Abstract
OBJECTIVE
Hemoglobin A1c (A1C) has emerged as a recommended diagnostic tool for identifying diabetes and subjects at risk for the disease. This recommendation is based on data in adults showing the relationship between A1C with future development of diabetes and microvascular complications. However, studies in the pediatric population are lacking.
RESEARCH DESIGN AND METHODS
We studied a multiethnic cohort of 1,156 obese children and adolescents without a diagnosis of diabetes (male, 40%/female, 60%). All subjects underwent an oral glucose tolerance test (OGTT) and A1C measurement. These tests were repeated after a follow-up time of ∼2 years in 218 subjects.
RESULTS
At baseline, subjects were stratified according to A1C categories: 77% with normal glucose tolerance (A1C <5.7%), 21% at risk for diabetes (A1C 5.7–6.4%), and 1% with diabetes (A1C >6.5%). In the at risk for diabetes category, 47% were classified with prediabetes or diabetes, and in the diabetes category, 62% were classified with type 2 diabetes by the OGTT. The area under the curve receiver operating characteristic for A1C was 0.81 (95% CI 0.70–0.92). The threshold for identifying type 2 diabetes was 5.8%, with 78% specificity and 68% sensitivity. In the subgroup with repeated measures, a multivariate analysis showed that the strongest predictors of 2-h glucose at follow-up were baseline A1C and 2-h glucose, independently of age, ethnicity, sex, fasting glucose, and follow-up time.
CONCLUSIONS
The American Diabetes Association suggested that an A1C of 6.5% underestimates the prevalence of prediabetes and diabetes in obese children and adolescents. Given the low sensitivity and specificity, the use of A1C by itself represents a poor diagnostic tool for prediabetes and type 2 diabetes in obese children and adolescents.
After years of debate, the American Diabetes Association (ADA) published revised recommendations to use hemoglobin A1c (A1C) to diagnose diabetes and to identify subjects at risk for developing diabetes in the future (1). The decision is based on numerous cross-sectional and longitudinal studies showing the correlation between A1C and diabetes at baseline or long-term association between A1C and risk of diabetes and diabetes-related comorbidities (1–6). Additional factors influencing this decision were as follows: A1C does not require a fasting state, reflects the usual 3–4 months before glycemia, has low intraindividual variability, and is a good predictor of diabetes-related complications (1,5). It should be noted that this decision was made only on studies performed in adults. Little is known about the use of the A1C test for the diagnosis of type 2 diabetes and prediabetes in childhood and adolescence. In view of the fact that both prediabetes and, more important, type 2 diabetes have recently emerged as early complications of childhood obesity (7), it is of critical importance to diagnose these forms of dysglycemia early in their development. Thus, the aim of this study was to assess the diagnostic utility of A1C for the diagnosis of prediabetes and type 2 diabetes in obese children and adolescents. We therefore conducted this study to evaluate the following: 1) the distribution of A1C levels in a multiethnic cohort of obese children and adolescents without known diabetes and 2) the sensitivity and specificity of A1C for type 2 diabetes and prediabetes diagnoses compared with the current oral glucose tolerance test (OGTT) gold standard.
RESEARCH DESIGN AND METHODS
Subjects
The Yale Pathophysiology of Type 2 Diabetes in Obese Youth Study is a long-term project that examines early alterations in glucose metabolism in obese children and adolescents. The subjects reported were recruited from our Pediatric Obesity Clinic from 2005 to 2010. To be eligible for the study, subjects had to be obese (>95th percentile for age and sex) and were excluded from this analysis if they were using medications that may affect glucose metabolism or had known type 2 diabetes. Children and adolescents who had previously shown fasting glucose or 2-h glucose indicative of type 2 diabetes were considered not eligible for the study. Type 2 diabetes was defined by glucose levels (fasting glucose >125 mg/dL or 2-h glucose ≥200 mg/dL) obtained during the OGTT (1). Type 1 diabetes was excluded by testing for GAD 65, islet cell antibody (ICA) 512, and IA. The obese children and adolescents of the current study are referred to the Yale Pediatric Obesity Clinic by local community pediatricians in the area of New Haven, Connecticut. The study protocol was approved by the institutional review board of the Yale University School of Medicine, and written parental consent and child assent were obtained before the study.
