda, which is a type of vegetable ghee and major source of edible oil in India, has a trans fat level of about 50% (19). Trans fat intake is associated with adverse cardiometabolic risk profiles and increased risk of heart disease, and it may also play a role in the development of insulin resistance and chronic inflammation (20,21).
Global trade liberalization has made food products, such as edible oil and sugar, more accessible and relatively cheaper than in the past (19). Major changes that have occurred in Asia in the last several decades include 1) a large shift from consumption of coarse grains to polished rice and refined wheat, especially in India and China; 2) reduced intake of cereals particularly among urban populations and higher-income groups; 3) higher energy intake among the poor, lower energy intake among the rich, and greater consumption of fat in all income groups; and 4) increased intake of meat, edible oil, and fruits and vegetables in China and increased consumption of dairy products, especially highly saturated ghee (clarified butter), and added sugar in India (7).
Globalization and economic development have spurred nutrition transitions in many developing nations. This nutritional shift typically involves increased consumption of animal fat and energy-dense foods, decreased fiber, and more frequent intake of fast foods. At the same time, the traditional diets of many Asian countries, which are largely based on polished white rice and refined wheat, have high glycemic index (GI) and GL values. Findings from the Shanghai Women's Health Study (SWHS) indicate that high intake of foods with a high GI or GL, especially white rice, is associated with increased risk of diabetes (RR comparing highest to lowest quintile of white rice intake: 1.78 [95% CI 1.48–2.15]) (22). Despite relatively low consumption of rice in the U.S. population, in the NHS, we found that higher consumption of white rice is associated with increased risk of diabetes, whereas consumption of brown rice, a whole grain, protects against the disease (23).
The adverse effects of high dietary GI and GL are more evident in overweight or obese individuals who are prone to insulin resistance (24). Data from the SWHS showed a stronger association of rice intake, GI, and GL with diabetes risk in women with higher waist-to-hip ratio and BMI (22). Prior to rapid urbanization throughout Asia, the metabolic effects of high GI/GL diets were offset by high levels of physical activity. As lower-income Asian countries shift away from agricultural labor toward employment in manufacturing services, energy expenditure has declined dramatically (25). The combination of excessive energy intake and reduced energy output leads to increased obesity and insulin resistance. Underlying insulin resistance can exacerbate adverse metabolic effects of high carbohydrate diets. The biological interaction between insulin resistance and carbohydrates may explain why a high intake of polished white rice does not appear to cause deleterious metabolic effects in lean and physically active individuals, such as Chinese peasants, but has become an important risk factor for diabetes in urbanized Asian populations.
Physical activity
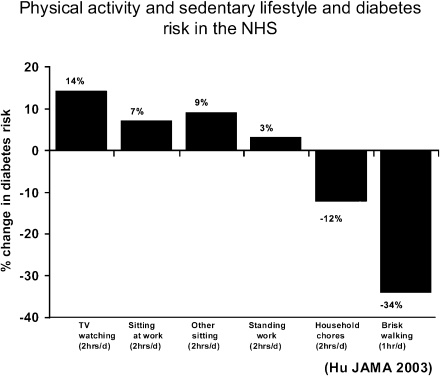
Numerous epidemiologic studies show that increased physical activity reduces risk of diabetes, whereas sedentary behaviors increase risk. In the NHS (26), each 2-h/day increment of time spent watching television (TV) was associated with a 14% increase in diabetes risk. Each 2-h/day increment of standing or walking around at home was associated with a 12% reduction in risk. Each 1-h/day increment of brisk walking was associated with a 34% reduction in risk (Fig. 3). These results indicate a continuum in the relationship between physical activity levels and diabetes risk. Among sedentary behaviors (TV watching, sitting at work, and other sitting), prolonged TV watching was associated with the highest risk.
Figure 3.
