Abstract
OBJECTIVE
Metreleptin has been efficacious in improving metabolic control in patients with lipodystrophy, but its efficacy has not been tested in obese patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We studied the role of leptin in regulating the endocrine adaptation to long-term caloric deprivation and weight loss in obese diabetic subjects over 16 weeks in the context of a double-blinded, placebo–controlled, randomized trial. We then performed detailed interventional and mechanistic signaling studies in humans in vivo, ex vivo, and in vitro.
RESULTS
In obese patients with diabetes, metreleptin administration for 16 weeks did not alter body weight or circulating inflammatory markers but reduced HbA1c marginally (8.01 ± 0.93–7.96 ± 1.12, P = 0.03). Total leptin, leptin-binding protein, and antileptin antibody levels increased, limiting free leptin availability and resulting in circulating free leptin levels of ∼50 ng/mL. Consistent with clinical observations, all metreleptin signaling pathways studied in human adipose tissue and peripheral blood mononuclear cells were saturable at ∼50 ng/mL, with no major differences in timing or magnitude of leptin-activated STAT3 phosphorylation in tissues from male versus female or obese versus lean humans in vivo, ex vivo, or in vitro. We also observed for the first time that endoplasmic reticulum (ER) stress in human primary adipocytes inhibits leptin signaling.
CONCLUSIONS
In obese patients with diabetes, metreleptin administration did not alter body weight or circulating inflammatory markers but reduced HbA1c marginally. ER stress and the saturable nature of leptin signaling pathways play a key role in the development of leptin tolerance in obese patients with diabetes.
Metreleptin has consistently been shown to dramatically improve insulin resistance and HbA1c in several clinical trials involving hypoleptinemic subjects with lipodystrophy, hypoleptinemia, insulin resistance, and the metabolic syndrome (1). No prior study has evaluated in detail the effect of metreleptin in obese subjects, with garden variety diabetes, obesity, and high circulating leptin levels, who are presumably resistant or tolerant to the effects of leptin (2). Furthermore, no prior study has evaluated mechanisms underlying such leptin tolerance.
In the context of a large, randomized, placebo–controlled trial, we examined for the first time the efficacy of metreleptin in regulating body weight, glycemic control, and immune function in hyperleptinemic obese subjects with type 2 diabetes. We subsequently examined whether the observed suboptimal efficacy of circulating leptin in regulating adiposity and immune function in obese diabetic individuals is attributable to specific, identifiable mechanisms at the cellular and molecular level. In this respect, we methodically explored mechanisms previously shown to underlie other hormone resistance syndromes, e.g., insulin resistance or underlying immunogenicity seen with use of other biologics. To further elucidate the role of leptin in regulating human adiposity and immune function and to study potential mechanisms underlying the development of leptin resistance or tolerance, we then performed detailed interventional and mechanistic signaling studies in humans in vivo, ex vivo, and in vitro.
More specifically, we first discovered that levels of leptin-binding protein (LBP) and antibodies against metreleptin increased in response to metreleptin treatment, limiting circulating free leptin to ∼50 ng/mL despite total leptin levels of ∼982.7 ng/mL in obese diabetic subjects. We then proceeded to study whether mechanisms that have been described to affect leptin signaling and thus leptin resistance in mice, i.e., endoplasmic reticulum (ER) stress (3–6), are also operative in humans. Subsequently, we investigated intracellular leptin signaling in vivo in response to metreleptin administration in lean and obese subjects by comparatively studying metreleptin signaling in human adipose tissue (hAT) and human peripheral blood mononuclear cells (hPBMCs) from both lean and obese humans in vivo. Finally, we extended these observations by studying leptin signaling in vitro and ex vivo in hAT and hPBMCs from lean and obese subjects to determine whether neuroendocrine changes induced by metreleptin in vivo or paracrine mechanisms ex vivo may differentially affect leptin signaling in humans in vivo versus ex vivo or in vitro.
RESEARCH DESIGN AND METHODS
Clinical study I: Body weight, metabolic, and immune responses to metreleptin versus placebo in obese hyperleptinemic subjects with diabetes.
We studied 71 obese subjects (41 male and 30 female; age, 53.3 ± 11.4 years; BMI, 33.2 ± 3.8 kg/m2) with diet-controlled type 2 diabetes who gave written informed consent to participate in the study. Inclusion criteria for participation in the study included HbA1c between 7 and 11%, BMI between 27 and 40 kg/m2, and adherence to a stable weight–maintaining diet for at least 4 weeks before the screening evaluation. Subjects could not have taken oral hypoglycemic agents or insulin in the 12 weeks preceding the screening evaluation. Subjects were randomized in a 2:1 ratio to receive metreleptin or placebo, respectively, at a dose of 10 mg twice daily (morning and evening) by subcutaneous injection for 4 months (16 weeks), resulting in a total daily dose of 20 mg metreleptin. Blood samples were obtained at baseline (before metreleptin or placebo treatment) and after 4 and 16 weeks of treatment (with the exception of nine subjects who received metreleptin and six subjects who received placebo because of insufficient serum). Samples were stored at −70°C until assayed for the measurement of leptin, LBP, free leptin, antibody titer, inflammatory marker, and HgbA1c.
Clinical study II: In vivo metreleptin signaling in hAT and hPBMCs from lean and obese subjects.
Normal volunteers were recruited from the community and screened at the Clinical Research Center at Beth Israel Deaconess Medical Center (BIDMC). Subjects were excluded if they had a history of any illness, other than obesity, that may affect insulin sensitivity, use of medications that are known to influence glucose metabolism, history of anaphylaxis or anaphylactoid-like reactions, or a known hypersensitivity to Escherichia coli derived proteins or anesthetic agents, such as lidocaine or procaine hydrochloride (Novocaine; Hospira, Inc., Lake Forest, IL). Subjects were provided with take-home meals and consumed an isocaloric diet, specifically designed for each subject, for 48 h before their main study visit to ensure stable dietary intake. On the morning of the main study visit, subjects attended the Clinical Research Center after a 12-h fast. An intravenous cannula was placed in each antecubital fossa, and samples were drawn for baseline laboratory tests. The skin of the lower abdomen was anesthetized using lidocaine, and by using an aseptic technique, a core of adipose tissue was obtained using a core biopsy instrument. The tissue sample was immediately placed in a cryotube and frozen in liquid nitrogen at the bedside. After baseline laboratory tests and baseline hAT biopsy, an intravenous bolus of metreleptin (dose 0.01 mg/kg body weight) or placebo (10 mL normal saline) was given by slow intravenous injection >1 min. The subject rested in supine position. Thirty minutes later, the adipose tissue biopsy was repeated for metreleptin signaling experiments in hAT, and a blood sample was taken for metreleptin signaling experiments in hPBMCs. Serum and hAT samples were stored in liquid nitrogen in batches until later analysis. For the in vivo metreleptin signaling study, we performed experiments with hAT and hPBMCs before and after a bolus of metreleptin was administered to six lean (BMI 23.7 + 0.66 kg/m2) and six obese (BMI 35.42 + 5.7 kg/m2) men. There was no difference in LBP (lean 33.2 + 6.38, obese 31.48 + 6.1, control 25.85 + 3.98 μg/mL, P = 0.07) or age (lean 41 + 13.3, obese 43.3 + 10.78, control 33.17 + 14.12 years, P = 0.37) between the groups. Subjects in the lean group had lower waist circumference (87 + 5.68 vs. 111.8 + 12 cm, P = 0.004) and leptin levels (2.94 + 3.16 vs. 11.3 + 9.28 ng/mL, P = 0.04) compared with the obese group at baseline. All subjects provided written informed consent to participate, and the study was approved by the institutional review board at BIDMC.