Testing procedures
Subjects were studied at the research unit at the Yale University School of Medicine at 8:00 a.m., after a 10-h overnight fast. A standard oral OGTT administering a dose of 1.75 g of glucose per kilogram of body weight (up to a maximum of 75 g) was performed in all subjects to establish glucose tolerance status, as previously described (7). The following categories of dysglycemia were considered according to ADA definition (1): Prediabetes is considered when an individual shows impaired fasting glucose (IFG) (fasting plasma glucose 100–125 mg/dL) or impaired glucose tolerance (IGT) (2-h glucose after the OGTT 140–199 mg/dL) or both; type 2 diabetes was defined as fasting glucose >125 mg/dL or 2-h glucose ≥200 mg/dL (1).
Biochemical analyses
Plasma glucose was determined using the YSI 2700 Stat Analyzer (Yellow Springs Instruments, Yellow Springs, OH). Fasting plasma insulin was measured using radioimmunoassays (Linco Research, St. Charles, MO), and lipid levels were measured with the use of an Auto-Analyzer (model 747–200, Roche–Hitachi, Indianapolis, IN). A1C levels were measured on the same day of the OGTT by the Yale Central Laboratory by using an assay based on latex immunoagglutination inhibition methodology (DCA Analyzers, Siemens, Berlin, Germany).
Calculations.
To determine the relationships between categories of A1C and phenotypes of relevance to the pathogenesis of type 2 diabetes, we calculated the following estimates of both insulin sensitivity and β-cell function during the OGTT as previously reported:
The whole body insulin sensitivity index (WBISI) is a surrogate measure of insulin sensitivity, which we have described and validated against the euglycemic–hyperinsulinemic clamp in obese children and adolescents (8).
The insulinogenic index (IGI) represents early-phase insulin secretion and is a commonly used surrogate index of β-cell function, as previously reported (9).
The disposition index (DI), which represents a measure of the insulin secretion adjusted for the insulin sensitivity, was calculated as the product of the IGI and WBISI, based on the curvilinear relation of these OGTT-derived variables, previously described by our group in obese children and adolescents.
Statistical analysis
The study population was described using mean/SD or frequency. Group comparisons across three A1C categories were made with ANOVA. Either raw mean/SD or least-squares means and 95% CI were estimated, as well as P value testing for linear trend after adjusting for sex, ethnicities (Caucasian, African American, and Hispanic), age, and BMI z score if applicable. Because the three ethnic groups tend to have a different prevalence of prediabetes and type 2 diabetes (7), we adjusted for fasting and 2-h glucose. Geometric means were presented for variables that were log transformed to meet the normal assumptions for analysis. Glucose tolerance status within each category of A1C was examined, and frequency and percentage were calculated. Agreement between A1C category and OGTT status according to fasting glucose or 2-h glucose was also assessed. κ and weighted κ coefficient were reported. Receiver operating characteristic (ROC) curve analysis was performed for A1C and fasting glucose to discriminate IGT from normal glucose tolerance (NGT) and type 2 diabetes from NGT and IGT using a logistic procedure. Area under the ROC curve (AUC) was considered as an effective measure of inherent validity of a diagnostic test. Sensitivity was the percentage of all patients with type 2 diabetes who are correctly identified by different cutoffs of A1C or fasting glucose. Specificity was the percentage of all patients without type 2 diabetes who are correctly identified as being free of the conditions by different cutoffs of A1C or fasting glucose. The ROC curve displayed the trade-off between the sensitivity and (1- specificity) across all observed cutoffs of A1C and fasting glucose. Then the optimal thresholds for A1C and fasting glucose that maximized sensitivity + specificity were identified, and corresponding sensitivity and specificity were presented. Two AUCs, for A1C and for fasting glucose, were compared using Mann-Whitney U statistic. Multivariate regression was conducted to evaluate the independent effect of baseline A1C on type 2 diabetes/prediabetes or NGT status at follow-up visit with the adjustment of potential confounders. A Pearson correlation was used to assess simple correlations. SAS 9.2 was used for the analysis (SAS Institute, Inc., Cary, NC).
