Abstract
OBJECTIVE
To examine the impact of withdrawing rosiglitazone and ramipril medication on diabetes incidence after closeout of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial.
RESEARCH DESIGN AND METHODS
The 3,366 DREAM subjects at trial end who had not developed diabetes while taking double-blind study medication were transferred to single-blind placebo for 2 to 3 months before undergoing an oral glucose tolerance test. Glycemic status was analyzed for the trial plus washout period and for the washout period alone.
RESULTS
Following median (interquartile range) 71 (63–86) days drug withdrawal, overall glycemic status remained modestly improved in those allocated ramipril during the trial with an 11% increase in regression to normoglycemia, compared with placebo. In those previously allocated rosiglitazone, glycemic status remained substantially improved with a 49% reduction of new-onset diabetes or death and a 22% increase in regression to normoglycemia, compared with placebo. However, during the washout phase alone the incidence of diabetes or death was identical for those allocated previously to ramipril or placebo, or to rosiglitazone or placebo.
CONCLUSIONS
In people allocated to ramipril compared with those not allocated ramipril during the trial, the postwashout normoglycemia incidence was higher. In people allocated to rosiglitazone compared with those not allocated rosiglitazone during the trial, the postwashout incidence of diabetes was significantly lower and the incidence of normoglycemia was higher. During the washout period, diabetes incidence was the same for ramipril versus placebo and for rosiglitazone versus placebo. Rosiglitazone delays disease progression during treatment but the process resumes at the placebo rate when the drug is stopped.
Type 2 diabetes prevention is a major public health issue. The Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial (1) showed that the new-onset diabetes rate in people with impaired glucose tolerance (IGT) and/or impaired fasting glucose (IFG) can be slowed substantially (60% relative to placebo) by the thiazolidinedione rosiglitazone (2) but not by the ACE inhibitor ramipril (3). The rosiglitazone results were similar to those seen in two trials with the now withdrawn thiazolidinedione troglitazone (4,5). DREAM also showed that both rosiglitazone and ramipril increased the rate of regression to normal glucose tolerance (2,3).
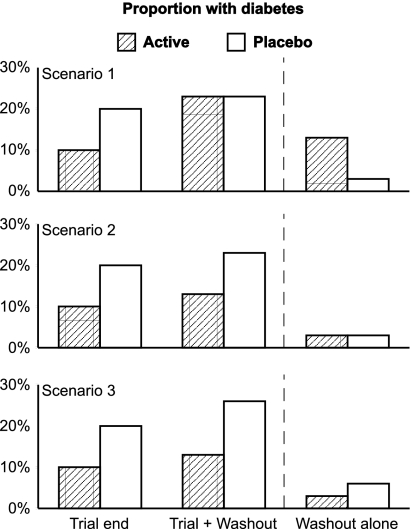
Because type 2 diabetes is a progressive disorder (6), it is of interest to know whether an intervention that delays diabetes onset is 1) masking diabetes appearance by suppressing glucose levels, 2) slowing diabetes development only while it is being administered, or 3) has sustained benefit even after withdrawal. These possibilities can be distinguished by washing out the intervention and then reassessing glycemic status (Fig. 1). If diabetes was being masked, postwashout diabetes incidence in the intervention group would exceed that in the placebo group and the overall (trial plus washout) diabetes prevalence in both groups would be similar (Fig. 1, scenario 1). If the underlying disease process only slowed during intervention, postwashout diabetes incidence would be similar in the intervention and placebo groups, with the overall prevalence of diabetes in the group formerly receiving the intervention remaining lower than in the placebo group (Fig. 1, scenario 2). Finally, if the intervention had a sustained effect after drug withdrawal, both the washout incidence and the overall diabetes prevalence in the group formerly receiving the intervention would be lower than in the placebo group (Fig. 1, scenario 3). Follow-up data from the truncated troglitazone arm of the Diabetes Prevention Program (DPP) suggested that scenario 2 may be the case for thiazolidinediones (5).
