Abstract
OBJECTIVE
We evaluated predictors of progression to diabetes in children with high-risk HLA genotypes and persistent islet autoantibodies.
RESEARCH DESIGN AND METHODS
The Diabetes Autoimmunity Study in the Young (DAISY) followed 2,542 children with autoantibodies measured to GAD, IA-2, and insulin.
RESULTS
Persistent islet autoantibodies developed in 169 subjects, and 55 of those progressed to diabetes. Children expressing three autoantibodies showed a linear progression to diabetes with 74% cumulative incidence by the 10-year follow-up compared with 70% with two antibodies and 15% with one antibody (P < 0.0001). Both age of appearance of first autoantibody and insulin autoantibody (IAA) levels, but not GAD or IA-2 autoantibodies, were major determinants of the age of diabetes diagnosis (r = 0.79, P < 0.0001).
CONCLUSIONS
In the DAISY cohort, 89% of children who progressed to diabetes expressed two or more autoantibodies. Age of diagnosis of diabetes is strongly correlated with age of appearance of first autoantibody and IAA levels.
Most of the trials to prevent type 1A diabetes target individuals in the preclinical phase of the disease, marked by the presence of persistent islet autoantibodies (1). Screening for autoantibodies to insulin (IAA) (2), GAD (3), and protein tyrosine IA-2 (ICA512) (4) is the mainstay of risk prediction (5). Factors correlating and potentially predictive of age of diagnosis of children followed from birth are less well characterized.
RESEARCH DESIGN AND METHODS
Study population
The Diabetes Autoimmunity Study in the Young (DAISY) has followed two cohorts of young children at increased risk of diabetes (n = 2,542), including relatives and general population children screened for susceptibility HLA-DR/DQ genotypes. The details of screening and follow-up were previously published (6). Autoantibodies to GAD, IA-2, and insulin (IAA) were measured in all samples at 9, 15, and 24 months of age, and annually thereafter; if positive, antibodies were measured every 3–6 months. Informed consent was obtained, and the Colorado Multiple Institutional Review Board approved all study protocols.
Islet autoantibodies
Measurement of islet autoantibodies was performed in the laboratory of Dr. George Eisenbarth at the Barbara Davis Center using radioimmunoassays, as described previously (7).
Statistical analysis
Statistical analyses were performed using PRISM (GraphPad Software, Inc., La Jolla, CA) and SAS software (SAS, Inc., Cary, NC). The IAA, GAD, and IA-2 levels were log-transformed for analyses. Survival analysis was performed for progression to diabetes using the log-rank test. Follow-up time was defined as the time from initial positive autoantibody test for each subject. Multiple linear regression was used to evaluate potential predictors of age of diabetes diagnosis in subjects who had their first autoantibody measurement before 1.5 years (n = 38).
RESULTS
During a median follow-up of 7.0 years, 169 children developed persistent islet autoantibodies (one or more autoantibody on at least two consecutive visits), and 55 of those progressed to diabetes. A total of 89% of children who progressed to diabetes, so far, expressed two or more autoantibodies.
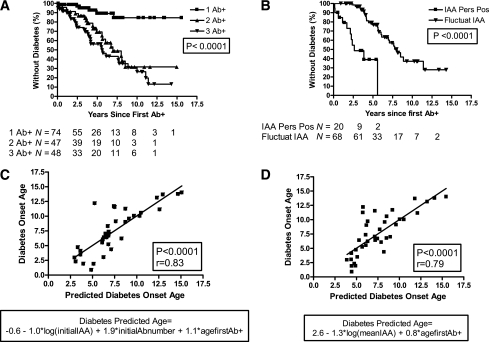
In a life-table analysis (Fig. 1A), children expressing two or more autoantibodies showed a nearly linear progression to diabetes. The cumulative incidence of diabetes by 10 years of follow-up differed significantly by the number of autoantibodies: 74, 70, and 15% in patients with three, two, and one autoantibodies, respectively (P < 0.0001). There was no significant difference in the progression to diabetes between relatives and general population subjects. The high-risk DR3/4-DQB1*0302 genotype was an additional predictor of a 10-year progression to diabetes in children expressing one autoantibody (30 vs. 13%; P = 0.035) or two autoantibodies (100 vs. 54%; P = 0.029), but not among patients expressing three autoantibodies (73.6 vs. 75.1%; P = 0.91). Children with persistently positive IAA levels had a higher progression rate to diabetes (100% by 5.6 years) than children with fluctuating IAA levels (63% by the 10-year follow-up) (P < 0.0001) (Fig. 1B).
