Abstract
Background:
We describe an intra-aneurysmal balloon-assisted technique to limit the coil volume in a large bilobulated paraophthalmic aneurysm. Our intent was to reduce the mass effect and presenting symptoms of diabetes insipidus (DI) with hypopituitarism.
Case Description:
A 32-year-old woman presented with symptoms of DI and her work-up demonstrated hypopituitarism and partial bitemporal visual field defects. Cerebral angiography revealed a large paraophthalmic aneurysm with two distinctive lobules, projecting toward the pituitary fossa. The patient declined craniotomy but consented for endovascular treatment. The plan was to limit the embolization to the proximal lobule only. Initially, we used a dual microcatheter technique with a microcatheter in each lobule. A framing coil in the distal lobule did not prevent coil migration from the proximal lobule. Instead, we elected to use a Hyperform balloon in the distal lobule and were able to successfully coil the proximal lobule only. Her 3-year follow-up angiogram revealed a completely occluded aneurysm. The patient experienced resolution of the DI and improvement of her visual fields. However, she remained in hypopituitarism.
Conclusion:
Intra-aneurysmal balloon-assisted coiling of proximal aneurysmal lobules might be an alternative for the reduction of mass effect related to the coil mass. Careful follow-up is needed because subtotal occlusion carries a future risk of growth, recanalization and rupture. Unruptured intracranial carotid aneurysms can present with reversible DI and usually permanent pituitary disturbances.
Keywords: Aneurysm, balloon-assisted coiling, diabetes insipidus, hypopituitarism, mass effect
INTRODUCTION
Controversy exists about the optimal treatment of intracranial aneurysms presenting with symptoms of mass effect. Total and subtotal endovascular occlusion results in improvement of mass effect and neural compression.[4,6,8,12,23,25,29]
It is well known that intrasellar and suprasellar aneurysms can present with hypopituitarism,[3,7,11,17] while aneurysm rupture and surgical clipping can lead to diabetes insipidus (DI).[15,16,21] However, there are few reports of unruptured carotid aneurysms presenting with DI.[1,5,22]
We report the case of a patient with an unruptured bilobulated aneurysm of the paraophthalmic carotid artery, presenting with DI, hypopituitarism and partial visual field defects. The patient underwent balloon-assisted coiling limited to the proximal aneurysmal lobule in an attempt to reduce mass effect on the adjacent pituitary gland, hypothalamus and optic chiasm. The report includes the technical challenges encountered and a review of the literature for mass effect associated with coiling. We also discuss the hypothalamic and pituitary disturbances seen with intracranial aneurysms.
CASE REPORT
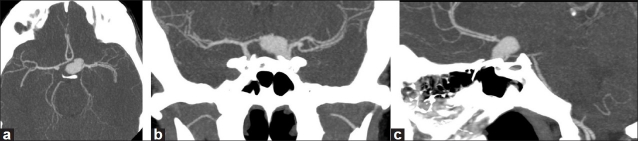
A 32-year-old woman presented with a 3-week history of persistent headaches, increased thirst, polyuria and blurred vision. Computed tomographic angiography (CTA) demonstrated a large suprasellar aneurysm [Figure 1]. Diagnostic angiography revealed a left supraclinoid segment bilobulated aneurysm projecting into the pituitary fossa. Formal visual field examination revealed partial bitemporal visual field defects. Her endocrine work-up confirmed the presence of hypopituitarism and DI. Supplementation was begun according to the endocrine service recommendations. After a careful discussion of all options, the patient declined surgical clipping/reconstruction but consented to endovascular occlusion.
Figure 1.