Percentage changes in risk of developing type 2 diabetes among nondiabetic women associated with TV watching, other sedentary behaviors, and walking. Adjusted for age, smoking, alcohol consumption, family history of diabetes, and dietary covariates. All sedentary behavior variables are included simultaneously in the model. Other sitting includes reading, eating meals, and time spent sitting at a desk. Error bars indicate 95% CIs. d, day. Adapted from Hu et al. (26).
At least two potential mechanisms account for the positive association between watching TV and obesity and diabetes risk (26). First, watching TV typically takes the place of physical activity, thereby reducing energy expended. However, the effects of TV watching are independent of physical activity because adjustment for recreational activities does not appreciably attenuate the increased risk for obesity or diabetes. Second, watching TV is associated with greater food and total energy intake, most likely because of increased exposure to food and beverage advertisements. In addition, those who spend more time watching TV tend to have unhealthy eating patterns characterized by increased consumption of snacks, sugary beverages, and fast foods.
Increased mechanization and driving have displaced physical activity over the last century in industrialized nations. This trend is increasing in developing countries as well. In China, increased use of automobiles instead of public transportation is associated with reduced physical activity. In a prospective study conducted in eight provinces in China (27), 14% of households acquired a car between 1989 and 1997. Compared with those whose vehicle ownership did not change, men who acquired a vehicle experienced a 1.8-kg greater weight gain (P < 0.05), and the likelihood of their becoming obese during that time period doubled. In addition, rapid shifts toward a service sector economy and growing use of new technologies have led to a marked decline in physical activity levels. According to Chinese National Health and Nutrition Surveys, average weekly physical activity levels among Chinese adults declined by 32% between 1991 and 2006 (28). This was mainly because of decreases in occupational activities as a result of urbanization.
Smoking
Cigarette smoking is an independent risk factor for type 2 diabetes. A meta-analysis found that current smokers had a 45% increased risk of developing diabetes compared with nonsmokers (29). Moreover, there was a dose-response relationship between the number of cigarettes smoked and diabetes risk.
Several possible biological mechanisms may explain the association between cigarette smoking and diabetes. First, although smokers tend to be leaner than nonsmokers, smoking has been associated with increased risk of central obesity or abdominal fat (30,31), an established risk factor for insulin resistance and diabetes. The accumulation of visceral adipose tissue among smokers may be because of increased plasma cortisol levels induced by stimulation of sympathetic nervous system activity (32). Second, smoking has anti-estrogenic effects in women and decreases plasma testosterone in men (33). These factors may promote abdominal fat accumulation and insulin resistance, especially in men. In addition, animal models have shown that nicotine exposure, particularly in the prenatal or neonatal phase, can cause β-cell dysfunction and increase β-cell apoptosis (34).
An estimated 50–60% of adult males in developing countries are regular smokers (18). Rates in Asia will continue to rise with aggressive marketing by multinational tobacco companies. Currently, China is the greatest producer and user of cigarettes in the world. One of every three cigarettes manufactured is consumed in China (35). India is the second largest producer and consumer of tobacco products worldwide (36). In India, many use smokeless tobacco products, such as betel quid, and 40% smoke bidis, which are small, typically flavored cigarettes. These are nontaxable, and their production provides employment for large numbers of urban poor (36). Reducing high smoking rates in most developing countries should be a key public health objective to prevent and control the global epidemic of diabetes and its complications.
Alcohol use
Light-to-moderate alcohol consumption is associated with reduced risk of diabetes. A meta-analysis of 370,000 individuals with 12 years of follow-up showed a U-shaped relationship, with a 30–40% reduced risk of the disease among those consuming 1–2 drinks/day compared with heavy drinkers or abstainers (37). The risk of diabetes among those who consumed three or more drinks/day was similar to that of abstainers (RR 1.04 [95% CI 0.84–1.29]). Possible mediators of beneficial effects of moderate alcohol consumption include improved insulin sensitivity, increased HDL cholesterol and adiponectin, and the anti-inflammatory effect of alcohol. On the other hand, heavy alcohol intake has multiple deleterious metabolic effects, including excess caloric intake and obesity, increased triglyceride levels, pancreatitis, disturbance of carbohydrate and glucose metabolism, and impairment of liver function (37).