Figure 1.
A: Progression to diabetes in children positive for anti-islet autoantibodies (n = 169). There was no significant difference in the progression rate between subjects with two or three positive antibodies. B: Progression to diabetes in children with persistently positive IAA levels and fluctuating IAA levels (n = 88). IAA Pers Pos, persistently positive IAA levels; Fluctuat IAA, fluctuating IAA levels. C: Predicted age of diagnosis of diabetes (initial IAA, GAD, and IA-2 levels) (n = 38). Analysis done in all subjects who had their first antibody measurement before 1.5 years and progressed to diabetes. D: Predicted age of diagnosis of diabetes (mean IAA, GAD, and IA-2 levels) (n = 38). Analysis was done in all subjects who had their first antibody measurement before 1.5 years and progressed to diabetes.
The age of appearance of autoantibody was a major determinant of the age at diabetes diagnosis, accounting for 47% of the variance (r = 0.69, P < 0.0001). The mean age of appearance of first autoantibody varied by group: 6.1, 5.5, and 3.8 years for one, two, or three antibodies, respectively (P = 0.0007).
We performed multiple regression analyses, including age at the first positive antibody, initial number of positive antibodies, family history, high-risk HLA-DR3/4, ethnicity, and IAA, GAD, and IA-2 levels (both initial and mean levels). In multiple regression analyses, including initial IAA, GAD, and IA-2 levels, the age at diabetes diagnosis was best predicted by initial IAA level, initial number of positive antibodies, and age at the first positive antibody (r = 0.83 and P < 0.0001) (Fig. 1C). When analyzing mean IAA, GAD, and IA-2 levels, only mean IAA levels and age of the first positive antibody were significant predictors of age of diabetes diagnosis (r = 0.79, P < 0.0001) (Fig. 1D).
CONCLUSIONS
In DAISY, 89% of children who progressed to diabetes expressed two or more autoantibodies with cumulative incidence of 74% by age 10 years for individuals expressing three autoantibodies. Risk of progression to diabetes in siblings has been shown to be influenced by age of diagnosis of proband, autoantibody number and levels, and genetic susceptibility markers (8–11). These results are consistent with studies from Finland (12,13) regarding high risk, and the most significant risk factors were young age at seroconversion, positivity for multiple autoantibodies, high autoantibody levels, and persistent positivity for IAA (11,14). In a Finnish study of siblings of diabetic children recruited at a mean age of 9.9 years, 76% of the variance in the age at diagnosis was explained by age at first sampling, initial IA-2 level, and initial number of detectable autoantibodies (11).
In this prospective study, major determinants of the age of diagnosis of diabetes were age at first positive autoantibody and IAA levels, but not GAD or IA-2 levels. It is likely that with time, additional children will progress to diabetes. However, initial IAA levels alone have limited predicted value (r = 0.36, P = 0.029), whereas mean levels over time strongly correlate with time to diabetes. The predictive value of mean levels of insulin autoantibodies, but not GAD or IA-2 levels, may relate to hypothesized unique biologic importance of insulin autoimmunity β-cell destruction (15).
The best predictive models to identify children at highest risk for diabetes depend on the genetic markers available, the age at which the screening commences, and our ability to repeat autoantibody testing over time. Potential public health applications will need to optimize combination of these factors and cost of screening.