Laboratory studies
Ex vivo metreleptin signaling study
hAT
For the ex vivo hAT metreleptin signaling study, we used discarded hAT from subjects undergoing laparoscopic adjustable gastric band, liposuction, or abdominoplasty at the BIDMC. The age and BMI of the subjects, but no other identifiable information, were recorded. Explants were solubilized for analysis of phosphorylation of signaling proteins as described previously (7).
hPBMCs
The hPBMCs were isolated as described previously (8).
In vitro metreleptin signaling study
Human primary adipocyte culture.
Subcutaneous and omental hAT samples were obtained from lean (aged 35–41 years, BMI 22–25 kg/m2) and obese (aged 34–48 years, BMI 39–50 kg/m2) men and women, respectively. The human primary adipocyte (hPA) culture was performed as described previously (9).
Protein extraction and Western blotting.
Protein extraction and Western blotting were performed as described previously (10).
Gene Expression Omnibus datasets analysis of published data.
To compare expression of inhibitors of leptin signaling in hAT from lean and obese subjects, including SOCS3 and PTP1B, we performed a search of publicly available data on the Gene Expression Omnibus datasets website (11) using the search terms “adipose tissue” and “adipocyte.” We excluded nonhuman datasets. We searched the results for datasets comparing gene expression profiles in lean and obese humans. We found one dataset with readily available data that matched our search criteria. We extracted gene expression data for SOCS3 and PTP1B from this and compared expression levels in lean and obese subjects.
Detection of antileptin antibodies and their functional/biochemical activity
Levels of serum antileptin antibodies.
Levels of serum antileptin antibodies were determined with an in-house developed colorimetric sandwich enzyme–linked immunosorbent assay (ELISA). In brief, 50 µL metreleptin at a final concentration of 10 µg/mL in PBS, pH 7.4, was plate-bound to a 96-well ELISA plate (PBI International SpA, Milano, Italy). After 16 h at 4°C, the plates were extensively washed with PBS-0.05% Tween 20, blocked with 200 µL PBS/10% FCS for 2 h, and repeatedly washed. Diluted sera in PBS-0.05% Tween 20/10% FCS (from 1/10 to 1/1,000) were added at 100 µL/well and incubated for 4 h at room temperature. After five washes, goat anti-human polyvalent immunoglobulins, such as alkaline phosphatase–conjugated antibodies (Sigma-Aldrich, St. Louis, MO), were added 100 μL/well for 1 h. The reaction was developed with Sigma-Fast p-nitrophenyl phosphate and alkaline phosphatase substrate (PNPP) (Sigma-Aldrich) and read after 30 min at 405 nm in an ELISA plate-reader (Bio-Rad Laboratories, Hercules, CA). Standard curves of antileptin antibodies were developed in each assay using an antileptin monoclonal antibody (mAb) generated in our laboratory (mAb 971212). Quantification of optical density values was performed after extrapolation from standard curves of known concentration of antileptin antibodies.
Functional activity of antileptin antibodies.
The functional activity of antileptin antibodies was assessed with the human leptin-receptor (hLepR)-transfected BAF3 cell line, provided by Dr. Arieh Gertler (The Hebrew University, Rehovot, Israel). In brief, because hLepR+BAF3 cell proliferation is leptin-dependent, hLepR+BAF3 cells were cultured in flat-bottom 96-well microtiter plates (Becton-Dickinson Falcon, Franklin Lakes, NJ) at a density of 5 × 103 cells/well in a total volume of 100 µL RPMI-1640 medium supplemented with 2% FCS (Hyclone-Pierce; Thermo Fisher Scientific, Inc., Rockford, IL), 2 mmol/L l-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Inc., Carlsbad, CA). Cells were cultured at 37°C in 100% humidity and 5% CO2 in the presence of increasing doses of metreleptin ranging from 0.01 to 10 ng/mL. Purified IgGs from sera of metreleptin-treated patients, placebo–treated patients, and healthy controls were added to cells in all the different conditions at a final concentration of 50 μg/mL. After 48 h, [3H]thymidine (0.5 μCi/well) (Perkin-Elmer, Milano, Italy) was added to the cultures, and cells were harvested after 12 h. Radioactivity was measured with a β-cell plate scintillation counter (Wallac, Gaithersburg, MD). As standard of leptin neutralization, anti-human leptin mAb 971212 was used at increasing concentrations usually from 0.1 to 25 μg/mL.
Capacity of antileptin IgGs at the biochemical level.
We also assessed at the biochemical level the capacity of antileptin IgGs, isolated from leptin or placebo-treated subjects and healthy controls, to affect LepR signaling in BAF3 cells. In brief, hLepR+BAF3 cells were incubated for 1 h at 37°C with recombinant leptin at 2 ng/mL in the presence or absence of IgGs (50 µg/mL) purified from leptin- or placebo-treated subjects and healthy controls. After a 1-h incubation, cell lysates were generated to perform Western blotting analyses for STAT3 phosphorylation as a readout of LepR signaling.
Immunocytochemistry.
Immunodetection was performed as described previously (12).
Blood sample measurements.
Leptin and LBP were analyzed by ELISA as described previously (13). Free leptin was measured using radioimmunoassay for study (Linco Research, Inc., St. Charles, MO; sensitivity, 0.5 ng/mL; coefficient of variation [CV], 6–7%) and by immunoradiometric assay for study (Diagnostics Systems Laboratory [DSL], Webster, TX; sensitivity, 0.1 ng/mL; CV, 3.7–6.6%). First, 100 µL of unknown serum samples were incubated at 37°C in a water bath for 2 h; 100 µL polyethelene glycol (Immucor, Inc. Norcross, GA) was then added to standards, subjects, and unknowns, vortexed for 1 min, and allowed to sit at room temperature for 10 min. Standards, samples, and subjects then were centrifuged for 15 min at 1,600 rpm; 50 μL supernatant was used from standards, subjects, and samples in duplicate to measure free leptin by radioimmunoassay. All samples were run in one assay to eliminate interassay variability.