In Asia, alcohol use is rising with rapid globalization and socioeconomic development. It is also worth noting that within-country consumption patterns are changing. Traditionally, heavy drinking was more prevalent in poor rural regions. However, high rates of heavy drinking are also starting to appear in the urban middle and upper classes as the alcohol industry increases social marketing to encourage more people to adopt Westernized lifestyles, which are symbolized by drinking and affluence. To date, many countries in Asia, including China and India, do not have public health policies regarding alcohol consumption. Concurrent alcohol and tobacco use, which could have a synergistic effect on diabetes risk, is highly common in Asian populations (38). These trends underscore the need for policy changes and effective education programs for tobacco and alcohol use in developing countries.
Linking Western dietary pattern, inflammation, and type 2 diabetes
Increasing evidence indicates that low-grade systemic inflammation is an underlying factor in the pathogenesis of type 2 diabetes (39). Epidemiologic studies have demonstrated a significant positive association between elevated plasma concentrations of inflammatory cytokines, such as tumor necrosis factor-α, interleukin-6, and C-reactive protein, and increased risk of diabetes. In the NHS, we used a reduced rank regression approach to identify a food consumption pattern that explains the largest amount of variance for markers of inflammation, including C-reactive protein, interleukin-6, intracellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin (39). This analysis was conducted in a nested case-control study of 656 cases of type 2 diabetes and 694 control subjects. The identified dietary pattern, which was strongly related to inflammatory markers, was characterized by high intakes of SSBs, refined grains, and red and processed meat, but low consumption of wine, coffee, cruciferous vegetables, and yellow vegetables. As expected, this proinflammatory pattern was strongly associated with an increased risk of diabetes (multivariate-adjusted odds ratio [OR] comparing extreme quintiles: 3.09 [95% CI 1.99–4.79]) in the nested case-control sample. We then calculated a proinflammatory dietary score for each individual in the main NHS cohort and examined the association between the dietary score and incident diabetes during 14 years of follow-up. These analyses were subsequently replicated in the NHS II. After adjustment for BMI and other potential lifestyle confounders, the RRs comparing extreme quintiles of the “proinflammatory dietary pattern” were 2.56 (95% CI 2.10–3.12, P for trend <0.001) in the NHS and 2.93 (95% CI 2.18–3.92, P for trend <0.001) in the NHS II. These data provide strong epidemiologic evidence linking overall dietary patterns, inflammation, and diabetes risk, suggesting that chronic inflammation may mediate the association between a Western dietary pattern and risk of type 2 diabetes.
GENETIC SUSCEPTIBILITY AND GENE-ENVIRONMENT INTERACTIONS
The recent advent of genome-wide association studies (GWAS) has led to major advances in the identification of common genetic variants contributing to diabetes susceptibility (40). To date, at least 40 genetic loci have been convincingly associated with type 2 diabetes, but these loci confer only a modest effect size and do not add to the clinical prediction of diabetes beyond traditional risk factors, such as obesity, physical inactivity, unhealthy diet, and family history of diabetes. Many diabetes genes recently discovered through GWAS in Caucasian populations have been replicated in Asians; however, there were significant interethnic differences in the location and frequency of these risk alleles. For example, common variants of the TCF7L2 gene that are significantly associated with diabetes risk are present in 20–30% of Caucasian populations but only 3–5% of Asians (41,42). Conversely, a variant in the KCNQ1 gene associated with a 20–30% increased risk of diabetes in several Asian populations (43,44) is common in East Asians, but rare in Caucasians. It is intriguing that most diabetes susceptibility loci that have been identified are related to impaired β-cell function, whereas only a few (e.g., peroxisome proliferator–activated receptor-γ, insulin receptor substrate 1, IGF-1, and GCKR) are associated with insulin resistance or fasting insulin, which points toward β-cell dysfunction as a primary defect for diabetes pathogenesis. It should be noted that most of the single nucleotide polymorphisms uncovered may not be the actual causal variants, which need to be pinpointed through fine-mapping, sequencing, and functional studies.
