Abstract
OBJECTIVE
Immunotherapy using peptides from the β-cell antigen GAD65 can preserve glucose homeostasis in diabetes-prone NOD mice; however, the precise mechanisms that arrest islet-reactive T cells remain unresolved. Our previous work revealed that a dominant GAD65 epitope contained two overlapping I-Ag7–restricted determinants, 524-538 and 530-543, with the former associated with amelioration of hyperglycemia. Here, we sought to discover whether p524-538–specific T cells could directly regulate islet-reactive T cells.
RESEARCH DESIGN AND METHODS
Prediabetic NOD mice were used to determine the relationship between peptide p524-538–induced interleukin (IL)-13 and regulation of islet autoimmunity. Pancreatic lymph node (PLN) cells from mice at distinct stages of islet inflammation, peri-insulitis versus invasive insulitis, were harvested to establish the expression pattern of IL-13 receptor α1 (IL-13Rα1) on islet-associated T cells.
RESULTS
Peptide p524-538 preferentially induced IL-13–producing T cells that antagonized the release of γ-interferon by spontaneously arising GAD65 autoimmunity, and recombinant human IL-13 inhibited proliferation of islet-reactive clonotypic T cells. A subset of CD4+ T cells in NOD and NOD.BDC2.5 T cell receptor transgenic mice expressed functional IL-13Rα1, which induced phosphorylation of signal transducer and activator of transcription 6 in the presence of cognate cytokine. Notably, the number of IL-13Rα1+ T cells was heightened in the PLN of young NOD mice when compared with older female counterparts with advanced insulitis. Immunization with p524-538 preserved IL-13Rα1 expression on PLN T cells.
CONCLUSIONS
IL-13 may be important for regulating autoimmunity in the early stages of insulitis, and the loss of IL-13Rα1 on islet-reactive T cells may be a biomarker for fading regional immune regulation and progression to overt diabetes.
The NOD mouse is a prototypical model of inflammatory autoimmune disease that displays many of the characteristics of human type 1 diabetes (1). No cure has been identified for the disease, but several interventions are able to arrest islet inflammation and prevent or delay the onset of hyperglycemia in NOD mice. Such findings and ongoing human clinical trials suggest that the targeting of autoantigen-specific T cells offers prospects for successful treatment of newly-diagnosed disease (2). Glutamic acid decarboxylase (GAD65) has persisted as a prominent therapeutic target, which when delivered as a protein (3,4), peptide (5,6), gene encoding plasmid (7), or major histocompatibility complex peptide:ligand (8) provides amelioration from insulitis and β-cell destruction. However, in spite of much success, a defined mode by which such therapies engage islet-reactive T cells is often lacking or has not extended beyond the notion of an altered cytokine milieu. Given the complexity of cytokine activities, a clear understanding of the mechanistic actions in GAD65-specific interventions is needed to improve upon existing therapies.
GAD65 peptide p524-543 is an immunogen that has been associated with both protection (9,10) and progression of diabetes (11,12) in NOD mice. Previously we showed that two I-Ag7–restricted determinants were contained within the p524-543 sequence, in which 530-543 (p530)-specific T cells appeared naturally in young naïve NOD mice (9,13), whereas 524-538 (p524)-specific T cells were primed and expanded following immunization with peptides p524-543 or p524 (9). Thus, the endogenous response focuses on sequences in the carboxyl-terminal portion of 524-543 (9,14), but active immunization with 524-543 switched the dominance to the amino portion of the molecule. Remarkably, active induction of an immune response to p524 significantly reduced the incidence of hyperglycemia in the aggressive cyclophosphamide-induced model of diabetes (9), and adoptive transfer of cloned p524-reactive T cells blocked the progression of insulitis and hyperglycemia (9). The appearance of a robust p524-specific response was also linked to a loss of the spontaneous p530-specific proliferative response (9), suggesting that the p524 immune response could antagonize islet-reactive T cells that gather in the early stages of insulitis.
In this study, we investigated the nature of the p524-induced regulation of spontaneous autoimmunity and found that the peptide preferentially induces interleukin (IL)-13 that is able to modulate β-cell–specific autoimmunity and directly alters the activities of islet-reactive T cells. Furthermore, expression of the cognate receptor, IL-13 receptor α1 (IL-13Rα1), was most prevalent among T cells in the pancreatic lymph nodes (PLNs) of young prediabetic NOD mice. Thus, although treatment with islet antigens may lead to the release of regulatory cytokines into the milieu, here we provide evidence that IL-13 can directly regulate T cells in the islets of young NOD mice.