Cytokines and inflammatory markers were measured in serum or plasma using commercially available ELISAs: tumor necrosis factor-α (Quantikine HS; R&D Systems, Minneapolis, MN; sensitivity, 0.06 pg/mL; CV, 5.3–8.8%), soluble tumor necrosis factor receptor (sTNFR)-I (Quantikine; R&D Systems; sensitivity, 3 pg/mL; CV, 2.7–6.9%), sTNFR-II (Quantikine; R&D Systems; sensitivity, 1 pg/mL; CV, 1.6–2.5%), interleukin (IL)-10 (Amersham Biosciences, Buckinghamshire, UK; high sensitivity, 0.1 pg/mL; CV, 10%), soluble intracellular adhesive molecule-1 (Quantikine; R&D Systems; sensitivity, 0.35 ng/mL; CV, 4.8–10.1%), C-reactive protein (CRP) (DSL; sensitivity, 1.6 ng/mL; CV, 1.7–3.9%), and IL-6 (Quantikine; R&D Systems; sensitivity, 0.04 pg/mL; CV, 6.9–7.8%).
Statistical analysis.
For clinical study I, between-group comparison of placebo- and metreleptin-treated subjects on change in study variable measures over time were determined using repeated-measures analysis, allowing for use of all available data. P values for difference between groups at baseline and each follow-up were determined by one-way ANOVA. P values for difference within group at baseline and each follow-up were determined by one-way ANOVA followed by a least significant difference test. Pearson correlation coefficients were calculated between changes of study variables. All data were presented as means ± SE. P < 0.05 was considered statistically significant. Intention-to-treat analysis revealed similar results (data not shown). For the in vivo signaling study, baseline comparisons among the three groups were made using a nonparametric Kruskal-Wallis test. The ratios of phosphorylated protein to total protein were compared before and 30 min after metreleptin bolus, using a nonparametric, paired Wilcoxon signed rank test. To compare pSTAT3 change between lean and obese subjects, the fold change was compared between the groups using a Wilcoxon rank sum test. For the ex vivo and the in vitro signaling study, data were analyzed with one-way ANOVA followed by post hoc test for multiple comparisons. Analyses were carried out using SPSS (version 11.5, SPSS, Inc., Chicago, IL) and SAS (version 9; SAS Institute, Inc., Cary, NC).
Results
Clinical study I: Body weight, metabolic, and immune responses to metreleptin versus placebo treatment in obese hyperleptinemic subjects with diabetes.
Circulating leptin levels increased significantly in men and women treated with metreleptin over the 4-month study period (Table 1, Supplementary Appendix 1). Non-neutralizing antileptin antibodies developed with increasing titers, and LBP levels decreased less in metreleptin-treated subjects compared with placebo-treated subjects. BMI remained unchanged throughout the study in both treatment groups. Free leptin levels were similar in both treatment groups at baseline but increased significantly in subjects treated with metreleptin despite the presence of antibodies against metreleptin. Although the development of antileptin antibodies was significantly and positively correlated with the increase in total circulating leptin levels (r = 0.99, P < 0.0001), circulating free leptin levels (r = 0.44, P = 0.012, SI 2) were also increased. This indicates that increasing doses of leptin can break through the resistance caused by increasing binding of leptin by leptin antibodies. The increase in free leptin levels to ∼48.4 ng/mL was not associated with any significant differential weight loss or changes in inflammatory markers in metreleptin-treated patients and resulted in only a small but differential improvement in glycemic control (HbA1c from 8.01 ± 0.93 to 7.96 ± 1.12, P = 0.03). Changes of leptin and free leptin levels were not correlated with changes of body weight or inflammatory markers studied (Table 2). Baseline TNFR-II and monocyte chemoattractant protein-1 levels were relatively similar at baseline in both treatment groups but increased in men and women treated with metreleptin. By contrast, IL-6, IL-10, CRP, sTNFR-I, and soluble intracellular adhesive molecule-I did not change significantly in any group (Table 1).
TABLE 1.
Clinical study I: Study variables for obese, diabetic subjects taking placebo (n = 21) or metreleptin (n = 50) at baseline and 4 and 16 weeks of follow-up and % change in study variables from baseline to 16 weeks
| Variable | Placebo-treateda |
Leptin-treated |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | % | n | Mean ± SE | % | P valueb | P valuec | |
| Female | 42.9 | 42.0 | ||||||
| BMI | 0.80 | |||||||
| Baseline | 21 | 32.8 ± 0.7 | 50 | 32.7 ± 0.5 | 0.84 | |||
| Week 4 | 21 | 32.6 ± 0.7 | 47 | 32.5 ± 0.5 | 0.85 | |||
| Week 16 | 19 | 32.8 ± 0.7 | 45 | 31.9 ± 0.5 | 0.36 | |||
| Change after 16 wk | 19 | −0.5 ± 0.2 | −1.6 | 45 | −0.7 ± 0.1 | −2.1 | 0.43 | |
| Leptin (ng/mL) | <0.0001 | |||||||
| Baseline | 18 | 38.0 ± 6.4 | 48 | 35.2 ± 3.5 | 0.27 | |||
| Week 4 | 18 | 35.5 ± 6.8 | 49 | 430.6 ± 47.4 | <0.0001 | |||
| Week 16 | 20 | 36.8 ± 6.5 | 48 | 987.1 ± 50.1 | <0.0001 | |||
| Change after 16 wk | 18 | −3.1 ± 3.2 | −8.2 | 47 | 957.2 ± 50.4 | 2,721 | <0.0001 | |
| LBP (ng/mL) | 0.04 | |||||||