Despite heterogeneity across populations in risk allele frequency or effect size in type 2 diabetes genes, the combined effects of multiple genetic variants using genetic scores based on the number of risk alleles appear to be similar across different ethnic groups. Typically, each risk allele increment is associated with a 10–20% increased risk of type 2 diabetes (41,42). These data suggest that the overall contribution of the identified genetic loci to type 2 diabetes is similar between Caucasians and other ethnic groups, and that these loci do not appear to explain ethnic differences in diabetes risk. In predicting future risk of diabetes, the clinical utility of these cumulative genetic risk scores appears to be limited in either high- or low-risk populations.
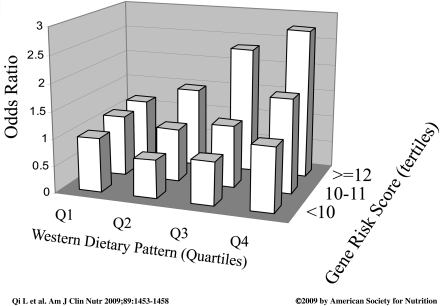
Like other multifactorial diseases, type 2 diabetes is a product of the interplay between genetic and environmental factors. It is likely that the genetic factors that underlie individual susceptibility are amplified in the presence of certain environmental triggers. On the other hand, given the same dietary and lifestyle factors, some individuals may be more prone to type 2 diabetes than others because of different genetic backgrounds. In the Health Professionals’ Follow-up Study, we found a significant interaction between a Western dietary pattern derived from principle component analysis of 40 food groups and a genetic risk score (GRS) of type 2 diabetes susceptibility based on 10 established single nucleotide polymorphisms (P = 0.02) (Fig. 4) (45). The multivariable ORs of diabetes across increasing quartiles of the Western dietary pattern were 1.00, 1.23 (95% CI 0.88–1.73), 1.49 (1.06, 2.09), and 2.06 (1.48, 2.88) among men with a higher GRS (≥12 risk alleles, P for trend = 0.01). Among those with a lower GRS, the Western dietary pattern was not associated with diabetes risk. In addition, intake of processed meat, red meat, and heme iron, which characterize the Western dietary pattern, showed significant interactions with GRS in relation to diabetes risk (P for interaction = 0.029, 0.02, and 0.0004, respectively). These results suggest that the detrimental effects of a Westernized diet may be enhanced by greater genetic susceptibility.
Figure 4.
ORs of diabetes risk according to joint classification of Western dietary pattern scores (in quartiles, Q) and genetic risk scores (<10, 10–11, and ≥12). ORs and 95% CIs were calculated by using an unconditional logistic regression model. The analyses were adjusted for age, BMI, smoking, alcohol consumption, physical activity, family history of diabetes, and total energy intakes (P for interaction = 0.02). From Qi et al. (45).
THE THRIFTY GENOTYPE VERSUS THE THRIFTY PHENOTYPE
The thrifty genotype hypothesis postulates that obesity and type 2 diabetes are caused by positive selection of genotypes for efficiency of metabolism and energy and fat storage, thereby conferring advantage in times of nutrient scarcity (46,47). This hypothesis has been widely used to explain the extraordinarily high rates of diabetes seen among Pima Indians and other indigenous populations. It has been suggested that these populations may have an enhanced genetic predisposition to obesity and diabetes because of overrepresentation of the thrifty genotypes, resulting from evolutionary selection by repeated feast and famine cycles. In contrast, Europeans are less likely to possess thrifty genotypes as they evolved in environments that were least affected by the feast and famine cycles.