RESULTS
Study cohort
At baseline, we studied a multiethnic cohort of 1,156 obese children and adolescents (Caucasian 36%/African American 35%/Hispanic 29%, 469 male and 687 female) (Supplementary Table 1). Their mean age was 13.2 ± 2.8 (range 4.8–23.1) years; except for the 23.1-year-old subject, the oldest subjects were aged 21 years. The median age of our population was 13.3 years, and the mode of the age was 14.7 years. The mean z score BMI was 2.39 ± 0.38.
Of 1,156 subjects, 31 (9 male and 22 female) had type 2 diabetes (10 Caucasians, 15 African Americans, and 6 Hispanics) according to the OGTT criteria. The mean age of this group was 13.7 ± 2.25 years, the mean z score BMI was 2.35 ± 0.41, the mean fasting glucose was 116.1 ± 20.2 mg/dL, and the mean 2-h glucose was 230.7 ± 29.4 mg/dL. None of them was positive for GAD 65, ICA 512, or IA.
The average A1C level in the entire cohort was 5.41 ± 0.42% (4.10–8.00). Of note, there was a significant ethnic difference in the mean A1C among the three ethnic groups, with African Americans showing the highest A1C levels (5.55 ± 0.45), Caucasians showing the lowest A1C levels (5.28 ± 0.36), and Hispanics showing middle A1C levels (5.38 ± 0.38) (P < 0.001). This ethnic difference persisted even after controlling for fasting and 2-h glucose levels (P < 0.001).
There was a modest, albeit significant, positive relationship between A1C and fasting glucose (r = 0.29; P < 0.001), and between A1C and 2-h glucose (r = 0.32; P < 0.001).
Baseline characteristics of the study cohort according to A1C categories
At baseline, we stratified the population according to A1C categories based on the ADA 2009 recommendations: NGT (A1C <5.7%), at risk for diabetes (A1C 5.7–6.4%), and type 2 diabetes (>6.5%) (Table 1).
Table 1.
Clinical features of the study population according to A1C categories at baseline
| <5.7 |
≥5.7 to ≤6.4 |
>6.4 |
||
|---|---|---|---|---|
| (n = 893) | (n = 247) | (n = 16) | P | |
| Anthropometrics | ||||
| Age (years) | 13.2 ± 2.84 | 13.1 ± 2.76 | 13.5 ± 2.33 | 0.81 |
| Sex (M/F) (%) | 41/59 | 42/58 | 28/72 | 0.70 |
| Race (Caucasian/African American/Hispanic) (%) | 42/29/29 | 18/54/28 | 28/56/17 | <0.001 |
| BMI z score | 2.38 ± 0.38 | 2.44 ± 0.38 | 2.52 ± 0.51 | 0.01 |
| BMI (kg/m2)# | 35.43 (34.98–35.88) | 35.88 (35.03–36.73) | 38.98 (35.73–42.23) | 0.08 |
| Glucose metabolism | ||||
| Fasting glucose (mg/dL)* | 92.07 (91.52–92.62) | 96.83 (95.74–97.94) | 106.64 (102.03–111.47) | <0.001 |
| 2-h Glucose (mg/dL)* | 117.58 (116.05–119.13) | 130.94 (127.70–134.26) | 188.09 (170.61–207.36) | <0.001 |
| Fasting insulin (μU/L)* | 29.10 (28.15–30.08) | 33.40 (31.36–35.56) | 41.81 (32.77–53.34) | <0.001 |
| WBISI* | 1.60 (1.54–1.66) | 1.35 (1.26–1.45) | 1.06 (0.81–1.39) | <0.001 |
| IGI* | 3.73 (3.55–3.92) | 3.79 (3.46–4.16) | 1.82 (1.28–2.61) | 0.13 |
| DI* | 5.99 (5.73–6.27) | 5.20 (4.77–5.67) | 1.60 (1.14–2.25) | <0.001 |
| HbA1c (%)* | 5.24 (5.22–5.26) | 5.88 (5.84–5.92) | 6.79 (6.62–6.97) | <0.001 |
| Lipids | ||||
| Triglycerides (mg/dL)* | 98.17 (94.49–102.01) | 86.36 (80.14–93.05) | 88.96 (65.41–120.98) | 0.0040 |
| Cholesterol (mg/dL)* | 156.27 (154.14–158.44) | 152.34 (148.31–156.48) | 156.36 (141.00–173.38) | 0.16 |
| HDL (mg/dL)* | 41.25 (40.62–41.90) | 42.22 (40.96–43.52) | 38.08 (33.76–42.97) | 0.57 |
| LDL (mg/dL)* | 90.01 (88.18–91.88) | 87.16 (83.74–90.72) | 91.83 (78.36–107.62) | 0.27 |
Mean and SD are presented for age, BMI, and z score.