| Baseline | 20 | 25.3 ± 1.7 | 49 | 25.0 ± 1.2 | 0.88 | |||
| Week 4 | 19 | 23.5 ± 1.4 | 49 | 24.4 ± 1.2 | 0.77 | |||
| Week 16 | 21 | 22.7 ± 1.4 | 49 | 24.4 ± 1.1 | 0.35 | |||
| Change after 16 wk | 20 | −2.2 ± 1.1 | −8.8 | 48 | −0.4 ± 0.5 | −1.5 | 0.07 | |
| Free leptin (ng/mL) | 0.002 | |||||||
| Baseline | 15 | 15.8 ± 3.3 | 32 | 22.6 ± 4.7 | 0.37 | |||
| Week 4 | 7 | 13.9 ± 4.2 | 26 | 30.0 ± 6.0 | 0.16 | |||
| Week 16 | 16 | 14.5 ± 3.9 | 33 | 61.6 ± 7.8 | 0.0002 | |||
| Change after 16 wk | 11 | −1.3 ± 1.6 | −8.1 | 21 | 53.2 ± 11.5 | 236.0 | 0.002 | |
| Antibody titer (µg/mL) | 0.0009 | |||||||
| Baseline | 7 | 0.0 ± 0.0 | 16 | 5.3 ± 4.0 | 0.41 | |||
| Week 4 | 1 | 0.0 ± 0.0 | 8 | 33.5 ± 11.4 | 0.56 | |||
| Week 16 | 6 | 0.0 ± 0.0 | 14 | 52.3 ± 2.7 | <0.0001 | |||
| Change after 16 wk | 4 | 0.0 ± 0.0 | 0 | 5 | 52.7 ± 3.9 | 987.4 | 0.0001 | |
| IL-6 (pg/mL) | 0.62 | |||||||
| Baseline | 20 | 3.2 ± 0.2 | 49 | 3.7 ± 0.4 | 0.25 | |||
| Week 4 | 19 | 3.7 ± 0.2 | 49 | 3.6 ± 0.5 | 0.78 | |||
| Week 16 | 21 | 3.5 ± 0.3 | 49 | 3.6 ± 0.5 | 0.78 | |||
| Change after 16 wk | 20 | −0.1 ± 0.6 | −3.2 | 48 | 0.2 ± 0.3 | 6.4 | 0.59 | |
| CRP (µg/L) | 0.93 | |||||||
| Baseline | 20 | 9,356 ± 2,059 | 48 | 9,138 ± 1,172 | 0.95 | |||
| Week 4 | 19 | 9,200 ± 2,026 | 49 | 9,702 ± 1,215 | 0.87 | |||
| Week 16 | 21 | 8,609 ± 2,013 | 49 | 9,512 ± 1,158 | 0.62 | |||
| Change after 16 wk | 20 | −555 ± 1,013 | −5.9 | 47 | 448 ± 883 | 4.9 | 0.52 | |
| sTNFR-I (pg/mL) | 0.31 | |||||||
| Baseline | 20 | 1,058 ± 63.1 | 49 | 1,129 ± 45.7 | 0.43 | |||
| Week 4 | 19 | 1,056 ± 68.0 | 50 | 1,147 ± 51.6 | 0.37 | |||
| Week 16 | 21 | 1,051 ± 69.7 | 49 | 1,134 ± 48.2 | 0.29 | |||
| Change after 16 wk | 20 | −8.6 ± 32.6 | −0.8 | 48 | 23.7 ± 23.8 | 2.1 | 0.47 | |
| sTNFR-II (pg/mL) | 0.09 | |||||||
| Baseline | 20 | 2,089 ± 186.9 | 49 | 2,470 ± 111.0 | 0.08 | |||
| Week 4 | 19 | 2,132 ± 217.7 | 50 | 2,493 ± 118.1 | 0.14 | |||
| Week 16 | 21 | 2,132 ± 172.1 | 49 | 2,533 ± 111.3 | 0.04 | |||
| Change after 16 wk | 20 | −4.4 ± 63.9 | −0.2 | 48 | 84.5 ± 57.1 | 3.4 | 0.39 | |
| sICAM-1 (ng/mL) | 0.31 | |||||||
| Baseline | 20 | 298.4 ± 12.5 | 49 | 313.2 ± 14.1 | 0.54 | |||
| Week 4 | 18 | 282.8 ± 13.0 | 48 | 305.6 ± 14.1 | 0.38 | |||
| Week 16 | 21 | 288.6 ± 12.4 | 48 | 317.7 ± 14.0 | 0.20 | |||
| Change after 16 wk | 20 | −7.0 ± 8.4 | −2.3 | 47 | −1.4 ± 9.8 | −0.4 | 0.77 | |
| IL-10 (pg/mL) | 0.42 | |||||||
| Baseline | 11 | 10.4 ± 2.0 | 27 | 8.9 ± 1.0 | 0.56 | |||
| Week 4 | 9 | 9.2 ± 2.5 | 18 | 8.5 ± 1.1 | 0.91 | |||
| Week 16 | 13 | 9.2 ± 1.6 | 25 | 9.2 ± 1.2 | 0.94 | |||
| Change after 16 wk | 8 | 0.2 ± 0.4 | 2.3 | 19 | 0.9 ± 1.2 | 10 | 0.71 | |
sICAM, soluble intracellular adhesive molecule.
aData presented are on treatment. The number of subjects is variable because of occasional missing blood test results from a few subjects in a small numbers of analytes as shown. The change after 16 weeks is based on pairwise difference of subjects who had data available for both baseline and week 16 time points. Means ± SEs are presented for each variable at each follow-up visit.
bP values are calculated from one-way ANOVA.
cP values are calculated from repeated-measures analysis evaluating the change in study variables over time, adjusted for age and sex.
TABLE 2.
Clinical study I: Pearson correlation coefficients of the changes in study variables before and after 16 weeks of treatment
| Change in variable | Leptin | Free leptin | Leptin antibody | LBP | IL-6 | CRP | sTNFR-I | sTNFR-II | MCP1 | sICAM-1 | IL-10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | |||||||||||
| r | −0.08 | −0.17 | −0.11 | 0.11 | 0.20 | 0.00 | −0.04 | −0.02 | 0.22 | 0.10 | −0.18 |
| P value | 0.531 | 0.372 | 0.812 | 0.395 | 0.122 | 0.991 | 0.754 | 0.866 | 0.113 | 0.432 | 0.424 |
| Leptin | |||||||||||
| r | 0.44 | 0.99 | 0.00 | 0.02 | −0.17 | 0.00 | −0.07 | 0.02 | −0.16 | −0.02 | |
| P value | 0.012 | <0.0001 | 0.975 | 0.9 | 0.16 | 0.999 | 0.583 | 0.85 | 0.203 | 0.932 | |
| Free leptin | |||||||||||
| r | 0.22 | −0.17 | −0.13 | −0.22 | −0.04 | 0.18 | 0.16 | −0.05 | 0.29 | ||
| P value | 0.601 | 0.363 | 0.494 | 0.236 | 0.808 | 0.325 | 0.4 | 0.782 | 0.274 | ||
| Leptin antibody | |||||||||||
| r | 0.31 | 0.09 | −0.25 | 0.14 | 0.15 | 0.18 | −0.07 | −0.13 | |||
| P value | 0.416 | 0.827 | 0.521 | 0.728 | 0.699 | 0.641 | 0.853 | 0.806 | |||
| LBP | |||||||||||
| r | 0.14 | 0.44 | 0.25 | 0.18 | −0.08 | 0.26 | −0.13 | ||||
| P value | 0.241 | 0.0002 | 0.043 | 0.132 | 0.522 | 0.031 | 0.503 | ||||
| IL-6 | |||||||||||
| r | 0.30 | 0.21 | 0.25 | 0.07 | 0.20 | 0.22 | |||||
| P value | 0.015 | 0.081 | 0.041 | 0.619 | 0.109 | 0.27 | |||||
| CRP | |||||||||||
| r | 0.43 | 0.47 | 0.05 | 0.49 | 0.26 | ||||||
| P value | 0.0003 | <0.0001 | 0.723 | <0.0001 | 0.186 | ||||||
| sTNFR-I | |||||||||||
| r | 0.62 | 0.09 | 0.40 | 0.50 | |||||||
| P value | <0.0001 | 0.513 | 0.0007 | 0.008 | |||||||
| sTNFR-II | |||||||||||
| r | 0.22 | 0.52 | 0.66 | ||||||||
| P value | 0.091 | <0.0001 | 0.0002 | ||||||||
| sICAM-1 | |||||||||||
| r | 0.43 | ||||||||||
| P value | 0.028 |
MCP, monocyte chemoattractant protein; sICAM, soluble intracellular adhesive molecule.