RESEARCH DESIGN AND METHODS
Mice and cell lines.
NOD mice were purchased from Taconic Farms (Germantown, NY) or bred in the University of Toledo Animal Care Facility. NOD.BDC2.5 T-cell receptor (TCR) transgenic (Tg) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The EL4 cell line (ATCC, Manassas, VA) was cultured in complete medium (RPMI-1640 supplemented with 10% horse serum).
Peptides and T-cell lines.
GAD65 peptides 524-543, 524-538 (p524), and 530-543 (p530), and hen egg white lysozyme peptide HEL 11–25 were synthesized as described previously (9). The p524-reactive T-cell clone GAD35Za was isolated from peptide-immunized mice (9). Briefly, spleen and lymph node cells from NOD mice immunized with p524-538 (20 μg of peptide p524 in complete Freund's adjuvant) were challenged in vitro with cognate peptide, and expanded with recombinant human IL-2 (rIL-2). T-cell clones were isolated by limiting dilution, and maintained by cycles of restimulation with p524-pulsed irradiated syngeneic spleen cells and expansion with rIL-2.
Cytokine enzyme-linked immunospot and enzyme-linked immunosorbent assay.
To enumerate endogenously primed p530-specific T cells via enzyme-linked immunospot (ELISPOT), spleen cells from naïve female NOD mice were cultured in 24-well plates with GAD65 peptide 524-543 as previously described (15). Alternatively, to detect responses by enzyme-linked immunosorbent assay (ELISA), spleen cells collected from the 24-well plates were resuspended in complete medium and added to 96-well round-bottom plates, with or without GAD65 peptide p530. Twenty-four to 72 h later, the culture supernatants were collected and tested for the presence of cytokines (9).
To evaluate p524 responses in vivo, 2- to 3-week-old NOD mice were immunized, once or twice, in the peritoneum with 100 μg of p524, HEL 11–25, or PBS, emulsified in incomplete Freund's adjuvant (IFA). Four or 9 weeks later, lymphoid tissues were collected and analyzed by ELISPOT or ELISA, or flow cytometry, respectively.
For transwell experiments, GAD35Za clonotypic cells (5 × 105 cells/mL) were coplated with irradiated syngeneic spleen cells (4 × 106 cells/mL) in the upper chamber of 24-transwell plates, and spleen cells from naïve 6- to 8-week-old NOD mice were plated at 1 × 107cells/mL in the lower chamber. Monoclonal antibody (10 μg/mL) to γ-interferon (IFN-γ) (R4–6A2), IL-5 (TRFK5), or IL-13 (MAB 413) was added to the upper wells; PBS or rat IgG (10 μg/mL) was added to control wells. GAD65 peptide 524-543 was then added to all upper chambers at a final concentration of 10 μg/mL. Three days later, the spleen cells in the lower chamber were recovered and IFN-γ spot-forming cells enumerated in duplicate wells by ELISPOT.
CD4+ T-cell purification.
NOD or NOD.BDC2.5.TCR.Tg spleen cells were labeled with anti-CD4–conjugated magnetic beads (Miltenyi Biotech, Auburn, CA) and purified according to the manufacturer’s instructions. CD4+ T-cell enrichment (>95%) was verified by flow cytometry.
Proliferation assay.
To assess the effect of rIL-13 on CD4+ T-cell proliferation, purified NOD or BDC2.5.TCR.Tg CD4+ T cells (6 × 104 cells/well) were cultured on anti-CD3ε–coated 96-well plates for 72 h in the presence or absence of rIL-13 (R&D Systems, Minneapolis, MN). PBS-coated wells served as negative controls. For the last 18 h of culture, wells were pulse-labeled with 1μCi/well 3H-thymidine (MP Biomedicals, Solon, OH), harvested, and counted as previously described (16).
RT-PCR.