#Adjusted for age, sex, and ethnicity.
*Log transformed and adjusted for age, sex, ethnicity, and BMI z score, and shown as geometric means and 95% CI. P values are from χ2 test for nominal variables or testing for linear trend for continuous variables across A1C categories.
According to this classification, 77% were in the NGT category, 21% were in the at risk for diabetes category, and only 1% were in the type 2 diabetes category (A1C >6.5%). Both age and sex distribution were not different among the three categories; however, it should be noted that there was a trend toward a greater number of female subjects in the higher categories of A1C. The ethnic differences among the A1C categories are particularly noteworthy; specifically, there was a higher prevalence of African Americans in the at risk for diabetes and type 2 diabetes categories (P < 0.0001). Across the A1C categories, subjects tended to be heavier in the at risk for diabetes and type 2 diabetes groups (P = 0.01). Moreover, subjects belonging to these two latter categories showed higher fasting glucose, fasting insulin, and 2-h glucose (P < 0.0001), lower insulin sensitivity (WBISI) (P < 0.0001), a trend toward lower first-phase insulin secretion (IGI, P = 0.13), and significantly lower DI (P < 0.0001) than subjects with A1C <5.7%. Plasma triglyceride levels were higher in the NGT category compared with both the prediabetic and type 2 diabetes categories (P = 0.004), probably because of the ethnic difference among the categories, with a lower prevalence of African Americans in the lower A1C group.
Baseline distribution of glucose tolerance status according to A1C categories
Table 2 describes the proportion of each type of glucose tolerance status (NGT, IGT, IFG, IFG/IGT, and type 2 diabetes, derived from the OGTT) within categories of A1Cs. First, although the majority (72%) of the subjects with an A1C <5.7% were classified as NGT by the OGTT, 27% were classified with prediabetes. Second, of the subjects in the at risk category, 47% of them showed laboratory values indicative of prediabetes or diabetes, whereas the majority (53%) were NGT. Last, the majority (62%) of subjects with an A1C >6.5% were classified as having type 2 diabetes by the OGTT; however, there were also 12.5% classified as NGT and 24% classified as having prediabetes (IFG and IGT). Thus, of the 247 subjects categorized as at risk for diabetes on the basis of their A1C value, only 103 (47%) were categorized as being at risk on the basis of their OGTT, and of the 16 classified with type 2 diabetes by A1C categories, only 10 (62%) would be indicated as having diabetes. On the other hand, of those considered as having type 2 diabetes by using A1C criteria, 6 of 16 (38%) were missed by the OGTT, whereas among those in the at risk category according to A1C criteria, 144 of 247 (58%) were missed by the OGTT. In other words, the OGTT missed approximately half of the subjects who were considered as at risk for diabetes or having type 2 diabetes by using the A1C criteria. κ and weighted κ coefficient calculated on the basis of Table 2 were 0.17 (95% CI 0.11–0.23) and 0.20 (0.14–0.26), respectively, which also indicated a poor agreement between A1C criteria and OGTT status.
Table 2.
Baseline glucose tolerance according to the A1C categories and OGTT
| A1C categories |
Total | |||
|---|---|---|---|---|
| OGTT | <5.7% (NGT) | 5.7–6.4% (at risk for diabetes) | >6.4% (type 2 diabetes) | |
| NGT | 644 | 132 | 2 | 778 |
| Prediabetes | 240 | 103 | 4 | 347 |
| Type 2 diabetes | 9 | 12 | 10 | 31 |
| Total | 893 | 247 | 16 | 1,156 |
κ coefficient 0.17 with 95% CI (0.11–0.23); weighted κ 0.20 with 95% CI (0.14–0.26).