Laboratory study I: Agonistic/stimulatory activity of antileptin antibodies generated during metreleptin administration.
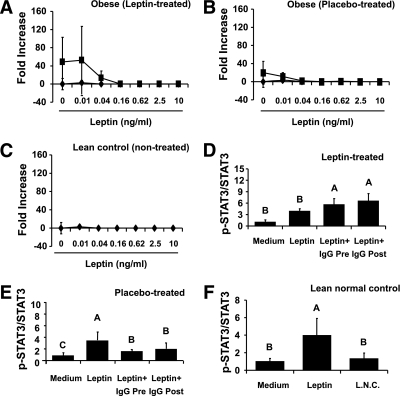
We asked whether the antibodies produced after treatment with metreleptin in clinical study I were able to affect (stimulate or neutralize) leptin-related proliferation and intracellular signaling. Thus, we used the BAF3 cell lines after transfection with the long form of the human leptin receptor (hLepR+BAF3). First, we developed standard curves of antileptin antibodies using an antileptin mAb generated in the laboratory (Supplementary Appendix 2A). Antibodies isolated from metreleptin-treated obese subjects were able to stimulate proliferation up to ∼50-fold more than leptin alone but only at lower leptin concentrations (Fig. 1A). On the contrary, no additional stimulatory effect was observed in the same subjects before treatment, in the placebo-treated subjects, or when using IgGs from normal subjects (Fig. 1B and C). These functional effects were also analyzed at the signaling level in terms of STAT3 phosphorylation. IgGs from metreleptin-treated obese subjects significantly increased p-STAT3 levels (Fig. 1D, Supplementary Appendix 2B), whereas no effect was observed after addition of either placebo-treated IgGs or IgGs isolated from normal controls (Fig. 1E and F, Supplementary Appendix 2B). These results suggest that metreleptin treatment results in the production of antileptin antibodies that are not neutralizing but may demonstrate minor agonistic activities on hLepR measured both as increased proliferation of a leptin-dependent cell line and as increased hLepR-mediated STAT3 signaling. The stimulatory effect of these antibodies is small, however, and easily overridden by relatively higher leptin doses.
FIG. 1.
Laboratory study I. Agonistic/stimulatory activity of antileptin antibodies generated during metreleptin administration. A–C: The functional activity of antileptin antibodies in hLepR+BAF3 cells was as described in detail in research design and methods. ■, Leptin + IgG posttreatment; ♦, Leptin + IgG pretreatment. D–F: The biochemical level of the capacity of antileptin IgGs in hLepR+BAF3 cells was studied as described in detail in research design and methods. All density values for each protein band of interest are expressed as a fold increase. Data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means (n = 6) ± SD. Means with different letters are significantly different, P < 0.05. L.N.C., lean normal control.
Clinical study II: In vivo metreleptin signaling in hAT and hPBMCs from lean and obese subjects.
To study whether intracellular signaling pathways are differentially activated (and thus underlie leptin resistance) in leptin-sensitive lean subjects versus leptin-resistant obese subjects in response to increasing leptin levels similar to those seen in clinical trials, we performed in vivo signaling experiments. In vivo metreleptin administration (0.01 mg/kg) induced an approximately 3.2-fold increase in p-STAT3 at 30 min versus baseline in both hAT (Supplementary Appendix 3A) and hPBMCs (Supplementary Appendix 3B), with no significant difference in p-STAT3 levels from lean versus obese subjects.
Laboratory study II: Comparative evaluation of ex vivo metreleptin signaling in hAT and hPBMCs from lean and obese subjects
No differential activation of STAT3 signaling by ex vivo metreleptin administration in hAT and hPBMC.
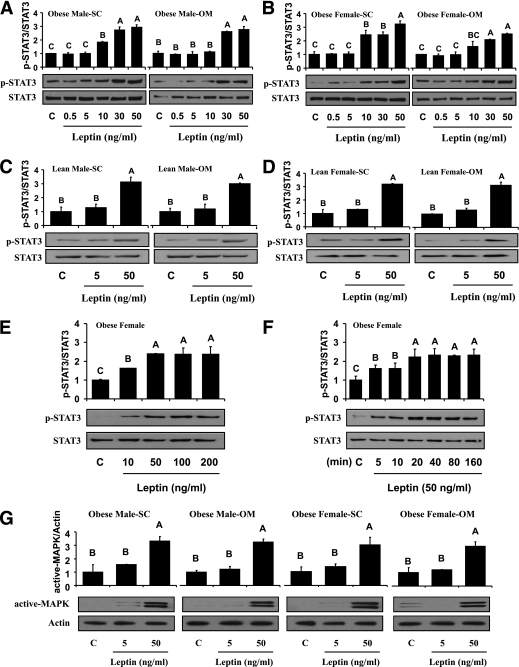
Dose-response curves showed that administration of up to 50 ng/mL metreleptin for 30 min significantly induced phosphorylation of STAT3 in subcutaneous and omental hAT from obese male and female subjects (Fig. 2A and B). These results were similar to what was observed in hAT and hPBMCs after in vivo metreleptin administration (Supplementary Appendix 3A and B). Similar to in vivo observations, there was no significant difference in p-STAT3 expression in hAT from obese versus lean subjects (Fig. 2C and D). The phosphorylated form of STAT3 was increased in metreleptin-stimulated hPBMCs (Fig. 2E and F) compared with control, showing that activation was evident as early as 5 min after ex vivo stimulation with metreleptin.
FIG. 2.
Laboratory study II. Comparative evaluation of ex vivo metreleptin signaling in hAT and hPBMCs from lean and obese subjects. Ex vivo metreleptin administration in hAT and hPBMCs was performed as described in detail in research design and methods. hAT (A–D) and hPBMCs (E) were incubated and stimulated with or without ex vivo metreleptin at the indicated concentrations for 30 min. F: hPBMCs were incubated and stimulated with or without ex vivo metreleptin at the indicated times. G: hAT was incubated and stimulated with or without ex vivo metreleptin at the indicated concentrations for 30 min. All lysates were examined by Western blotting as described in detail in research design and methods. All density values for each protein band of interest are expressed as a fold increase. Data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means (n = 3) ± SD. Means with different letters are significantly different, P < 0.05. OM, omental; SC, subcutaneous.