Acknowledgments
This research was supported by the National Institutes of Health grants R37-DK32493, DK320083, DK050979, DK57516, AI050864, and N01-AI15416. A.K.S. was supported by the Juvenile Diabetes Research Foundation Early Career Patient-Oriented Research Award 11-2010-206.
No potential conflicts of interest relevant to this article were reported.
A.K.S. wrote the manuscript and contributed to discussion. K.J., K.J.B., D.M., and L.Y. researched data. J.C.H. contributed to discussion. G.S.E. and M.J.R. contributed to discussion and reviewed and edited the manuscript.
Footnotes
A.K.S. and K.J. contributed equally to this work.
References
- 1.Yu L, Cuthbertson DD, Maclaren N, et al. Expression of GAD65 and islet cell antibody (ICA512) autoantibodies among cytoplasmic ICA+ relatives is associated with eligibility for the Diabetes Prevention Trial-Type 1. Diabetes 2001;50:1735–1740 [DOI] [PubMed] [Google Scholar]
- 2.Greenbaum CJ, Palmer JP, Kuglin B, Kolb H. Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the Fourth International Workshop on the Standardization of Insulin Autoantibody Measurement. J Clin Endocrinol Metab 1992;74:1040–1044 [DOI] [PubMed] [Google Scholar]
- 3.Baekkeskov S, Aanstoot H-J, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 1990;347:151–156 [erratum in Nature 1990;347:782] [DOI] [PubMed] [Google Scholar]
- 4.Gianani R, Rabin DU, Verge CF, et al. ICA512 autoantibody radioassay. Diabetes 1995;44:1340–1344 [DOI] [PubMed] [Google Scholar]
- 5.Bingley PJ, Bonifacio E, Ziegler AG, Schatz DA, Atkinson MA, Eisenbarth GS; Immunology of Diabetes Society Proposed guidelines on screening for risk of type 1 diabetes. Diabetes Care 2001;24:398. [DOI] [PubMed] [Google Scholar]
- 6.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 1996;39:807–812 [DOI] [PubMed]
- 7.Yu L, Simone E. Modification to combined GAD65/ICA512 radioassay for general population screening. Diabetes 1996;45
- 8.Orban T, Sosenko JM, Cuthbertson D, et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter M, Albert E, Conrad M, et al. IDDM2/insulin VNTR modifies risk conferred by IDDM1/HLA for development of type 1 diabetes and associated autoimmunity. Diabetologia 2003;46:712–720 [DOI] [PubMed]
- 10.Bonifacio E, Hummel M, Walter M, Schmid S, Ziegler AG. IDDM1 and multiple family history of type 1 diabetes combine to identify neonates at high risk for type 1 diabetes. Diabetes Care 2004;27:2695–2700 [DOI] [PubMed] [Google Scholar]
- 11.Mrena S, Virtanen SM, Laippala P, et al. Models for predicting type 1 diabetes in siblings of affected children. Diabetes Care 2006;29:662–667 [DOI] [PubMed] [Google Scholar]
- 12.Siljander HT, Veijola R, Reunanen A, Virtanen SM, Akerblom HK, Knip M. Prediction of type 1 diabetes among siblings of affected children and in the general population. Diabetologia 2007;50:2272–2275 [DOI] [PubMed]
- 13.Knip M, Korhonen S, Kulmala P, et al. Prediction of type 1 diabetes in the general population. Diabetes Care 2010;33:1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siljander HT, Simell S, Hekkala A, et al. Predictive characteristics of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility in the general population. Diabetes 2009;58:2835–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenbarth GS. Banting Lecture 2009: An unfinished journey: Molecular pathogenesis to prevention of type 1A diabetes. Diabetes 2010;59:759–774 [DOI] [PMC free article] [PubMed] [Google Scholar]