Comparisons between A1C and fasting glucose: AUC analysis at baseline
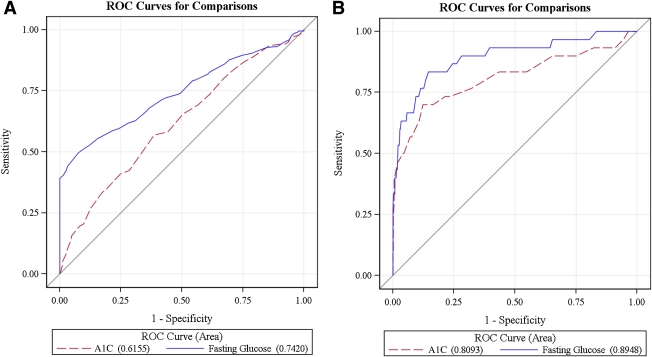
The AUCs shown in Fig. 1A and B represent the diagnostic accuracy of the A1C, compared with fasting glucose, for IGT and type 2 diabetes, respectively. For IGT, the AUC for A1C was 0.60 (95% CI 0.56–0.65) and the AUC for fasting glucose was 0.67 (0.63–0.72) (Fig. 1A). The optimal threshold of A1C was 5.5%, with a specificity of 59.9% and sensitivity of 57.0%. The two areas differed significantly from each other (P = 0.01). In contrast, in the type 2 diabetes category, the diagnostic accuracy of A1C and fasting glucose was not significantly different (P = 0.13). The AUC for A1C was 0.81 (0.70–0.92), and the AUC fasting glucose was 0.89 (0.82–0.97) (Fig. 1B). The optimal threshold of A1C was 5.8% in identifying type 2 diabetes, with a specificity of 87.64% and sensitivity of 67.7%.
Figure 1.
Comparison between the AUCs of the A1C and fasting glucose for IGT (A) and type 2 diabetes (B) at baseline. The red discontinuous line indicates the curve defining the area for the A1C, and the blue continuous curve defines the area for fasting glucose. The value of each area is specified next to the two variables (A1C and fasting glucose) at the bottom of the figure.
Follow-up data
To test the predictive value of A1C to diagnose prediabetes and type 2 diabetes, we analyzed data from 218 subjects on whom we had repeated measures after a mean of 1.68 ± 0.92 years. The follow-up group did not differ from the subjects lost to follow-up in terms of ethnicity (Caucasian 41.5%/African American 30.2%/Hispanic 28.3%, P = 0.2), sex distribution (male 36.3%, female 63.7%, P = 0.16), and A1C levels (5.39 ± 0.39 vs. 5.41 ± 0.42, range 4.3–6.5, P = 0.3).
At baseline, 139 subjects (63.76%) had NGT, 26 subjects (6.88%) had IFG only, 23 subjects (10.55%) had IGT only, 26 subjects (11.92%) had IGT and IFG, and 4 subjects (1.83%) had type 2 diabetes. Subjects in the follow-up group tended to be younger (mean age 12.53 ± 2.83 years, range 5.8–21; P = 0.001) than those who did not come to follow-up visits (mean age 13.36 ± 2.8 years, range 4.8–23.1).
Baseline and follow-up A1C were highly correlated (r = 0.78, P < 0.0001). The correlation between baseline A1C and follow-up fasting and 2-h glucose (r = 0.33; P < 0.0001 and r = 0.32; P < 0.0001, respectively) were similar to those observed in the whole population at baseline. A multivariate analysis showed that the strongest predictors of 2-h glucose at follow-up were baseline A1C and baseline 2-h glucose levels (P = 0.001 and P < 0.0001, respectively) independently of age, ethnicity, sex, baseline fasting glucose, changes in BMI z score, and follow-up time. Baseline A1C strongly predicted follow-up prediabetes/diabetes; the results from multivariate analysis showed a 1% increase in baseline A1C corresponding to 13-fold (95% CI 4.79–36.88) increases in the likelihood of having prediabetes/diabetes at follow-up, after adjusting for age, sex, race, and follow-up time. After additional adjusting for baseline fasting glucose and 2-h glucose, the strong effect of A1C still existed (odds ratio 6.6 [2.27–19.24]). Subjects with a baseline A1C ≥5.7 had a greater chance (odds ratio 5.7 [CI 1.54–10.31]) of having prediabetes/diabetes at the follow-up visit than their peers with a lower A1C (<5.7) at baseline, after controlling for age, sex, ethnicity, and follow-up time.