CT angiogram demonstrates a large suprasellar bilobulated aneurysm with a larger distal lobule and a small left middle cerebral bifurcation aneurysm (a, axial; b, coronal; c, sagittal)
Intervention
Under general anesthesia, the patient was fully heparinized and a 6-French shuttle sheath (Cook, Bloomington, IN, USA) was placed in the cervical segment of the left internal carotid artery. Initially, and in order to prevent coil migration from the proximal lobule, two Prowler Select Plus microcatheters (Cordis Endovascular, Miami Lakes, FL, USA) were navigated into the distal and proximal aneurysmal lobules, respectively. Then, an undeployed GDC (Guglielmi Detachable Coil, Boston Scientific, Natick, MA, USA) was advanced in the distal lobule with the intent of tamponading the coils deployed in the proximal lobule.[2,18,19] Multiple attempts at coiling the proximal lobule only failed, and we employed the reported approach.
A Hyperform balloon (MicroTherapeutics, Inc., Irvine, CA, USA) was positioned inside the distal lobule, and a Prowler Select Plus catheter was navigated in the proximal one. The balloon was inflated to its maximum capacity and brought to the entrance of the distal lobule. Then, a GDC coil was advanced into the proximal lobule but not deployed. A follow-up imaging run demonstrated appropriate coil positioning, sparing the distal lobule [Figure 2]. This coil was deployed and the proximal lobule was successively coiled with a combination of GDC and Hydrocoils (Micro Vention, Inc., Aliso Viejo, CA, USA). Next, the Prowler Select Plus was withdrawn and the distal lobule deflated by deflating and back bleeding the intra-aneurysmal balloon [Figure 3a].
Figure 2.
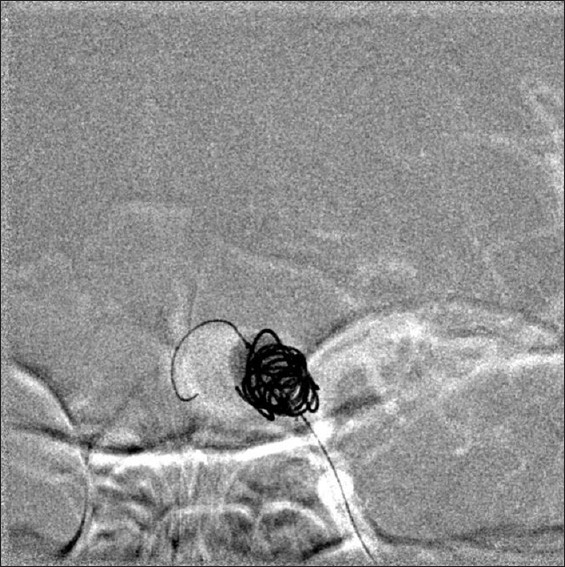
Digital subtraction mask with balloon inflated. The tail of the balloon is the wire in the larger distal lobule
Figure 3.
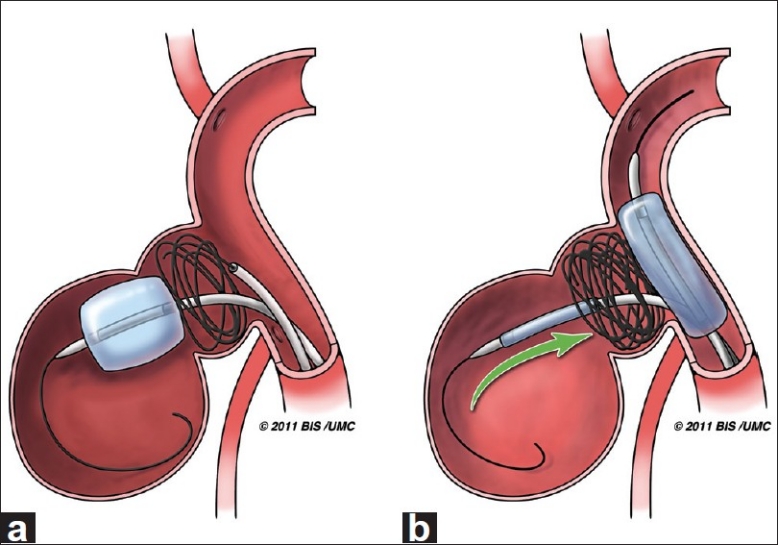
Artist illustration of the intra-aneurysmal balloon-assisted coiling technique. (a) Inflated Hyperform balloon at the entrance of the larger distal aneurysmal lobule, limiting coiling to the proximal lobule. (b) A Hyperglide balloon is inflated across the aneurysm neck as the deflated intra-aneurysmal balloon is slowly removed to prevent coil mass disturbance (Artist: W. Kyle Cunningham, Medical Illustrator at the University of Mississippi Medical Center)
Finally, in order to withdraw the intra-aneurysmal balloon without disturbing the coil mass, a second balloon (Hyperglide, MicroTherapeutics, Inc., Irvine, CA, USA) was advanced and inflated across the aneurysm neck and the intra-aneurysmal balloon was slowly removed [Figure 3b].