Figure 1.
Three possible scenarios showing the relative proportions of subjects with new-onset diabetes at the end of a diabetes prevention trial, after the trial plus a drug washout period, and after the washout period alone. Scenario 1 illustrates a treatment that merely masks the appearance of diabetes by suppressing glucose levels. Scenario 2 demonstrates slowing the development of diabetes while the treatment is being given. Scenario 3 shows sustained benefit even after the treatment has been withdrawn.
We conducted a prospective 2- to 3-month post-trial medication washout to evaluate the impact on the new-onset type 2 diabetes rate in consenting DREAM trial participants who at trial end had not developed diabetes, were taking their double-blind study medication, and underwent an oral glucose tolerance test (OGTT).
RESEARCH DESIGN AND METHODS
The design and primary results of the DREAM trial have been published (1–3). Between July 2001 and August 2003, 5,269 participants aged 30 years or more with impaired fasting plasma glucose (FPG; ≥6.1 but <7.0 mmol/L) and/or IGT (2-h post 75 g oral glucose load plasma glucose ≥7.8 but <11.1 mmol/L) were allocated at random to receive ramipril (titrated to a maximum of 15 mg) or matching placebo and, simultaneously, rosiglitazone (titrated to a maximum of 8 mg) or matching placebo, in a two-by-two balanced factorial design. Participants had OGTTs done after 2 years and at final visit, and at other yearly visits if FPG or HbA1c values were elevated (2), and were followed for a median of 3 years. All participants provided informed written consent for the whole study, including the washout phase.
Consenting participants who underwent an OGTT at their last study visit, who had not developed diabetes, and who were taking their study medication were entered into the post-trial washout. They were given single-blind placebo rosiglitazone and ramipril medication and scheduled for an OGTT 2 to 3 months later. No other clinical assessments were done at the end of this period. The primary composite outcome for the washout was the same as the main trial (new-onset diabetes or death), but the diagnosis of diabetes was based on one, not two, successive abnormal OGTTs or FPG values ≥7.0 mmol/L. As before, development of diabetes in an individual was accepted if a physician outside the study diagnosed diabetes on the basis of a FPG level ≥7.0 mmol/L or a non-FPG level ≥11.1 mmol/L and had prescribed an antidiabetic agent.
Statistical analysis
Data were collected and analyzed at the Population Health Research Institute, McMaster University, using an intention-to-treat approach according to marginal groups: ramipril versus placebo and rosiglitazone versus placebo. Participants who entered the washout phase but who did not return for an OGTT and whose postwashout diabetes status was unknown were assumed not to have developed diabetes. Differences in trial-end prewashout characteristics by prior randomization were compared using t tests for continuous variables and χ2 tests for categorical variables. Hazard ratios (HRs) and 95% CIs for the effect of prior treatment with each study drug (stratified by the other drug) on the primary outcome, and on the secondary outcome of regression to normoglycemia, were calculated using Cox proportional hazards models. Possible statistical interactions between ramipril and rosiglitazone treatments were assessed by the inclusion of an interaction term in the models. Trial plus washout analyses were done for all randomized participants from the time of randomization until the end of the washout period. Participants who did not enter the washout phase were analyzed according to their trial-end glycemic status. Additional washout alone analyses were restricted to those participants who entered the washout period. Median changes in fasting and 2-h plasma glucose levels during the washout phase were compared according to prior treatment with each study drug or respective placebo using Wilcoxon signed rank tests (with no adjustment for multiple testing).