The expression of IL-4, IL-10, transforming growth factor (TGF)-β, and β-actin was evaluated in GAD35Za T cells via RT-PCR. Kits containing primers for IL-10, TGF-β, and β-actin were purchased from R&D Systems. Primers for IL-4 (forward CATCGGCATTTTGAACGAGGTCA, and reverse CTTATCGATGAATCCAGGCATCG) were purchased from Operon (Alameda, CA). Briefly, GAD35Za T cells were stimulated with irradiated NOD spleen cells in the presence of p524 (10 μg/mL) for 24 to 48 h. mRNA was harvested with an RNeasy kit (Invitrogen, Carlsbad, CA) and used as a template for complimentary DNA (cDNA) synthesis. RT-PCR was performed according to the manufacturer’s instructions for the primer kits, using Platinum Taq polymerase (Invitrogen).
For analysis of IL-13Rα1 expression, CD4+ T cells purified from NOD or NOD.BDC2.5.TCR.Tg splenocytes were stimulated on anti-CD3ε–coated plates for 0, 24, 48, or 72 h. The cells were harvested, and mRNA was extracted with TRIzol (Invitrogen) and converted to cDNA. cDNA was used for quantitative real-time RT-PCR using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Normalized results using the Δ Ct method of quantification are expressed as % GAPDH. The PCR primer sequences were as follows: GAPDH forward CCAGGTTGTCTCCTGCGACT, reverse ATACCAGGAAATGAGCTTGACAAAGT; IL-13Rα1 forward GAATTTGAGCGTCTCTGTCGAA, reverse GGTTATGCCAAATGCACTTGAG; IL-4R forward AGTGAGTGGAGTCCTAGCATC, reverse GCTGAAGTAACAGAACAGGC.
Flow cytometry.
For direct ex vivo analysis of IL-13Rα1 expression on T-cell populations, spleens, inguinal lymph nodes (ILNs), and PLNs were harvested from age-matched mice and stained with anti–TCR-phycoerythrin (PE)/Cy5 (BioLegend), anti–CD4-PE (BioLegend), and purified goat anti–IL-13Rα1 (Santa Cruz Biotechnology, Santa Cruz, CA) or purified goat IgG. The cells were then washed and stained with donkey anti–goat IgG-fluoroisothiocyanate (Southern Biotech, Birmingham, AL), washed, and analyzed by flow cytometry.
For analysis of IL-13 signaling, PLNs from 4-week-old female NOD mice were harvested and stained with anti–TCR-fluoroisothiocyanate (BioLegend), serum-starved in RPMI for 1 h, and then exposed to rIL-13 for 30 min at 37°C. The cells were washed, fixed, permeabilized, and then stained with an antibody to phosphorylated signal transducer and activator of transcription (STAT) 6 (Santa Cruz Biotechnology) or goat isotype control, followed by biotinylated donkey anti–goat IgG and strepavidin-PE before analysis by flow cytometry.
Histological evaluation.
The pancreas was collected at the same time as lymphoid tissue harvest for IL-13Rα1 evaluation. The pancreas was fixed in 10% formalin, sectioned (5 micron sections, 20 microns apart), and stained with hematoxylin and eosin. Islets were graded as normal or as having peri-insulitis (inflammation only around the periphery of the islet) or invasive insulitis (inflammation was greater than 33% of the islet and disrupted the typical round/oval shape).
RESULTS
Injection with p524-538 or p524-responsive T cells reduces the endogenous p530 response in NOD mice.
p524-543 and p530-specific IFN-γ–producing cells were prevalent in the spleens of naïve prediabetic NOD mice (Fig. 1), but were undetectable in healthy BALB/c or C57BL/6 mice (data not shown). However, treatment of young 2-week-old NOD mice with p524-538/IFA led to a significant reduction in the endogenous p530-specific IFN-γ response, regardless of whether p524-543 or p530 was used in the challenge (Fig. 2A). In addition to blocking hyperglycemia (10), treatment with p524 enhanced or primed an IL-5 response to p524-543, which was restricted to the p524 epitope and could not be recalled by the p530 peptide (Fig. 2B). Taken together, these observations indicate that p524/IFA primed a p524-specific Th2 response while concomitantly antagonizing p530-specific Th1 cells; this correlated well with prior observations of lost proliferative responses to p530 in p524-treated mice (9). Furthermore, these findings support the hypothesis that the active induction of p524-reactive T cells directly regulates the activities of endogenously primed GAD65-specific T cells.