No differential activation of MAPK signaling by ex vivo metreleptin administration in hAT.
Ex vivo metreleptin administration stimulated activation of MAPK by ∼3.1-fold in hAT (Fig. 2G) from obese male and female subjects. There was no difference in MAPK activation from subcutaneous versus omental, male versus female, and obese versus lean subjects.
No differential expression of inhibitors of ex vivo metreleptin signaling in hAT.
We observed no activation of SOCS3 by ex vivo metreleptin administration in hAT (Supplementary Appendix 4). There was no difference in SOCS3 activation from subcutaneous versus omental, male versus female, and obese versus lean subjects.
No differential activation of AMPK signaling by ex vivo metreleptin administration in hAT and hPBMC.
Ex vivo metreleptin administration stimulated phosphorylation of AMPK in both subcutaneous and omental hAT, and hPBMCs from obese female subjects (Supplementary Appendix 5). We observed no difference in AMPK activation from subcutaneous versus omental, obese versus lean, and male versus female subjects.
Laboratory study III: In vitro metreleptin signaling in subcutaneous and omental hPAs from lean and obese subjects
No differential activation of STAT3 signaling by in vitro metreleptin administration in hPA.
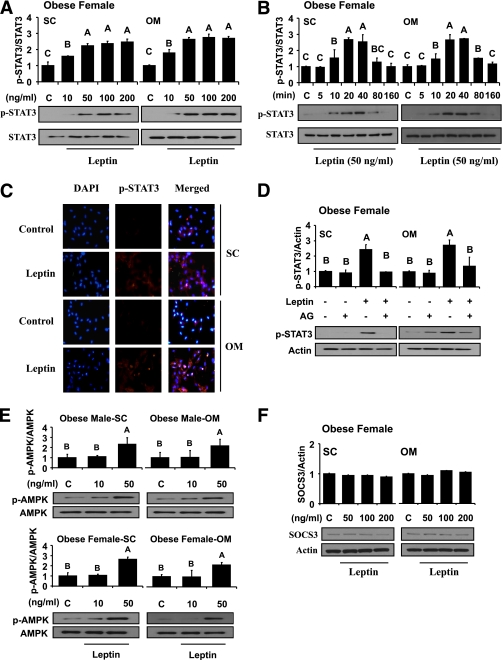
In vitro metreleptin administration significantly induced phosphorylation of STAT3 by ∼2.1-fold at 10 min with a trend toward greater induction at 20–40 min (Fig. 3A and B). A considerable amount of p-STAT3 in metreleptin-treated cells, but only background levels of p-STAT3 in control cells, was detected in both subcutaneous and omental hPA (Fig. 3C). However, these effects were totally blocked by pretreatment with AG490, a STAT3 inhibitor (Fig. 3D), suggesting that metreleptin stimulation activates STAT3 signaling in hPA. In addition, we observed nuclear translocation of STAT3 by in vitro metreleptin administration in a dose-dependent manner (Supplementary Appendix 6). Metreleptin signaling pathways in hPA were saturable at a level of ∼50 ng/mL, and these results are consistent with those observed in hAT in vivo and ex vivo. There were no significant differences in STAT3 activation from subcutaneous versus omental, male versus female, and obese versus lean subjects.
FIG. 3.
Laboratory study III. In vitro metreleptin signaling in subcutaneous (SC) and omental (OM) hPA from lean and obese subjects. In vitro metreleptin administration in hPA was performed as described in detail in research design and methods. A: Cells were treated with metreleptin at the indicated concentrations for 30 min. B: Cells were treated with metreleptin at the indicated times. C: Cells were treated with metreleptin (50 ng/mL) for 30 min. Immunodetection was carried out as described in detail in research design and methods. All pictures were ×40 magnification. D: Cells were pretreated with the STAT3 inhibitor AG490 (AG, 1 μmol/L) for 1 h, followed by treatment with 50 ng/mL metreleptin for 30 min. E and F: Cells were treated with metreleptin at the indicated concentrations for 30 min. All lysates were examined by Western blotting as described in detail in research design and methods. All density values for each protein band of interest are expressed as a fold increase. Data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means (n = 3) ± SD. Means with different letters are significantly different, P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
No differential activation of AMPK signaling by in vitro metreleptin administration in hPA.
In vitro metreleptin administration increased AMPK phosphorylation by ∼2.5-fold in both subcutaneous and omental hPA from male and female subjects (Fig. 3E). We observed no differences in AMPK phosphorylation from subcutaneous versus omental, male versus female, and obese versus lean subjects.
No differential expression of inhibitors of metreleptin signaling in hPA.
We observed no early activation of SOCS3 by in vitro metreleptin administration in hPA (Fig. 3F). In addition, we observed no differences in expression of inhibitors from subcutaneous versus omental, obese versus lean, and male versus female subjects.
Downregulation of in vitro metreleptin-stimulated STAT3 signaling by ER stress in hPA.
Stimulation of the cells with metreleptin led to a marked increase in phosphorylation of STAT3, but when challenged with ER stress (tunicamycin and dithiothreitol), the metreleptin-activated STAT3 phosphorylation was abolished totally (Supplementary Appendix 7). We observed no differences in ER stress–mediated STAT3 phosphorylation from subcutaneous versus omental, male versus female, and obese versus lean subjects.
Laboratory study IV: Gene Expression Omnibus datasets analysis of published data on expression of inhibitors of metreleptin signaling.
To validate our in vivo, ex vivo, and in vitro metreleptin signaling data, we used publicly available datasets to examine expression of potential inhibitors of leptin signaling in hAT from lean and obese nondiabetic humans. We analyzed a dataset comparing gene expression profiles from subcutaneous adipocytes from 20 lean and 19 obese nondiabetic Pima Indians (14), and there was no significant difference in the expression levels of PTP1B or SOCS3.