Post-procedural angiography demonstrated a stable coil mass and complete occlusion of the aneurysm.
Postoperative course
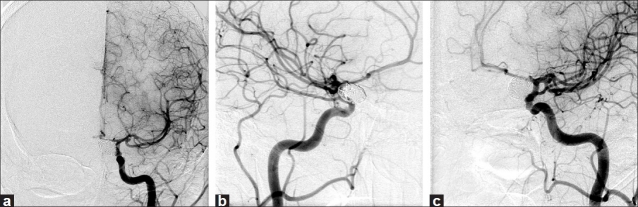
The patient was discharged 5 days later. Over the following weeks, she had progressive resolution of polyuric episodes. Three years after treatment, her angiogram revealed a stable coil mass and persistent aneurysmal obliteration [Figure 4]. The patient was no longer in DI, but remained in hypopituitarism. Her blurred vision has resolved, and the visual field deficits have improved.
Figure 4.
Digital subtraction angiogram 3 years after embolization demonstrates complete occlusion of the proximal lobule and a stable small left middle cerebral bifurcation aneurysm (a, anteroposterior; b, lateral; c, oblique)
DISCUSSION
Panhypopituitarism and intracranial aneurysms
Intrasellar and suprasellar aneurysms represent an uncommon cause of hypopituitarism, accounting for only 0.17% of the cases.[3,7,11,17] Pituitary deficiency may result from compression of the hypothalamus or the pituitary stalk.[7,28] Adrenal, thyroid and gonadal deficiencies along with hyperprolactinemia are the more prevalent abnormalities. DI is very uncommon. Regardless of the therapeutic approach, hypopituitarism is usually permanent, with only a few cases of pituitary function recovery after surgical decompression.[7,10,28]
When DI results from clipping ruptured anterior cerebral or anterior communicating artery aneurysms, it is caused by vasospasm-related ischemia of the anterior portions of the hypothalamus.[15,16,21] We found three cases reported in the literature of unruptured intrasellar and suprasellar aneurysms presenting with DI. In these cases, the proposed mechanism for DI has been direct compression of the hypothalamus, pituitary stalk or posterior pituitary gland.[1,5,22]
In a majority of cases, the DI usually resolves or improves within 3 weeks. DI can be seen in three different patterns: transient with normalization 12–36 hours after onset, prolonged with most returning to normal or near normal at 1 year, and the least frequent triphasic response.[1,5,15,16,21,22] In the case presented, we are unable to conclude whether the resolution of DI was the result of decompression and reduced pulsatility after limited coiling, or merely a reflection of this natural progression.
Technical aspects and mass effect considerations
In a recent report, Fiorella et al. described a double balloon technique to coil a large superior cerebellar artery (SCA) aneurysm. They used an intra-aneurysmal Hyperform balloon to preserve the origin of the SCA at the aneurysm neck. A second balloon (Hyperglide) was used to protect the parent basilar artery and trap the smaller intra-aneurysmal balloon during coiling.[9]
Our patient's bilobulated aneurysm had a smaller proximal lobule. It was reasonable to obliterate the aneurysm and at the same time decrease its mass effect/coil volume by limiting coiling to the proximal lobule. The previously described double catheter technique[2,18,19] with a larger undeployed coil in the distal lobule was employed first and it failed to prevent coil migration into the distal lobule. This led us to use the intra-aneurysmal balloon technique. We do not advocate the use of intra-aneurysmal balloons in ruptured aneurysms and would consider their use only in cases where the distal lobule is larger. In this setting, the proximal lobule can be considered as a “stand alone” aneurysm. In aneurysms with smaller distal lobules, proper occlusion of the proximal lobule only can be done by adequate selection of coils, proper positioning of the microcatheter and manipulation of the catheter-coil system.