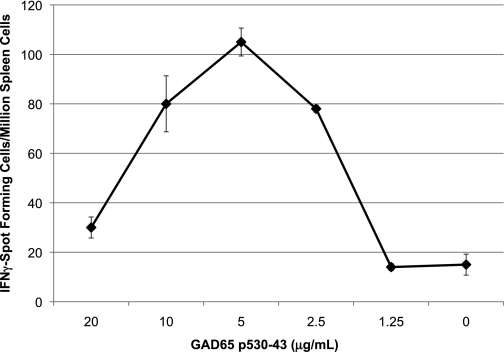
FIG. 1.
Naïve NOD mice mount Th1 immune responses to GAD65 peptide p530-543. Spleen cells pooled from three 6-week-old female NOD mice were stimulated in vitro with peptide p524-543 (10 μg/mL) in 24-well plates. Five days later, the cells were resuspended in IFN-γ ELISPOT plates (1 × 106 cells/well) with the indicated concentration of peptide GAD65 530-543 (p530). The number of spot-forming cells was determined using a stereomicroscope, and the results are representative of four experiments.
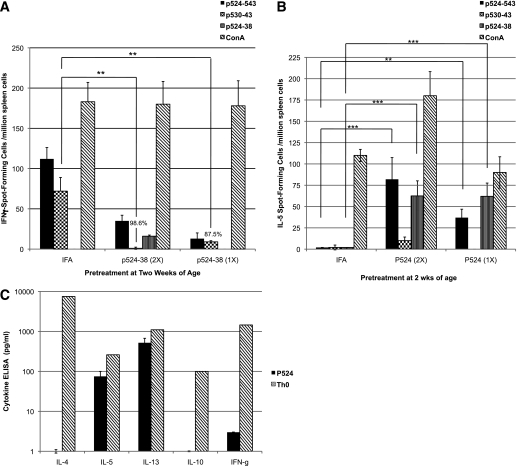
FIG. 2.
Immunization with GAD65 peptide p524–538 antagonizes the endogenous Th1 response to p530–43. A and B: Six 2-week-old NOD mice were immunized once (1×) or twice (2×) with 100 μg of p524–538 or PBS emulsified in IFA. Four weeks later, the spleen cells were collected and analyzed (as in Fig. 1) to enumerate (A) IFN-γ spot-forming cells or (B) IL-5 spot-forming cells, in response to challenge with p524-543 (black bar), p530-543 (checkered), p524-538 (vertical stripes), or Concanavalin A (ConA) (diagonal stripes). Background responses to control peptide HEL 11–25 have been subtracted. The results are representative of two experiments that yielded similar findings. In all experiments, the mean response in peptide-treated mice was compared with control mice (IFA alone) using Student t test (**P < 0.005; ***P < 0.0005). The percentages noted in the chart indicate the reduction in spot-forming cells compared with IFA-immunized control animals. C: Three 4-week-old NOD mice were immunized once with 100 μg of p524-538/IFA. The individual spleens (p524) were collected 12 days later and challenged in vitro with p524-538 (10 μg/mL, black bar). Forty-eight hours later, IL-4, IL-5, IL-13, IL-10, and IFN-γ levels in the supernatants were measured by ELISA. A Th0 cell line stimulated with anti-CD3 (1.0 μg/mL, diagonal stripes) served as a positive control. The bars represent the mean concentration detected in cultures from the three mice (minus medium controls). The data are representative of four experiments.
Because it was possible that the observed suppression of the p530 Th1 responses was mediated through a regulatory cytokine, we wanted to determine whether p524 preferentially induced a cytokine(s) known to regulate diabetes in NOD mice. Although IL-13 and IL-5 were consistently detected in recall responses of 4-week-old NOD mice immunized with p524/IFA, IL-4, IL-10, and IFN-γ, specific responses were marginal and rarely reached significant levels (Fig. 2C). Interestingly, GAD35Za secreted IFN-γ, IL-5, and IL-13 upon antigenic challenge (Table 1), but failed to produce IL-4 or IL-10 (Table 1). The expression of IL-4, IL-10, and TGF-β was also undetectable by RT-PCR in antigen-challenged GAD35Za T cells (data not shown). Therefore, although susceptibility to autoimmune disease in NOD mice has often been linked to a poor Th2 response, as judged by modest IL-4 responses, here we found NOD mice to be quite capable of generating both IL-5 and IL-13 specific responses to p524.