CONCLUSIONS
In a large clinic-based multiethnic cohort of obese children and adolescents, regardless of age and sex, an A1C of 6.5% had relatively low sensitivity and specificity for classifying type 2 diabetes. There was poor agreement between A1C and OGTT criteria in classifying subjects with glucose values suggestive of type 2 diabetes. The optimal threshold of A1C was 5.8% for identifying type 2 diabetes, with a specificity of 87.64% and sensitivity of 67.7%, and 5.5% for identifying IGT. The diagnostic utility of A1C was examined according to ADA criteria (1) with OGTT as the reference. We observed that the use of an A1C of 6.5% would largely underestimate the prevalence of prediabetes and type 2 diabetes. Our results suggest that, although A1C could be used as a clinical tool to identify type 2 diabetes, along with fasting and 2-h glucose, the use of A1C by itself to pinpoint prediabetes and type 2 diabetes is not recommended. Our data are in agreement with those who reported using the National Health and Nutrition Examination Survey of 14,611 individuals aged ≥20 years, clearly showing that an A1C of 6.5% has a lower capacity to detect prediabetes and undiagnosed type 2 diabetes than the OGTT (10).
Studies in adults have clearly shown the utility of A1C in predicting type 2 diabetes (11–14) and cardiovascular disease even in nondiabetic adults (6). Nevertheless, concerns in the use of A1C for diagnosing type 2 diabetes have been recently raised (15) in view of the poor relationship with fasting glucose (16), the overall lower diagnostic performance in some groups such as pregnant women and elderly, and the risk of overdiagnosing patients with anemia and those predisposed to rapid glycosylation (15). In addition, as previously stated (10), it should be noted that, despite the numerous advantages, the use of A1C as a diagnostic tool would largely affect national surveillance of prediabetes and type 2 diabetes.
Different cutoff points have been reported when the ROC curve was used to identify the cutoff point for diagnosing type 2 diabetes or prediabetes (11). A review on A1C as a screening tool for diabetes (11) showed that three cutoff points (5.9, 6.1, and 6.3%) of A1C were advised as cutoff points for detecting diabetes in at least two different studies, and most studies identified a cutoff point of ≥6.1% as optimum for the detection of type 2 diabetes. In addition, the authors concluded that at equivalent cutoff points, sensitivity was generally lower in detecting IGT for both A1C and fasting plasma glucose in both community- and hospital-based studies (11). Thus, the cutoff point identified in our study of 5.8% is somewhat lower than oftentimes reported, which might indicate that our population is of especially high risk.
Although only a small percentage of subjects had a repeated A1C and OGTT after a follow-up of 2 years, we believe that the data are important, indicating that the best predictors of future diabetes or prediabetes are A1C and the 2-h glucose from the OGTT. Thus, both the cross-sectional and longitudinal data would argue in favor of the utility of performing both tests in obese youth for predicting future development of diabetes.
Ethnic disparities in A1C in adults have been suggested by others (17). Although previous studies attributed ethnic differences in A1C to a poorer glycemic control among ethnic minorities (18), studies in nondiabetic subjects showed that these differences are not related, or at least not only related, to the different glycemic control (19–21). Other studies have clearly shown that factors influencing glycemia, such as adiposity, fasting, postglucose load glucose levels, β-cell function, and insulin resistance, do not explain ethnic differences in A1C.
It is known that conditions characterized by altered erythrocyte physiology may influence the utility of A1C in diabetes diagnosis (22). Recently it has been shown that some common genetic variations resulting in alterations in iron levels or hemoglobin concentration can also affect A1C levels (23).
A few limitations are worth noting. There is no lean control group, a clinic-based cohort was studied, and the follow-up group is small. Strengths include the large group of obese youngsters without known diabetes and the existence of data derived on the same day for both the OGTT and A1C.