RESULTS
Subject disposition
Of the 5,269 randomized participants the primary outcome occurred by trial end in 995, comprising the 992 reported previously (2,3) plus 3 more with delayed reports. An additional 907 people were ineligible for the washout because they had no final-visit OGTT (n = 64) or were no longer taking study medication (n = 843). Of the eligible 3,367, 105 declined to participate. The 3,262 (96.9%) participants completing the washout period were followed for a median (interquartile range [IQR]) of 71 (63–86) days. When compared with the 105 individuals who declined participation, these individuals had a similar duration of trial follow-up, age, sex, weight, waist-to-hip ratio, blood pressure, and mean FPG level (P all > 0.1), but lower 2-h postchallenge glucose value (7.13 mmol/L vs. 7.77 mmol/L; P = 0.0024).
Trial-end characteristics
Table 1 lists the participant characteristics at trial end according to their prior allocation to ramipril or placebo and to rosiglitazone or placebo. These reflect the glycemic impact of these therapies before commencing the washout phase. Participants allocated previously to ramipril (n = 1,632), compared with those allocated to placebo (n = 1,630), had lower fasting and 2-h postchallenge plasma glucose values and lower systolic and diastolic blood pressure values. Participants allocated previously to rosiglitazone (n = 1,773), compared with those allocated to placebo (n = 1,489), had lower fasting and 2-h postchallenge plasma glucose values and lower systolic and diastolic blood pressure values. They were also less likely to be women and were heavier, and the females had a lower waist-to-hip ratio.
Table 1.
Demographic, clinical, and biochemical characteristics of the 3,367 subjects without diabetes who entered the drug washout period
| All | Ramipril | Placebo | P | Rosiglitazone | Placebo | P | |
|---|---|---|---|---|---|---|---|
| n | |||||||
| Entering washout | 3,367 | 1,677 | 1,690 | 1,833 | 1,534 | ||
| Completing washout | 3,262 (96.9%) | 1,632 (97.3%) | 1,630 (96.5%) | 1,773 (96.7%) | 1,489 (97.1%) | ||
| Female | 1,897 (58.2%) | 972 (59.6%) | 925 (56.8%) | 0.11 | 1,003 (56.67%) | 894 (60.0%) | 0.046 |
| Follow-up to trial end (years) | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.2 ± 0.5 | 0.28 | 3.2 ± 0.5 | 3.2 ± 0.5 | 0.89 |
| Mean age at trial end (years) | 58.1 ± 10.7 | 58.1 ± 10.8 | 58.0 ± 10.6 | 0.83 | 57.9 ± 10.5 | 58.3 ± 10.9 | 0.30 |
| FPG (mmol/L) | 5.56 ± 0.72 | 5.54 ± 0.70 | 5.59 ± 0.74 | 0.029 | 5.46 ± 0.69 | 5.69 ± 0.74 | <0.0001 |
| 2HPG (mmol/L) | 7.13 ± 1.96 | 7.04 ± 1.94 | 7.22 ± 1.98 | 0.013 | 6.76 ± 1.82 | 7.57 ± 2.03 | <0.0001 |
| BP (mmHg) | |||||||
| Systolic | 129.2 ± 17.1 | 127.0 ± 17.1 | 131.5 ± 16.8 | <0.0001 | 128.4 ± 16.8 | 130.2 ± 17.4 | 0.0025 |
| Diastolic | 78.4 ± 10.6 | 77.2 ± 10.7 | 79.6 ± 10.3 | <0.0001 | 77.7 ± 10.4 | 79.2 ± 10.6 | <0.0001 |
| BMI (kg/m2) | 31.0 ± 5.7 | 31.0 ± 5.7 | 31.0 ± 5.7 | 0.94 | 31.6 ± 5.7 | 30.4 ± 5.6 | <0.0001 |
| Weight (kg) | 85.3 ± 19.4 | 85.1 ± 19.3 | 85.5 ± 19.5 | 0.56 | 87.3 ± 19.7 | 83.0 ± 18.7 | <0.0001 |
| Waist/hip | |||||||
| Male | 0.96 ± 0.08 | 0.96 ± 0.08 | 0.96 ± 0.08 | 0.58 | 0.96 ± 0.08 | 0.96 ± 0.07 | 0.62 |
| Female | 0.87 ± 0.12 | 0.87 ± 0.13 | 0.87 ± 0.10 | 0.60 | 0.86 ± 0.13 | 0.88 ± 0.11 | 0.0075 |
Categorical variables are expressed as n (%) and continuous variables as mean ± 1 SD.