TABLE 1.
The T-cell clone GAD35Za produces IL-13, IL-5, and IFN-γ
| IL-13 ng/mL | IL-5 ng/mL | IFN-γ μg/mL | IL-4 ng/mL | IL-10 ng/mL | |
|---|---|---|---|---|---|
| GAD35Za* | 13.5 | 1.75 | 308 | 0 | 0 |
| OVA-1* | 0 | 0 | 130 | 0.5 | 0 |
*I-Ag7–restricted T clones GAD35Za and OVA-1 were plated (5 × 104/well) with irradiated syngeneic spleen cells (5 × 105/well) in the presence or absence of cognate antigen, 10 μg/mL of p524-538 or 50 μg/mL of ovalbumin, respectively. Forty-eight hours later, the supernatants were collected and cytokine levels determined by ELISA, as described in research design and methods, with background levels subtracted (T cells + antigen-presenting cells).
p524-specific T cells regulate the p530 response via IL-13.
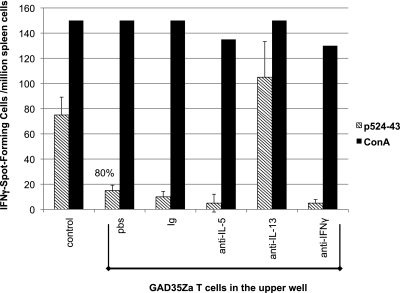
To demonstrate that p524-reactive T cells could indeed alter the spontaneous p530-specific response via release of IL-5 or IL-13 we performed experiments in transwell plates where the upper and lower chambers are separated by a semipermeable membrane. Naïve NOD spleen cells were added to the lower chamber, and GAD35Za T cells and irradiated syngeneic spleen cells were added to the upper chamber. p524-543 was added to the upper chamber along with monoclonal antibody to IL-5, IL-13, or IFN-γ, or an IgG control monoclonal antibody (Fig. 3). The p524-543–specific IFN-γ response among the spleen cells recovered from the lower chamber was reduced by 80% when cultured in the presence of GAD35Za T cells (Fig. 3, control vs. PBS), consequently confirming that the inhibitory effect induced by the p524-reactive cells was mediated by a soluble factor. Importantly, this inhibition was completely neutralized by antibodies to IL-13 but not by anti–IL-5, anti–IFN-γ, or rat IgG (Fig. 3).
FIG. 3.
IL-13 produced by p524-538–reactive T cells can antagonize the endogenous Th1 response to p530-543. GAD35Za clonotypic T cells were plated (5 × 105 mL) with irradiated syngeneic spleen cells in the upper chamber of a semipermeable transwell, whereas spleen cells from naïve 6- to 8-week-old NOD mice were plated (1 × 107/mL) in the lower chamber. PBS, rat IgG (10 μg/mL), monoclonal antibody (10 μg/mL) to IL-5, IL-13, or IFN-γ was added to the upper wells. GAD65 peptide 524-543 (diagonal striped bar) or Concanavalin A (ConA) (black) was then added to all upper chambers at a final concentration of 10 μg/mL or 2.5 μg/mL, respectively. The control well contained the NOD spleen cells plus peptide antigen in the lower chamber, but no GAD35Za cells in the upper chamber. Three days later, spleen cells in the lower chamber were recovered and analyzed for IFN-γ spot-forming cells (as in Fig. 1). The p524-543–specific results represent the mean and SD of triplicate wells and are representative of three experiments.