We do not recommend having a second deflated balloon within the parent vessel during coil embolization, as it would be a third instrument raising the possibility of thromboembolic events or vascular damage. Nonetheless, it is advised to have a second balloon ready to deploy on the instrument table, in the event of an intraoperative rupture.
Currently available is the Ascent balloon catheter (Micrus endovascular, San Jose, CA, USA), which can also function as a microcatheter/delivery system. If this catheter were available at the time of our reported technique, it would have functioned as the proximal lobule microcatheter and parent vessel balloon. This would have simplified our method to two micro-instruments instead of three, with the added benefit of having a balloon to secure the aneurysmal neck if needed.
Cranial nerve dysfunction, obstructive hydrocephalus, brainstem, and visual pathway compression constitute the more prevalent symptoms of mass effect related to intracranial aneurysms.[8,12,13,20,24,25] Contributing factors for this mass effect include aneurysmal volume, its pulsatile blood flow, and the presence of perianeurysmal edema. It is known that mass effect related to small and large aneurysms improves following total and subtotal coil embolization. Aneurysm size and preoperative duration of mass effect may affect the likelihood of symptoms improving after coil embolization.[4,8,12,13,20,23,24,29]
Having said all this, there seems to be a role for surgical decompression of the coil mass when the mass effect symptoms persist after endovascular coiling.[12,20,24,26,27]
CONCLUSIONS
Balloon-assisted remodeling of the coil mass is a technique available in the treatment of geometrically complex aneurysms. The primary goal of any method of treating intracranial aneurysms is to prevent aneurysm rupture. Considerations of mass effect response are secondary to considerations of the safety, efficacy, and durability of aneurysm obliteration. Whether there is greater reduction of mass effect with a lesser coil mass and distal dome deflation requires further investigation. Careful follow-up is needed because subtotal occlusion carries a future risk of growth, recanalization and rupture.[6,8,12,14,25,27,30]
Acknowledgments
We would like to thank Mrs. Renea Hays, Department of Radiology, for her help in the preparation of the images.
Contributor Information
Ludwig D. Orozco, Email: lorozco-castillo@umc.edu.
Razvan F. Buciuc, Email: buciuc@umc.edu.
REFERENCES
- 1.Abe K. Mental symptoms and diabetes insipidus in a case of intrasellar saccular aneurysm. Folia Psychiatr Neurol Jpn. 1961;15:72–6. doi: 10.1111/j.1440-1819.1961.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 2.Baxter BW, Rosso D, Lownie SP. Double microcatheter technique for detachable coil treatment of large, wide-necked intracranial aneurysms. Neuroradiology. 1998;19:1176–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Cartlidge NE, Shaw DA. Intrasellar aneurysm with subarachnoid hemorrhage and hypopituitarism. Case report. J Neurosurg. 1972;36:640–3. doi: 10.3171/jns.1972.36.5.0640. [DOI] [PubMed] [Google Scholar]
- 4.Chen PR, Amin-Hanjani S, Albuquerque FC, McDougall C, Zabramski JM, Spetzler RF. Outcome of oculomotor nerve palsy from posterior communicating artery aneurysms: Comparison of clipping and coiling. Neurosurgery. 2006;58:1040–6. doi: 10.1227/01.NEU.0000215853.95187.5E. [DOI] [PubMed] [Google Scholar]