Ramipril outcomes
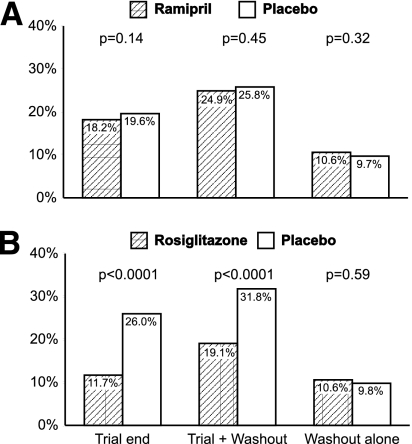
At trial-end plus washout, the proportion of participants in whom the primary outcome had occurred (Fig. 2A and Table 2) did not differ between those allocated to ramipril or to placebo (24.9 vs. 25.8%, respectively; P = 0.45); the proportion of participants regressing to normoglycemia was greater (HR 1.11 [95% CI 1.01–1.21]; P = 0.031) for those allocated to ramipril (36.0%) compared with placebo (33.2%). During the washout period alone, both the primary and secondary outcome occurred at the same rate in both groups (10.6 vs. 9.7%, P = 0.32; and 23.0 vs. 22.7%, P = 0.66, respectively).
Figure 2.
Proportions of subjects with new-onset diabetes at the end of the trial, after the trial plus drug washout, and after the washout period (median 71 days) alone for ramipril vs. placebo (A) and rosiglitazone vs. placebo arms of the trial (B).
Table 2.
HRs (95% CI) for development of diabetes or death at trial end, at trial end plus washout, and during washout period alone for the ramipril vs. placebo and for the rosiglitazone vs. placebo arms of the trial
| Ramipril | Placebo | HR (95% CI) | P | Rosiglitazone | Placebo | HR (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| Primary outcome | ||||||||
| Trial end | 476/2,623 | 519/2,646 | 0.91 (0.80–1.03) | 0.14 | 309/2,635 | 686/2,634 | 0.40 (0.35–0.46) | <0.0001 |
| Trial + washout | 654/2,623 | 683/2,646 | 0.95 (0.84–1.08) | 0.45 | 501/2,635 | 836/2,634 | 0.51 (0.45–0.57) | <0.0001 |
| Washout alone | 178/1,677 | 164/1,690 | 1.12 (0.90–1.38) | 0.32 | 192/1,833 | 150/1,534 | 1.06 (0.86–1.32) | 0.59 |
| Secondary outcome* | ||||||||
| Trial end | 1,117/2,623 | 1,013/2,646 | 1.17 (1.07–1.27) | 0.0002 | 1,329/2,635 | 801/2,634 | 1.69 (1.55–1.85) | <0.0001 |
| Trial + washout | 944/2,623 | 878/2,646 | 1.11 (1.01–1.21) | 0.031 | 1,032/2,635 | 790/2,634 | 1.22 (1.11–1.34) | <0.0001 |
| Washout alone | 164/714 | 183/807 | 1.05 (0.85–1.30) | 0.66 | 144/669 | 203/852 | 0.90 (0.73–1.12) | 0.33 |
*Regression to normoglycemia.
Rosiglitazone outcomes
At the end of the trial plus washout, the proportion of participants in whom the primary outcome had occurred (Fig. 2B and Table 2) was less (HR 0.51 [95% CI 0.45–0.57]; P < 0.0001) for those allocated to rosiglitazone (19.0%) compared with placebo (31.7%); the proportion of participants regressing to normoglycemia was greater (HR 1.22 [1.11–1.34]; P < 0.0001) for those allocated to rosiglitazone (39.2%) compared with placebo (30.0%). During the washout period alone, both the primary and secondary outcome occurred at the same rate in both groups (10.5 vs. 9.8%, P = 0.59; and 21.5 vs. 23.8%, P = 0.33, respectively).