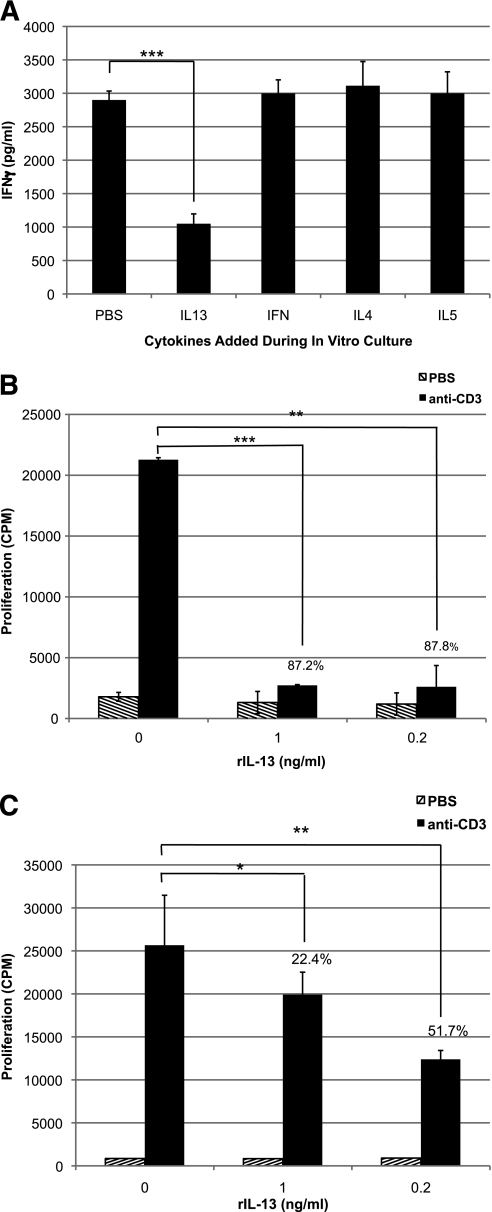
To determine whether IL-13 alone could mimic the suppressive effect of GAD35Za T cells, mouse rIL-13, rIFN-γ, rIL-5, or rIL-4 was added to naïve NOD spleen cells during in vitro challenge with the peptide p524-543. rIL-13 was able to significantly reduce the p530-specific secretion of IFN-γ (Fig. 4A), whereas neither rIL-4, rIL-5, nor rIFN-γ had a similar inhibitory effect (Fig. 4A). The rIL-13–mediated inhibition was not the result of cellular toxicity, since there was no reduction in the number of spleen cells recovered from wells cultured in the presence of this cytokine (data not shown).
FIG. 4.
Recombinant IL-13 directly antagonizes T-cell activities. A: Pooled spleen cells (8 × 106/mL) from three 6-week-old female NOD mice were stimulated in vitro with 10 μg/mL of p524-543 in the presence or absence of recombinant cytokine (10 μg/mL) (IFN-γ, IL-4, IL-5, or IL-13). Three days later, the cells were resuspended and challenged with p530-543–pulsed irradiated spleen cells. Forty-eight hours later, the supernatants were tested by ELISA for the presence of IFN-γ. The results are representative of five experiments. B and C: Clonotypic spleen T cells from NOD.BDC2.5.TCR.Tg mice (B) or CD4+ T cells purified from NOD mice (C) were stimulated in triplicate on anti-CD3ε–coated plates (1.0 μg/mL, black bar) in the presence or absence of murine rIL-13 (0.2 or 1.0 ng/mL). Control wells were coated with PBS (diagonal stripes). Two days later, the wells were pulsed with 3H-thymidine, then harvested and counted after an additional 18 h of culture. The results are representative of two experiments. The percentages in the chart indicate the reduction in proliferation (counts per minute [cpm]) compared with cells stimulated in the absence of rIL-13. The P values were calculated using Student t test (*P = 0.05; **P < 0.005; ***P < 0.0005).
To establish whether IL-13 could directly suppress the activities of islet-reactive T cells, NOD.BDC2.5 TCR clonotypic spleen cells were cultured in wells coated with anti-CD3 in the presence or absence of rIL-13. Even in the presence of robust antigen receptor engagement, BDC2.5, clonotypic proliferation was inhibited by up to 87% when stimulated in the presence of rIL-13 (Fig. 4B). Although this approach should eliminate influences by antigen-presenting cells, to further confirm that rIL-13 was having a direct impact on T cells and that the inhibitory response was not peculiar to the BDC2.5 clonotypic cells, CD4+ T cells were purified from the spleens of naïve NOD mice and stimulated with anti-CD3 in the presence of rIL-13. Again, rIL-13 caused significant suppression of the T cell proliferative response (Fig. 4C). Similar results were observed when the experiment was performed with purified NOD.BDC2.5.TCR.Tg CD4+ T cells (data not shown).
Subsets of NOD and NOD.BDC2.5 T cells express a functional IL-13Rα1.