- 5.Fujii M, Tone O, Tomita H, Tamaki M, Akimoto H, Shigeta K, et al. Endosaccular embolization of an intrasellar aneurysm with hypopituitarism: Case report. No Shinkei Geka. 2008;36:329–37. [PubMed] [Google Scholar]
- 6.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: A 7-year, single-center experience. Neurosurgery. 1999;45:793–803. doi: 10.1097/00006123-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Heshmati HM, Fatourechi V, Dagam SA, Piepgras DG. Hypopituitarism caused by intrasellar aneurysms. Mayo Clin Proc. 2001;76:789–93. doi: 10.1016/S0025-6196(11)63222-9. [DOI] [PubMed] [Google Scholar]
- 8.Kazekawa K, Tsutsumi M, Aikawa H, Iko M, Kodama T, Go Y, et al. Internal carotid aneurysms presenting with mass effect symptoms of cranial nerve dysfunction: Efficacy and imitations of endosaccular embolization with GDC. Radiat Med. 2003;21:80–5. [PubMed] [Google Scholar]
- 9.Kelly ME, Gonugunta V, Woo HH, Turner R, 4th, Fiorella D. Double-balloon trapping technique for embolization of a large wide-necked superior cerebellar artery aneurysm: Case report. Neurosurgery. 2008;63:291–2. doi: 10.1227/01.NEU.0000316432.05038.F1. [DOI] [PubMed] [Google Scholar]
- 10.Kita Y, Kawato M, Nakabayashi H, Takeda R, Usukura N, Hasatani K. A case of reversible hypopituitarism with hyperprolactinemia caused by a large suprasellar aneurysm. Nippon Naika Gakkai Zasshi. 1986;75:1756–63. doi: 10.2169/naika.75.1756. [DOI] [PubMed] [Google Scholar]
- 11.Klose S, Kopf D, Lehnert H. Giant intrasellar carotid aneurysm - An unusual cause of panhypopituitarism. Exp Clin Endocrinol Diabetes. 2005;113:551–3. doi: 10.1055/s-2005-865808. [DOI] [PubMed] [Google Scholar]
- 12.Malisch TW, Guglielmi G, Viñuela F, Duckwiler G, Gobin YP, Martin NA, et al. Unruptured aneurysms presenting with mass effect symptoms: Response to endosaccular treatment with Guglielmi detachable coils. Part I Symptoms of cranial nerve dysfunction. J Neurosurg. 1998;89:956–61. doi: 10.3171/jns.1998.89.6.0956. [DOI] [PubMed] [Google Scholar]
- 13.Mansour N, Kamel MH, Kelleher M, Aquilina K, Thornton J, Brennan P, et al. Resolution of cranial nerve paresis after endovascular management of cerebral aneurysms. Surg Neurol. 2007;68:500–4. doi: 10.1016/j.surneu.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 14.Mericle RA, Wakhloo AK, Lopes DK, Lanzino G, Guterman LR, Hopkins LN. Delayed aneurysm regrowth and recanalization after Guglielmi detachable coil treatment. Case report. J Neurosurg. 1998;89:142–5. doi: 10.3171/jns.1998.89.1.0142. [DOI] [PubMed] [Google Scholar]
- 15.McMahon AJ. Diabetes insipidus developing after subarachnoid haemorrhage from an anterior communicating artery aneurysm. Scott Med J. 1988;33:208–9. doi: 10.1177/003693308803300107. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen BN, Yablon SA, Chen CY. Hypodipsic hypernatremia and diabetes insipidus following anterior communicating artery aneurysm clipping: Diagnostic and therapeutic challenges in the amnestic rehabilitation patient. Brain Inj. 2001;15:975–80. doi: 10.1080/02699050110063459. [DOI] [PubMed] [Google Scholar]
- 17.Ooi TC, Russell NA. Hypopituitarism resulting from an intrasellar carotid aneurysm. Can J Neurol Sci. 1986;13:70–1. doi: 10.1017/s0317167100035836. [DOI] [PubMed] [Google Scholar]