Postwashout fasting and 2-h plasma glucose levels
At the end of the washout, there was no difference in either fasting or 2-h postchallenge median plasma glucose levels between people originally allocated to ramipril versus placebo (Table 3). At the same time median FPG levels did not differ between those allocated to rosiglitazone or placebo, despite lower FPG levels in the rosiglitazone group at trial end (Table 3). This was the result of a greater increase in FPG levels during the washout in participants formerly on rosiglitazone versus placebo (0.30 vs. 0.10 mmol/L, respectively; P < 0.0001). Conversely, 2-h postchallenge plasma glucose levels remained lower after the washout (7.1 vs. 7.4 mmol/L; P = 0.005) despite a greater increase in the rosiglitazone group (0.5 mmol/L) compared with the placebo group (0.0 mmol/L; P < 0.0001).
Table 3.
Median (IQR) fasting and 2-h plasma glucose levels (mmol/L) at trial end, at trial end plus washout, and during washout period alone for the ramipril vs. placebo and for the rosiglitazone vs. placebo arms of the trial
| Ramipril |
Placebo |
P | Rosiglitazone |
Placebo |
P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | IQR | n | Median | IQR | n | Median | IQR | n | Median | IQR | |||
| Fasting glucose | ||||||||||||||
| Baseline | 2,623 | 5.9 | 5.4–6.3 | 2,646 | 5.9 | 5.4–6.4 | 0.45 | 2,635 | 5.9 | 5.4–6.4 | 2,634 | 5.9 | 5.4–6.3 | 0.52 |
| Trial end | 2,418 | 5.7 | 5.2–6.3 | 2,440 | 5.7 | 5.2–6.5 | 0.033 | 2,431 | 5.5 | 5.1–6.1 | 2,427 | 5.9 | 5.3–6.5 | <0.0001 |
| Washout | 1,630 | 5.7 | 5.2–6.2 | 1,628 | 5.7 | 5.3–6.2 | 0.090 | 1,769 | 5.7 | 5.2–6.2 | 1,489 | 5.7 | 5.2–6.2 | 0.72 |
| 2-h Glucose | ||||||||||||||
| Baseline | 2,623 | 8.6 | 8.0–9.6 | 2,646 | 8.8 | 8.0–9.8 | 0.064 | 2,635 | 8.7 | 8.0–9.7 | 2,634 | 8.7 | 8.0–9.7 | 0.85 |
| Trial end | 2,032 | 7.1 | 5.8–8.7 | 2,033 | 7.3 | 5.9–8.9 | 0.020 | 2,168 | 6.7 | 5.6–8.3 | 1,897 | 7.7 | 6.2–9.4 | <0.0001 |
| Washout | 1,624 | 7.2 | 5.9–8.6 | 1,610 | 7.3 | 6.0–8.9 | 0.052 | 1,754 | 7.1 | 5.9–8.6 | 1,480 | 7.4 | 6.0–9.0 | 0.0051 |
Interaction effects
No statistical interactions were observed between ramipril and rosiglitazone with respect to FPG levels, 2-h plasma glucose levels, or glycemic status at the end of the trial plus washout or during the washout period alone.
Further analyses
No differences were seen in the results for individuals entering the study with IFG as opposed to IGT.
CONCLUSIONS
The DREAM study primary outcome (new-onset diabetes or death) results remained essentially similar when reanalyzed after a median 71-day study medication washout period. With respect to the washout period itself, because ramipril did not reduce diabetes incidence in the main trial (2) it is not surprising that the postwashout new-onset diabetes rates did not differ between those allocated ramipril or placebo during the trial. The lack of any differential impact on post-trial diabetes incidence between those allocated rosiglitazone or placebo during the trial, despite the major within-trial reduction in diabetes incidence (3), suggests that this agent does not have a sustained effect on the underlying disease pathophysiology.