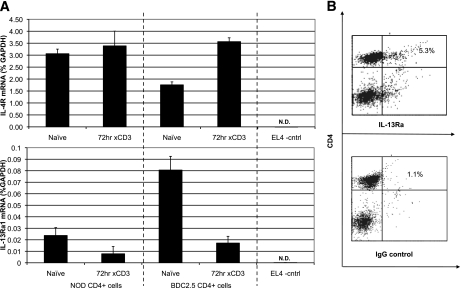
Because IL-13 seemed to directly regulate the T cells, it was necessary to demonstrate that NOD and BDC2.5 clonotypic T cells expressed IL-13Rα1 and IL-4Rα, which heterodimerize to form the functional complex for IL-13–mediated signaling (17,18). cDNA produced from purified NOD or NOD.BDC2.5.TCR.Tg CD4+ splenocytes (naïve or activated) was analyzed by quantitative real-time PCR using primers specific for mouse IL-13Rα1, IL-4Rα, or IL-13Rα2. IL-13Rα1 and IL-4Rα transcripts were detected in both naïve and activated T cells (Fig. 5A). IL-13Rα1 mRNA expression was reduced following TCR engagement in both sets of T cells (Fig. 5A), but IL-13Rα1 and IL-4Rα were not detectable in the EL-4 thymoma control cell line (Fig. 5A). Next, we were able to validate the expression of IL-13Rα1 chain on NOD T cells, as approximately 5% of the TCR+CD4+ cells in the spleens of untreated mice were positive for the cytokine receptor (Fig. 5B). These findings support the contention that IL-13 can deliver influential signals directly to effector T cells. IL-13Rα2, a decoy receptor that negatively regulates IL-13 signaling (19), was not detected in any of the samples above (data not shown).
FIG. 5.
NOD and BDC2.5.TCR.Tg T cells express IL-13Rα1. A: cDNA produced from purified NOD or BDC2.5.TCR.Tg CD4+ cells, before or after culture on anti-CD3–coated plates (72 h) was evaluated by real-time PCR to detect expression of IL-4R mRNA (upper panel) or IL-13Rα1 mRNA (lower panel), relative to GAPDH. The CD4+ EL4 thymoma line served as a negative control – transcripts for GAPDH were detected, whereas mRNA for IL-13Rα1 or IL-4Rα was not detected (N.D.). B: NOD splenocytes were stained with antibodies to TCRαβ, CD4, and IL-13Rα1 (upper plot) or isotype control (lower plot). The plots are gated on TCR+ populations. The numbers in the upper right quadrant indicate percentages of TCR+ cells that are also positive for the designated parameters. The plots shown are a representative experiments performed six times with similar results.
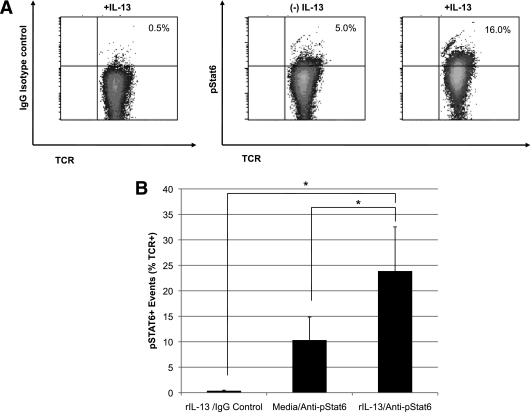
In human cells, ligation of IL-13 to the high affinity IL-13Rα1/IL-4Rα heterodimer activates janus kinase 2 tyrosine kinase and subsequently the phosphorylation of STAT6 (20). Here, we show that TCR+ T cells recovered from the PLNs of young NOD mice respond to IL-13 by increasing the phosphorylation of STAT6 (Figs. 6A and B), demonstrating that the IL-13Rα1 expressed on the T cells was functional and capable of transmitting intracellular signals following ligand binding.
FIG. 6.
IL-13 stimulates the phosphorylation of STAT6 in T cells from the PLN. A: Serum-starved cells collected from the PLNs of three 4-week-old NOD mice were cultured in the absence (middle plot) or presence of rIL-13 (1 ng/ml, left and right plots) for 30 min, and then stained with antibodies to TCRαβ and the phosphorylated form of STAT6 (pSTAT6) (right plot) or isotype control (left plot). B: The mean number of TCR+/pSTAT6+ cells was calculated from three independent experiments. P values were calculated using Student t test (*P < 0.05).
T-cell–specific IL-13Rα1 expression is prevalent in the PLNs of young NOD mice.