- 18.Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: Initial experience. Radiology. 2001;221:318–26. doi: 10.1148/radiol.2212010474. [DOI] [PubMed] [Google Scholar]
- 19.Raymond J, Salazkin I, Georganos S, Guilbert F, Desfaits AC, Gevry G, et al. Endovascular treatment of experimental wide neck aneurysms: Comparison of results using coils or cyanoacrylate with the assistance of an aneurysm neck bridge device. Am J Neuroradiol. 2002;23:1710–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Russell SM, Nelson PK, Jafar JJ. Neurological deterioration after coil embolization of a giant basilar apex aneurysm with resolution following parent artery clip ligation.Case report and review of the literature. J Neurosurg. 2002;97:705–8. doi: 10.3171/jns.2002.97.3.0705. [DOI] [PubMed] [Google Scholar]
- 21.Savin IA, Popugaev KA, Oshorov AV, Goriachev AS, Moldotasheva AK, Kurdiumova NV, et al. Diabetes insipidus in acute subarachnoidal hemorrhage after clipping of aneurysm of the anterior cerebral artery and the anterior communicating artery. Anesteziol Reanimatol. 2007;2:56–9. [PubMed] [Google Scholar]
- 22.Shucart WA, Wolpert SA. An aneurysm in infancy presenting with diabetes insipidus.Case report. J Neurosurg. 1978;37:368–70. doi: 10.3171/jns.1972.37.3.0368. [DOI] [PubMed] [Google Scholar]
- 23.Stiebel-Kalish H, Maimon S, Amsalem J, Erlich R, Kalish Y, Rappaport HZ. Evolution of oculomotor nerve paresis after endovascular coiling of posterior communicating artery aneurysms: A neuro-ophthalmological perspective. Neurosurgery. 2003;53:1268–74. doi: 10.1227/01.neu.0000093495.70639.ae. [DOI] [PubMed] [Google Scholar]
- 24.Tawk RG, Villalobos HJ, Levy EI, Hopkins LN. Surgical decompression and coil removal for the recovery of vision after coiling and proximal occlusion of a clinoidal segment aneurysm: Technical case report. Neurosurgery. 2006;58:1217. doi: 10.1227/01.NEU.0000215995.09860.0A. [DOI] [PubMed] [Google Scholar]
- 25.Thornton J, Aletich VA, Debrun GM, Alazzaz A, Misra M, Charbel F, et al. Endovascular treatment of paraclinoid aneurysms. Surg Neurol. 2000;54:288–99. doi: 10.1016/s0090-3019(00)00313-x. [DOI] [PubMed] [Google Scholar]
- 26.Thornton J, Dovey Z, Alazzaz A, Misra M, Aletich VA, Debrun GM, et al. Surgery following endovascular coiling of intracranial aneurysms. Surg Neurol. 2000;54:352–60. doi: 10.1016/s0090-3019(00)00337-2. [DOI] [PubMed] [Google Scholar]
- 27.Tirakotai W, Sure U, Yin Y, Benes L, Schulte DM, Bien S, et al. Surgery of intracranial aneurysms previously treated endovascularly. Clin Neurol Neurosurg. 2007;109:744–52. doi: 10.1016/j.clineuro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Verbalis JG, Nelson PB, Robinson AG. Reversible panhypopituitarism caused by a suprasellar aneurysm: The contribution of mass effect to pituitary dysfunction. Neurosurgery. 1982;10:604–11. doi: 10.1227/00006123-198205000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Vargas ME, Kupersmith MJ, Setton A, Nelson K, Berenstein A. Endovascular treatment of giant aneurysms which cause visual loss. Ophthalmology. 1994;101:1091–8. doi: 10.1016/s0161-6420(94)31213-9. [DOI] [PubMed] [Google Scholar]
- 30.Yasui T, Komiyama M, Iwai Y, Yamanaka K, Matsusaka Y, Morikawa T, et al. Regrowth and fatal rerupture despite proximal occlusion after coil embolization of a ruptured large basilar bifurcation aneurysm-case report. Neurol Med Chir (Tokyo) 2004;44:587–90. doi: 10.2176/nmc.44.587. [DOI] [PubMed] [Google Scholar]