Although this hypothesis has conceptual appeal, the identification of the thrifty genes has so far remained elusive. Based on an analysis of 17 type 2 diabetes loci and 13 obesity loci, Southam et al. (48) found no clear evidence for overrepresentation of derived alleles (vs. ancestral alleles) for the obesity or diabetes loci or greater concentration of these loci in a particular ethnic group. These analyses, albeit preliminary, provide little support for the thrifty gene hypothesis. Speakman (49) proposed the drifty genotype hypothesis as an alternative to the thrifty genotype hypothesis. He argued that the presence or absence of obesity or diabetes gene variants is simply because of random genetic drift over millions of years as there is little evidence that humans (including some indigenous populations such as Pacific Islanders) were under strong selective pressure by famine during the evolutionary history. Whether the identified obesity or diabetes loci represent thrifty or drifty genotypes is still under debate. Nonetheless, ongoing fine-mapping efforts and whole-genome sequencing analyses will shed more light on the genetic architecture of type 2 diabetes in different ethnic groups.
While the thrifty genotype hypothesis points to a mismatch between the ancestral genes and modern environment, the thrifty phenotype hypothesis postulates a mismatch between intrauterine and adult life environments. This hypothesis has been widely used to explain the role of nutrition transition in the development of chronic diseases. It postulates that adaptations in response to fetal undernutrition lead to metabolic and structural changes (e.g., decreased β-cell mass and function and increased insulin resistance) that are beneficial for early survival, but may increase the risk of chronic diseases, such as type 2 diabetes, in adulthood (50,51). The risks of adverse long-term consequences are likely to be exacerbated in a nutritionally rich environment in later life. Indirect support for this hypothesis comes from studies showing consistent associations between low birth weight (LBW) and increased risk of type 2 diabetes. LBW promotes a thrifty phenotype during intrauterine and early life that induces an insulin resistant state and low β-cell function. This might explain early life origins of insulin resistance and type 2 diabetes in India and other Asian countries, where rapid nutrition transition has led to the coexistence of undernutrition and overnutrition. Both fetal undernutrition (LBW) and overnutrition (the baby of a diabetic mother) are associated with increased future risk of diabetes (52). These effects may be meditated by the regulation of gene expression through chromatin modification or DNA methylation (epigenetics) because of intrauterine or early childhood exposures rather than changes in genotypes (53).
Results from the Dutch Famine Study show that adults exposed to famine conditions during World War II had increased insulin resistance than those who had not been exposed (54). However, this association was not observed in another famine cohort study, the Leningrad Siege Study (55). The Great Chinese Famine, which lasted from the late 1950s to the early 1960s and caused millions of deaths, provided a unique opportunity to examine long-term metabolic effects of famine exposure. The famine period with the highest mortality rate was between 1959 and 1961 (56). Recently, we examined the association between famine exposure in fetal life and adult hyperglycemia in the Chinese National Nutrition and Health Survey (57). We found that the prevalence of hyperglycemia was highest (18.9%) in fetal-exposed subjects who consumed an affluent/Western diet during adulthood. Compared with the relatively nonexposed cohort, the ORs of hyperglycemia in the fetal-exposed cohort were 7.63 (95% CI 2.41–24.1, P = 0.0005) for those who had an affluent/Western dietary pattern, and 2.34 (95% CI 0.82–6.70, P = 0.112) for those with a traditional dietary pattern. These findings indicate that exposure to severe famine in fetal life increases the risk of hyperglycemia in adulthood, and that this association is exacerbated by a nutritionally “rich” environment in later life. Because many Asian adults who experienced undernutrition in early life are now adapting Westernized diets and lifestyles, their risk of developing diabetes and other metabolic diseases is substantially elevated.