DISCUSSION
Several open-label trials have demonstrated that administering metreleptin to correct overt hypoleptinemia significantly improves the metabolic abnormalities and insulin resistance in patients with congenital lipodystrophy and insulin resistance (1). Likewise, randomized placebo-controlled trials in patients with human immunodeficiency virus–induced lipodystrophy and the metabolic syndrome who are also hypoleptinemic, although less so than subjects with congenital lipodystrophy, have shown significant, albeit less pronounced, effects of metreleptin (15,16). In contrast, obese hyperleptinemic subjects do not respond to exogenously administered leptin (15,16). We studied whether metreleptin administration would be effective in hyperleptinemic obese subjects with garden variety insulin resistance and diabetes who are considered leptin-resistant or -tolerant. We observed only minor improvements in the glycemic control of hyperleptinemic/diabetic subjects in this randomized trial, which, albeit statistically significant, are one order of magnitude less pronounced than those observed in hypoleptinemic, lipodystrophic subjects and apparently are not of major clinical importance (2). Final body weight and levels of inflammatory markers remained completely unaltered in metreleptin-treated hyperleptinemic obese diabetic subjects. This lack of metreleptin’s efficacy is consistent with a state of “resistance” or “tolerance” to leptin action, defined as an inability of increasing leptin levels to reduce body weight in obese individuals (17). This is the first study in obese diabetic subjects that proves the existence of clinical leptin resistance or tolerance similar to the only prior study in obese nondiabetic subjects (17). Moreover, this study demonstrated no inflammatory response to leptin, with only a minor glycemic response of no apparent clinical significance. Thus, we initiated studies to elucidate mechanisms underlying leptin resistance or tolerance.
We first explored whether mechanisms similar to those involved in other hormone resistance syndromes, such as the increase in binding protein levels, the development of antibodies, the presence of a saturable signaling system, or the presence of in vivo or ex vivo signaling inhibitors in humans, may underlie leptin resistance. A novel finding of this study was that LBP, the extracellular cleaved part of the long isoform of the leptin receptor, and the titers of antileptin antibodies increased significantly in response to metreleptin administration, but not in response to placebo administration. We previously demonstrated that short-term caloric deprivation (72 h) significantly decreases leptin levels but significantly increases LBP by >100% (18). The current study shows that LBP levels increase in response to several months of pharmacologic metreleptin versus placebo treatment. We also observed for the first time the development of non-neutralizing, leptin–binding antibodies, the titers of which increased in the circulation over time in the majority of subjects treated with metreleptin. The distinct possibility exists that other formulations of leptin that do not have the amino acid (methionine) substitution, which in the case of metreleptin was added to improve protein folding, but have full sequence homology with human leptin may not induce leptin antibodies; this possibility remains to be studied. In addition, because pharmacologic metreleptin doses (∼0.2 mg/kg in the current study) may have different effects than physiologic doses in terms of generating antibodies (C.S.M., unpublished data), future placebo–controlled randomized studies involving metreleptin administration in lower, physiologic replacement doses are needed. In any case, the apparent importance of this novel finding is that the majority of circulating leptin is bound to antileptin antibodies, whereas only a small fraction of circulating leptin is free leptin. Thus, we proceeded to explore whether this observation could also be of clinical significance.
Despite increasing LBP and antibody levels, the levels of free leptin increased and remained relatively higher (i.e., at levels ∼40–50 ng/mL) in metreleptin-treated subjects. Although these levels are higher than the putative threshold for saturating the blood-brain–barrier leptin transport system (19,20), circulating free leptin levels did not correlate with weight loss, indicating clinical ineffectiveness of free leptin levels in the 40–50 ng/mL range. Thus, we decided to study whether metreleptin administration in doses within or above the physiologic leptin range, and encompassing the ∼50 ng/mL levels seen in our clinical studies described, can differentially activate signaling pathways in humans in vivo, ex vivo, and in vitro. It has been shown that leptin induces STAT3 phosphorylation in various mouse cell lines and tissues and is generally accepted that this represents a major signaling pathway through which leptin exerts its actions (7, 21–26). Despite minor differences in the timing of signaling pathway activation, we observed no major differences in the magnitude of STAT3 activation in response to metreleptin administration in the human peripheral tissues studied in vivo, ex vivo, or in vitro. We observed no differences in leptin-activated signaling pathways when comparing obese versus lean subjects or men versus women in vivo, ex vivo, or in vitro. More important, metreleptin signaling pathways were saturable at a level of ∼50 ng/mL, suggesting that above that level, i.e., the level clinically seen in obese subjects at baseline, no additional signaling effect can be observed. This explains the effectiveness of metreleptin in subjects with very low circulating leptin levels and the tolerance to metreleptin’s actions when the baseline circulating level is closer to the 40–50 ng/mL range.
We then focused on ER stress, which has recently been shown to play a role in the development of leptin resistance in the hypothalamus of rodents (2). It has been suggested that ER capacity is directly related to leptin sensitivity (3,4,27), and thus, it has been proposed that ER stress reversal could be used as a strategy to sensitize obese mice and, by extension, humans to leptin. These previous studies have shown that the reduction in ER function creates ER stress, blocks leptin action, and generates leptin resistance in mice, suggesting that ER stress provides a potential mechanism for the development of leptin resistance in which increased ER stress antagonizes and inhibits leptin-mediated STAT3 signaling at a step upstream of STAT3 phosphorylation (27). Because ER stress cannot yet be studied in humans in vivo, we performed in vitro metreleptin signaling studies in hPAs to explore whether ER stress could underlie the development of leptin resistance in human primary cells. We report for the first time that ER stress limits leptin signaling in hPAs in vitro, indicating that ER stress may induce leptin resistance in humans similar to induction of leptin resistance in mice in vivo (3,4) and suggesting that improving ER stress could be used as a strategy to sensitize not only obese mice (3,27) but also humans to metreleptin. Because in vivo leptin actions may differ in comparison with in vitro, studies of in vivo leptin signaling in humans are needed to prove or disprove this hypothesis, but it is currently impossible to perform human in vivo ER stress studies.
The blinded and simultaneous administration of metreleptin or placebo in the current study provides a clear and contemporaneous assessment of the role of metreleptin in regulating body weight, metabolic, and immune function in obese diabetic individuals. It could be argued that metreleptin may have different actions in the pharmacologic range (as used in the current study) than in the physiologic range, in terms of generating antileptin antibodies or possibly by resulting in differential downregulation of leptin receptors. It is unlikely that the latter is the case in the current study because we observed an increase in LBP, which reflects an increased number of cell surface receptors (18). The possible differences between the effects of pharmacologic and physiologic doses of metreleptin, however, need to be studied by future well-designed clinical trials in which significant weight loss will be induced and replacement dose of metreleptin will be administered and studied in relation to weight maintenance. Finally, although it is difficult to perform in vivo time course signaling experiments in humans, this is an area that also needs to be addressed in future studies. Future in vivo leptin signaling studies involving additional signaling pathways and other peripheral human tissues are also needed. However, leptin signaling in central nervous system tissues (i.e., hypothalami of humans) cannot be studied in vivo or ex vivo, and thus we and others have initiated indirect studies of leptin’s actions in the brain using functional magnetic resonance imaging techniques. It is also possible that inducers of leptin resistance other than those in the current study may exist in humans and corresponding leptin sensitizers, if any, remain to be identified.