The incidence of diabetes after therapy cessation with either of the agents evaluated did not differ from the incidence observed with placebo. These findings suggest that the underlying disease process is slowed only while the intervention is being given (scenario 2). The fact that most (but not all) of their effects on glucose levels disappear with drug discontinuation also suggests that underlying β-cell defects are not reversed or repaired and that the minor metabolic effects of ramipril and the profound effects of rosiglitazone are likely secondary to increased sensitivity to insulin.
The rosiglitazone results are consistent with the observations made in a similar population of 585 people with IGT who took troglitazone for a median period of 0.9 years in the DPP trial (5). When compared with placebo, troglitazone reduced the incidence of diabetes by 75% while it was being taken, but when discontinued (because of emerging concerns about liver toxicity) diabetes incidence was similar to, but not greater than, that in the placebo group. Therefore, in the context of the full 3-year follow of the DPP, study participants who had taken troglitazone for 0.9 years were only ∼17% less likely to have developed diabetes. By contrast, these findings are inconsistent with a study in women with a history of gestational diabetes (4) where troglitazone reduced the incidence of diabetes by 55% compared with placebo while it was being taken, but the effect persisted during an 8-month washout period. Postwashout glucose tolerance testing, however, was only done in a subset of randomized people who completed the trial without diabetes and who then returned after the washout. The possibility that responders were more likely to return for reassessment than nonresponders cannot be excluded.
The prospectively planned DREAM washout was completed while participants were taking single-blind placebo medication. Moreover, 97% of eligible participants completed this phase of the study with completion rates the same in those previously on active therapies or placebo (Table 1). These strengths support the robustness of the DREAM washout findings but there are several limitations. First, the ∼10% primary outcome incidence during a median washout of 71 days is higher than expected given the 26% 3-year incidence in the rosiglitazone placebo group during the trial (Fig. 2B). This is likely because of the fact that during the washout phase incident diabetes was based on only a single abnormal fasting or 2-h plasma glucose level as opposed to two successive abnormal values during the trial. Because of regression to the mean (7), many of those classified with diabetes based on a single abnormal glucose value during washout would not have had this diagnosis confirmed on a second test. Similar discrepancies in post-trial diabetes incidence rates have been noted in other diabetes prevention trials, which reported high diabetes incidence rates based on one abnormal glucose value following a short washout phase (5,8). Any overestimate of the absolute incidence of diabetes would, however, affect all treatment groups to the same extent so should not invalidate between-group comparisons. A second limitation is the relatively short washout period of a median 71 days. This may have been insufficient to totally wash out rosiglitazone, which may require a longer period of study medication. Finally, the effect of the washout on other secondary measurements such as liver function tests, blood pressure, body weight, and edema was not assessed.
In summary, rosiglitazone delays disease progression during treatment, but the process resumes at the placebo rate when the drug is stopped, as has been seen previously with metformin (9). Additional longer-term assessments of glucose tolerance in epidemiologic follow-up studies may yield further insights in the context of the effect of these interventions on the natural history of IFG, IGT, and type 2 diabetes. At this time the data indicate that rosiglitazone can substantially reduce the incidence of type 2 diabetes while it is being administered, but this effect is not sustained when the drug is withdrawn.
Acknowledgments
The trial was funded by the Canadian Institutes of Health Research (MCT41548), sanofi-aventis, GlaxoSmithKline, and King Pharmaceuticals. J.J.B., S.Y., H.C.G., B.Z., and M.J.D. report having received consulting and lecture fees from sanofi-aventis. S.Y., H.C.G., R.R.H., and B.Z. report having received consulting and lecture fees from GlaxoSmithKline. G.R.D. received consulting and lecture fees from sanofi-aventis and GlaxoSmithKline and reports holding a patent for the use of ramipril. J.J.B., S.Y., H.C.G., G.R.D., and J.M.P. report holding a patent for the use of ramipril to prevent diabetes and assigning all rights to sanofi-aventis in 2003. No other potential conflicts of interest relevant to this article were reported.