PREVENTABILITY OF TYPE 2 DIABETES
Many epidemiologic studies have implicated individual dietary and lifestyle factors in the development of type 2 diabetes in diverse populations, yet few studies have investigated multiple risk factors simultaneously. In the NHS, we defined a low-risk group according to five variables: 1) BMI <25; 2) a diet high in cereal fiber and polyunsaturated fat and low in trans fat and GL; 3) moderate-to-vigorous physical activity for at least half an hour/day; 4) no current smoking; and 5) an average intake of at least a half-serving of an alcoholic beverage/day. Compared with the rest of the cohort, women in the low-risk group (3.4%) had an RR of diabetes of 0.09 (95% CI 0.05–0.17). A total of 91% of the cases of diabetes (95% CI 83–95%) could be attributed to the five factors listed above. These data provide strong epidemiologic evidence that the majority of cases of type 2 diabetes could be prevented by a healthier lifestyle.
Several randomized clinical trials have demonstrated that diabetes is preventable. One of the first diabetes prevention trials was conducted in Daqing, China (58). After 6 years of active intervention, risk was reduced by 31, 46, and 42% in the diet-only, exercise-only, and diet-plus-exercise groups, respectively, compared with the control group. In a subsequent 14-year follow-up study, the intervention groups were combined and compared with control subjects to assess how long the benefits of lifestyle change can extend beyond the period of active intervention (59). Compared with control subjects, individuals in the combined lifestyle intervention group had a 51% lower risk of diabetes during the active intervention period, and a 43% lower risk over a 20-year follow-up.
Randomized controlled trials from other populations have demonstrated similar results. In both the Finnish Diabetes Prevention Study (60) and the U.S. Diabetes Prevention Program (DPP) (61), lifestyle intervention significantly reduced diabetes incidence by 58%. A follow-up of the Finnish Diabetes Prevention Study showed a 43% reduction in diabetes risk over a median of 7 years after discontinuation of active counseling (62). In the Indian Diabetes Prevention Program (IDPP) (63), after 3 years of follow-up, the RR reduction was 28.5% with lifestyle management, 26.4% with metformin, and 28.2% with the combined interventions compared with the control group.
Together, these clinical trials demonstrate that diet and lifestyle modification is highly effective in preventing type 2 diabetes in different ethnic and racial groups. There is an urgent need to translate the findings from these trials into clinical and public health practice. Emphasis should be placed on early adoption of healthy habits in pediatric populations because these practices track through to adulthood.
CONCLUSIONS
Type 2 diabetes is a global crisis that threatens the health and economy of all nations, particularly developing countries. This epidemic is primarily driven by rapid urbanization, nutrition transition, and increasingly sedentary lifestyles. The diabetes epidemic in Asia is characterized by onset at lower BMI levels and younger ages compared with Caucasian populations. Although the average BMI is still relatively low in Asian populations, abdominal or central obesity is highly prevalent, creating the widespread “metabolically obese” phenotype. Poor nutrition in utero and in early life plus overnutrition in later life may also contribute to the current diabetes epidemic in Asian populations.
Recent advances in GWAS have substantially improved our understanding of the pathophysiology of diabetes, but the currently identified genetic susceptibility loci are insufficient to explain differences in diabetes risk across different ethnic groups or the rapid rise in diabetes prevalence over the past several decades. Clinical utility of these loci in predicting future risk of diabetes is also limited.
Accumulating evidence strongly demonstrates that the majority of type 2 diabetes cases can be prevented through diet and lifestyle modification. However, the adoption of a healthy diet and lifestyle requires not only individual behavioral changes, but also changes in our food, built, and social environments. Public health strategies that target the obesogenic environment are critical. Translating clinical and epidemiologic findings into practice requires fundamental shifts in public policies and health systems. To curb the diabetes epidemic, primary prevention through the promotion of a healthy diet and lifestyle should be a global public policy priority.
Acknowledgments
F.B.H.’s research has been supported by an American Diabetes Association Research Award and National Institutes of Health grants DK58845 and HL60712.