We believe that beginning to elucidate the mechanisms underlying the physiologic and pharmacologic actions of metreleptin, using studies with direct relevance to humans as those presented in this article, offers distinct advantages over the studies in rodents that have been published to date. These initial translational studies in humans are of direct potential clinical and therapeutic significance.
In summary, in obese patients with diabetes, metreleptin administration for 16 weeks did not alter body weight or circulating inflammatory markers but reduced HbA1c marginally. Furthermore, total leptin, LBP, and antileptin antibody levels increased, limiting free leptin availability and resulting in circulating free leptin levels of ∼50 ng/mL. These data identify several steps mediating leptin “tolerance” in humans. Most important, we demonstrate for the first time the saturable nature of leptin signaling pathways in humans and the inhibition of leptin signaling by environmental factors inducing ER stress that contribute significantly to the development of leptin tolerance in obese humans. The mechanisms reported lend themselves to future studies with an ultimate goal of identifying and overcoming leptin resistance in the path toward developing novel therapies for the treatment of excess adiposity and associated abnormalities in humans.
Supplementary Material
ACKNOWLEDGMENTS
The current study was supported by grant F32-DK64550-01A1 (G.K.S.); the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants DK081913, DK79929, DK58785, and AG032030 (C.S.M.); and a Veterans Affairs Merit Review grant (C.S.M.). The Mantzoros Laboratory is also supported by a discretionary grant from the Beth Israel Deaconess Medical Center (BIDMC). G.M. is supported by grants from the EU Ideas Programme, ERC-Starting Independent Grant “LeptinMS” 202579, and Telethon-Juvenile Diabetes Research Foundation Grant GJT08004. The studies were also supported by Amgen and grants UL1-RR-025758 and M01-RR-01032, Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
A.M.D. was an employee of Amgen. Amgen and Amylin Pharmaceuticals supplied metreleptin for this study and approved the clinical studies presented but had no role in interpretation of the data or the preparation, review, or approval of the article. No other potential conflicts of interest relevant to this article were reported.
H.S.M. and C.S.M. wrote the article; H.S.M., G.M., A.M.B., J.P.C., X.L., G.H.M., C.J.W., L.A., F.C., B.E.S., A.M.D., C.G.F., and C.S.M. participated in the performance and coordination of the study; A.M.D. conceived an initial version of clinical study I; and C.S.M. conceived and designed the studies as presented. All authors read and approved the final article.
The authors thank Dr. Young-Bum Kim, Division of Endocrinology, Diabetes, and Metabolism, BIDMC, Harvard Medical School, for technical assistance in the ex vivo study, and Dr. Anke Neuwirth, Division of Endocrinology, Diabetes, and Metabolism, BIDMC, Harvard Medical School, for contributions to early phases of this study. The authors also thank the nurses, technicians, and nutritionists at the BIDMC, General Clinical Research Center and Core Laboratory, for assistance in the conduct of the study.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1791/-/DC1.
Clinical trial reg. no. NCT01275053, clinicaltrials.gov.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
REFERENCES
- 1.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346:570–578 [DOI] [PubMed] [Google Scholar]
- 2.Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab 2005;90:1618–1624 [DOI] [PubMed] [Google Scholar]
- 3.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8:519–529 [DOI] [PubMed] [Google Scholar]
- 4.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 5.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic β-cell apoptosis. Endocrinology 2006;147:3398–3407 [DOI] [PubMed] [Google Scholar]
- 6.Marchetti P, Bugliani M, Lupi R, et al. The endoplasmic reticulum in pancreatic β cells of type 2 diabetes patients. Diabetologia 2007;50:2486–2494 [DOI] [PubMed] [Google Scholar]
- 7.Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology 2000;141:2328–2339 [DOI] [PubMed] [Google Scholar]
- 8.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968;97:77–89 [PubMed] [Google Scholar]
- 9.Ribet C, Montastier E, Valle C, et al. Peroxisome proliferator-activated receptor-alpha control of lipid and glucose metabolism in human white adipocytes. Endocrinology 2010;151:123–133 [DOI] [PubMed] [Google Scholar]
- 10.Moon HS, Chamberland JP, Diakopoulos KN, et al. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care 2011;34:132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett T, Edgar R. Gene expression omnibus: microarray data storage, submission, retrieval, and analysis. Methods Enzymol 2006;411:352–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moon HS, Lee HG, Seo JH, et al. Lipolysis is stimulated by PEGylated conjugated linoleic acid through the cyclic adenosine monophosphate-independent signaling pathway in 3T3-L1 cells: activation of MEK/ERK MAPK signaling pathway and hyper-secretion of adipo-cytokines. J Cell Physiol 2008;214:283–294 [DOI] [PubMed] [Google Scholar]
- 13.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 2004;351:987–997 [DOI] [PubMed] [Google Scholar]
- 14.Lee YH, Nair S, Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia 2005;48:1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantzoros CS. W(h)ither metreleptin for lipodystrophy and the metabolic syndrome? Endocr Pract 2010;29:1–18 [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab 2006;91:2605–2611 [DOI] [PubMed] [Google Scholar]
- 17.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 1999;282:1568–1575 [DOI] [PubMed] [Google Scholar]
- 18.Chan JL, Blüher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes 2002;51:2105–2112 [DOI] [PubMed] [Google Scholar]
- 19.Banks WA. The blood-brain barrier as a cause of obesity. Curr Pharm Des 2008;14:1606–1614 [DOI] [PubMed] [Google Scholar]
- 20.Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res 2002;950:130–136 [DOI] [PubMed] [Google Scholar]
- 21.Catalano S, Giordano C, Rizza P, et al. Evidence that leptin through STAT and CREB signaling enhances cyclin D1 expression and promotes human endometrial cancer proliferation. J Cell Physiol 2009;218:490–500 [DOI] [PubMed] [Google Scholar]
- 22.Cernkovich ER, Deng JB, Bond MC, Combs TP, Harp JB. Adipose-specific disruption of signal transducer and activator of transcription 3 increases body weight and adiposity. Endocrinology 2008;149:1581–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee SD, Sung YY, Jung WH, Cheon HG. Leptin inhibits rosiglitazone-induced adipogenesis in murine primary adipocytes. Mol Cell Endocrinol 2008;294:61–69 [DOI] [PubMed] [Google Scholar]
- 24.Kanda N, Watanabe S. Leptin enhances human beta-defensin-2 production in human keratinocytes. Endocrinology 2008;149:5189–5198 [DOI] [PubMed] [Google Scholar]
- 25.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 2009;58:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Séron K, Corset L, Vasseur F, et al. Distinct impaired regulation of SOCS3 and long and short isoforms of the leptin receptor in visceral and subcutaneous fat of lean and obese women. Biochem Biophys Res Commun 2006;348:1232–1238 [DOI] [PubMed] [Google Scholar]
- 27.Won JC, Jang PG, Namkoong C, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–1865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.