R.R.H. wrote the manuscript and researched data. B.Z. contributed to discussion and reviewed and edited the manuscript. S.Y. reviewed and edited the manuscript. P.M.S. and S.S.A. contributed to discussion. J.J.B. researched data and reviewed and edited the manuscript. I.C., M.J.D., and V.P. reviewed and edited the manuscript. G.R.D. researched data and reviewed the manuscript. J.M.P. researched data and reviewed and edited the manuscript. P.Z.Z. reviewed and edited the manuscript. H.C.G. researched data and wrote the manuscript.
APPENDIX
Writing committee: Rury R. Holman, FRCP1; Bernie Zinman, MD2; Salim Yusuf, DPhil3; Patrick M. Sheridan, MSc4; Sonia S. Anand, MD5; Jackie J. Bosch, MSc6; Ignacio Conget, MD7; Melanie J. Davies, FRCP8; Valdis Pirags, MD9; Gilles R. Dagenais, MD10; Janice M. Pogue, MSc11; Paul Z. Zimmet, PhD12; Hertzel C. Gerstein, FRCPC13; on behalf of the DREAM Trial Investigators.
From the 1Diabetes Trials Unit, University of Oxford, Oxford, U.K.; the 2Samuel Lunenfeld Research Institute, Mount Sinai Hospital and University of Toronto, Toronto, Ontario, Canada; the 3Hamilton Health Sciences Corporation, McMaster University, Hamilton, Ontario, Canada; the 4McMaster University, Hamilton, Ontario, Canada; the 5McMaster University, Hamilton, Ontario, Canada; the 6Institute for Applied Health Sciences, McMaster University, Hamilton, Ontario, Canada; the 7Endocrinology and Diabetes Unit, Hospital Clínic i Universitari, Barcelona, Spain; the 8Department of Cardiovascular Sciences, Diabetes Research, University of Leicester, Leicester Royal Infirmary, Leicester, U.K.; the 9University of Latvia, Riga, Latvia; the 10Institut Universitaire de Cardiologie et Pneumologie de Québec, Québec City, Québec, Canada; the 11Population Health Research Institute, McMaster Clinic, Hamilton General Hospital, Hamilton, Ontario, Canada; the 12Baker IDI, Heart & Diabetes Institute, Melbourne, Victoria, Australia; and the 13McMaster University, Hamilton, Ontario, Canada.
Footnotes
Clinical trial reg. no. NCT00095654, clinicaltrials.gov.
A complete list of the DREAM Trial Investigators can be found in the appendix.
References
- 1.Gerstein HC, Yusuf S, Holman RR, Bosch J, Pogue J; DREAM Trial Investigators Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: the DREAM trial. Diabetologia 2004;47:1519–1527 [DOI] [PubMed] [Google Scholar]
- 2.Gerstein HC, Yusuf S, Bosch J, et al. ; DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 3.Bosch J, Yusuf S, Gerstein HC, et al. ; DREAM Trial Investigators Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–1562 [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Hamman RF, Edelstein SL, et al. ; Diabetes Prevention Program Research Group Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005;54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner RC, Cull CA, Stratton IM, et al. U.K. Prospective Diabetes Study 16. Overview of 6 years' therapy of type 2 diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 7.Yudkin PL, Stratton IM. How to deal with regression to the mean in intervention studies. Lancet 1996;347:241–243 [DOI] [PubMed] [Google Scholar]
- 8.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 9.Diabetes Prevention Program Research Group Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003;26:977–980 [DOI] [PMC free article] [PubMed] [Google Scholar]