No potential conflicts of interest relevant to this article were reported.
The author would like to thank Vasanti Malik and Vanessa Boulanger (Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts) for helpful comments.
References
- 1.International Diabetes Federation IDF Diabetes Atlas. Epidemiology and Mobidity. In: International Diabetes Federation. Available from http://www.idf.org/. Accessed on 1 March 2011 [Google Scholar]
- 2.Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:293–301 [DOI] [PubMed] [Google Scholar]
- 3.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–2140 [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Lu J, Weng J, et al. ; China National Diabetes and Metabolic Disorders Study Group Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101 [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care 2008;31:893–898 [DOI] [PubMed] [Google Scholar]
- 6.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437 [DOI] [PubMed] [Google Scholar]
- 7.Popkin BM, Horton S, Kim S, Mahal A, Shuigao J. Trends in diet, nutritional status, and diet-related noncommunicable diseases in China and India: the economic costs of the nutrition transition. Nutr Rev 2001;59:379–390 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31:177–188 [DOI] [PubMed] [Google Scholar]
- 9.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681–1688 [DOI] [PubMed] [Google Scholar]
- 10.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 2007;86:353–359 [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–797 [DOI] [PubMed] [Google Scholar]
- 12.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med 2007;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA 2002;288:2569–2578 [DOI] [PubMed] [Google Scholar]
- 15.Stanhope KL, Havel PJ. Fructose consumption: recent results and their potential implications. Ann N Y Acad Sci 2010;1190:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel K, Narayan KM, Kinra S. Finding a policy solution to India's diabetes epidemic. Health Aff (Millwood) 2008;27:1077–1090 [DOI] [PubMed] [Google Scholar]
- 18.Shetty PS. Nutrition transition in India. Public Health Nutr 2002;5:175–182 [DOI] [PubMed] [Google Scholar]
- 19.Popkin BM. The nutrition transition and obesity in the developing world. J Nutr 2001;131:871S–873S [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Garcia E, Schulze MB, Meigs JB, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr 2005;135:562–566 [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Pischon T, Hankinson SE, et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr 2004;79:606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villegas R, Liu S, Gao YT, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med 2007;167:2310–2316 [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, Spiegelman D, van Dam RM, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170:961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding EL, Malik VS. Convergence of obesity and high glycemic diet on compounding diabetes and cardiovascular risks in modernizing China: an emerging public health dilemma. Global Health 2008;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popkin BM. Nutrition in transition: the changing global nutrition challenge. Asia Pac J Clin Nutr 2001;10(Suppl.):S13–S18 [PubMed] [Google Scholar]
- 26.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 2003;289:1785–1791 [DOI] [PubMed] [Google Scholar]
- 27.Bell AC, Ge K, Popkin BM. The road to obesity or the path to prevention: motorized transportation and obesity in China. Obes Res 2002;10:277–283 [DOI] [PubMed] [Google Scholar]
- 28.Ng SW, Norton EC, Popkin BM. Why have physical activity levels declined among Chinese adults? Findings from the 1991-2006 China Health and Nutrition Surveys. Soc Sci Med 2009;68:1305–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–2664 [DOI] [PubMed] [Google Scholar]
- 30.Barrett-Connor E, Khaw KT. Cigarette smoking and increased central adiposity. Ann Intern Med 1989;111:783–787 [DOI] [PubMed] [Google Scholar]
- 31.Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat. III. Effects of cigarette smoking. JAMA 1989;261:1169–1173 [PubMed] [Google Scholar]
- 32.Grassi G, Seravalle G, Calhoun DA, Bolla G, Mancia G. Cigarette smoking and the adrenergic nervous system. Clin Exp Hypertens A 1992;14:251–260 [DOI] [PubMed] [Google Scholar]