In conclusion, the use of A1C alone may result in missed or delayed individuation of prediabetes/type 2 diabetes given the limited sensitivity of the A1C test. Further investigation on the role of A1C in the diagnosis of prediabetes and diabetes in children and adolescents is needed. Prospective studies are especially important to examine the utility of A1C in pediatric populations in the prediction of diabetes-related comorbidities later in life.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) (grants R01-HD-40787, R01-HD-28016, and K24-HD-01464 to S.C.), the Tegger Foundation (to P.N.), and the National Center for Research Resources, NIH (Clinical and Translational Science Award grant UL1-RR-0249139).
No potential conflicts of interest relevant to this article were reported.
P.N. researched data and wrote the manuscript. N.S. researched data and contributed to discussion. H.L. researched data and reviewed and edited the manuscript. D.L., M.M.S., R.G., C.G., M.S., and P.R. contributed to discussion. S.C. wrote, reviewed, and edited the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1984/-/DC1.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohlfing CL, Little RR, Wiedmeyer HM, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 2000;23:187–191 [DOI] [PubMed] [Google Scholar]
- 3.Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ. Utility of hemoglobin A1c in predicting diabetes risk. J Gen Intern Med 2004;19:1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med 2007;120:720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002;346:802–810 [DOI] [PubMed] [Google Scholar]
- 8.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab 2004;89:1096–1101 [DOI] [PubMed] [Google Scholar]
- 9.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. Beta-cell function across the spectrum of glucose tolerance in obese youth. Diabetes 2005;54:1735–1743 [DOI] [PubMed] [Google Scholar]
- 10.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care 2010;33:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med 2007;24:333–343 [DOI] [PubMed] [Google Scholar]
- 12.Ko GT, Chan JC, Woo J, et al. Glycated haemoglobin and cardiovascular risk factors in Chinese subjects with normal glucose tolerance. Diabet Med 1998;15:573–578 [DOI] [PubMed] [Google Scholar]
- 13.Droumaguet C, Balkau B, Simon D, et al. ; DESIR Study Group Use of HbA1c in predicting progression to diabetes in French men and women: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2006;29:1619–1625 [DOI] [PubMed] [Google Scholar]
- 14.Shimazaki T, Kadowaki T, Ohyama Y, Ohe K, Kubota K. Hemoglobin A1c (HbA1c) predicts future drug treatment for diabetes mellitus: a follow-up study using routine clinical data in a Japanese university hospital. Transl Res 2007;149:196–204 [DOI] [PubMed] [Google Scholar]
- 15.Lippi G, Mattiuzzi C, Targher G. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:2030–2031 [DOI] [PubMed]
- 16.Kramer CK, Araneta MR, Barrett-Connor E. A1C and diabetes diagnosis: The Rancho Bernardo Study. Diabetes Care 2010;33:101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman WH, Ma Y, Uwaifo G, et al. ; Diabetes Prevention Program Research Group Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirk JK, Bell RA, Bertoni AG, et al. Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes. Ann Pharmacother 2005;39:1489–1501 [DOI] [PubMed] [Google Scholar]
- 19.Summerson JH, Konen JC, Dignan MB. Race-related differences in metabolic control among adults with diabetes. South Med J 1992;85:953–956 [DOI] [PubMed] [Google Scholar]
- 20.Wisdom K, Fryzek JP, Havstad SL, Anderson RM, Dreiling MC, Tilley BC. Comparison of laboratory test frequency and test results between African-Americans and Caucasians with diabetes: opportunity for improvement. Findings from a large urban health maintenance organization. Diabetes Care 1997;20:971–977 [DOI] [PubMed] [Google Scholar]
- 21.Brown AF, Gregg EW, Stevens MR, et al. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care 2005;28:2864–2870 [DOI] [PubMed] [Google Scholar]
- 22.Roberts WL, Safar-Pour S, De BK, Rohlfing CL, Weykamp CW, Little RR. Effects of hemoglobin C and S traits on glycohemoglobin measurements by eleven methods. Clin Chem 2005;51:776–778 [DOI] [PubMed] [Google Scholar]
- 23.Soranzo N, Sanna S, Wheeler E, et al. Common variants at 10 genomic loci influence hemoglobin A1C levels via glycemic and nonglycemic pathways. Diabetes 2010;59:3229–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.