- 33.Meikle AW, Liu XH, Taylor GN, Stringham JD. Nicotine and cotinine effects on 3 alpha hydroxysteroid dehydrogenase in canine prostate. Life Sci 1988;43:1845–1850 [DOI] [PubMed] [Google Scholar]
- 34.Bruin JE, Petre MA, Raha S, Morrison KM, Gerstein HC, Holloway AC. Fetal and neonatal nicotine exposure in Wistar rats causes progressive pancreatic mitochondrial damage and beta cell dysfunction. PLoS ONE 2008;3:e3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L. Tobacco control necessary. China Daily. 2011. Available from http://www.chinadaily.com.cn/opinion/2011-01/10/content_11815752.htm. Accessed on 1 March 2011 [Google Scholar]
- 36.Kabir Z, Clancy L, Connolly GN. Tobacco control efforts: where is India now? Lancet 2007;370:134. [DOI] [PubMed] [Google Scholar]
- 37.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 2005;28:719–725 [DOI] [PubMed] [Google Scholar]
- 38.Gupta PC, Maulik PK, Pednekar MS, Saxena S. Concurrent alcohol and tobacco use among a middle-aged and elderly population in Mumbai. Natl Med J India 2005;18:88–91 [PubMed] [Google Scholar]
- 39.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–684; quiz 714–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med 2010;363:2339–2350 [DOI] [PubMed] [Google Scholar]
- 41.Ng MC, Park KS, Oh B, et al. Implication of genetic variants near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in type 2 diabetes and obesity in 6,719 Asians. Diabetes 2008;57:2226–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y, Li H, Loos RJ, et al. Common variants in CDKAL1, CDKN2A/B, IGF2BP2, SLC30A8, and HHEX/IDE genes are associated with type 2 diabetes and impaired fasting glucose in a Chinese Han population. Diabetes 2008;57:2834–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unoki H, Takahashi A, Kawaguchi T, et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet 2008;40:1098–1102 [DOI] [PubMed] [Google Scholar]
- 44.Yasuda K, Miyake K, Horikawa Y, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 2008;40:1092–1097 [DOI] [PubMed] [Google Scholar]
- 45.Qi L, Cornelis MC, Zhang C, van Dam RM, Hu FB. Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. Am J Clin Nutr 2009;89:1453–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962;14:353–362 [PMC free article] [PubMed] [Google Scholar]
- 47.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? 1962. Bull World Health Organ 1999;77:694–703; discussion 692–693 [PMC free article] [PubMed] [Google Scholar]
- 48.Southam L, Soranzo N, Montgomery SB, et al. Is the thrifty genotype hypothesis supported by evidence based on confirmed type 2 diabetes- and obesity-susceptibility variants? Diabetologia 2009;52:1846–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the ‘drifty gene’ hypothesis. Int J Obes (Lond) 2008;32:1611–1617 [DOI] [PubMed] [Google Scholar]
- 50.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 2001;60:5–20 [DOI] [PubMed] [Google Scholar]
- 51.Gluckman PD, Hanson MA, Bateson P, et al. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet 2009;373:1654–1657 [DOI] [PubMed] [Google Scholar]
- 52.Yajnik CS. Nutrient-mediated teratogenesis and fuel-mediated teratogenesis: two pathways of intrauterine programming of diabetes. Int J Gynaecol Obstet 2009;104(Suppl. 1):S27–S31 [DOI] [PubMed] [Google Scholar]
- 53.Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr 2010;30:315–339 [DOI] [PubMed] [Google Scholar]
- 54.Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998;351:173–177 [DOI] [PubMed] [Google Scholar]
- 55.Stanner SA, Bulmer K, Andrès C, et al. Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. BMJ 1997;315:1342–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang DT. China's agricultural crisis and famine of 1959–1961: a survey and comparison of Soviet famines. Comparative Economic Studies 2008;50:1–29 [Google Scholar]
- 57.Li Y, He Y, Qi L, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes 2010;59:2400–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 59.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 60.Tuomilehto J, Lindström J, Eriksson JG, et al. ; Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 61.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 63.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]