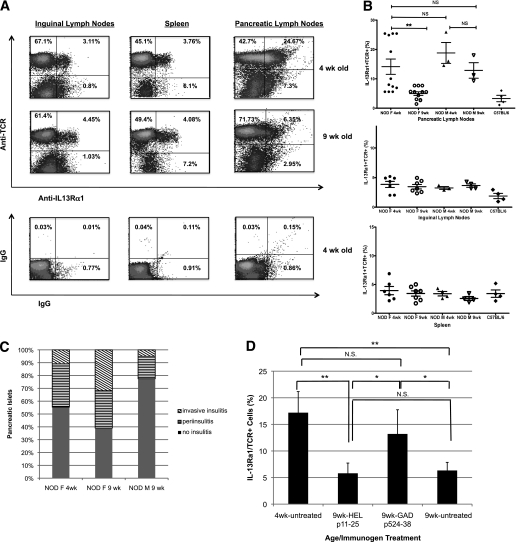
Islet inflammation in NOD mice is characterized by distinct temporal and phenotypic stages. The early stage of insulitis, at 4–5 weeks of age, is characterized by peri-insulitis with very limited β-cell destruction. This benign period is followed by stages of progressively enhanced islet destruction and infiltration at 9–12 weeks of age, and sets the stage for the extensive β-cell destruction that gives rise to hyperglycemia. To explore the possibility of IL-13–mediated T-cell regulation in the context of natural type 1 diabetes, we compared patterns of IL-13Rα1+ expression on T cells in 4-week-old mice and 9- to 11-week-old mice. We found that the frequency of IL-13Rα1+ T cells was enhanced in the PLNs of the young mice, compared with their ILNs or spleens (Fig. 7I). Strikingly, there were significantly more IL-13Rα1–expressing T cells in the PLNs of young mice compared with the older animals (Fig. 7B). On the other hand, there was no significant difference between 4- and 9-week-old mice in the frequency of IL-13Rα1+ T cells in the ILNs or splenic populations (Fig. 7B). Although the actual percentages of IL-13Rα1+ T cells varied, in individual experiments (containing equal numbers of young and older mice) the percentage of receptor positive cells was always significantly higher in the younger mice (Fig. 7B).
FIG. 7.
IL-13Rα1 expression on T cells in the PLNs is enhanced in young prediabetic NOD mice. ILNs, PLNs, and spleens from 4- or 9-week-old NOD mice were analyzed by fluorescence-activated cell sorter for TCRαβ and IL-13Rα1 expression. A: Representative density plots from 4-week-old (top plots) and 9-week-old (bottom plots) female NOD tissues. The plots shown are ungated populations, with the numbers indicating the percentages of cells in each quadrant. B: Quantitative analysis of IL-13Rα1+ T cells in individual 4-week-old or 9–11-week-old NOD mice, female (F) and male (M), or female C57BL/6 controls—each marker indicates an individual mouse. The means were calculated from seven experiments performed under identical conditions; statistical analysis was performed using Student t test (**P < 0.005). C: The pancreas was collected from three NOD mice, individuals in (B), fixed, processed, and stained as described in the research design and methods. At least 50 islets were evaluated for the severity of leukocyte infiltration: normal (filled bar), peri-insulitis (horizontal stripes), and invasive (diagonal stripes). D: PLNs from female NOD mice immunized at 3 weeks of age with p524-538/IFA or HEL 11–25/IFA, were collected at 9 weeks of age and analyzed by flow cytometry. Untreated 4- and 9-week-old mice were used as controls. The data represents two experiments, with three mice per group. Statistical analysis was performed using Student t test (*P < 0.05, **P < 0.005). N.S., not significant.
The findings above offer the possibility that the loss of IL-13Ra1+ T cells in the PLNs of NOD mice could correlate with the increased severity of insulitis. Female NOD mice bred and housed in our facility have a diabetes incidence rate of greater than 80%, whereas less than 20% of the male counterparts progress to hyperglycemia. When we evaluated 4- and 9-week old male NOD mice (littermates of the females) we found that both young and adult males displayed a pattern similar to that of 4-week-old females—significantly more IL-13Rα1+ T cells in the PLNs than in the corresponding inguinal or splenic lymphoid compartments (Fig. 7B). Conversely, the frequency of IL-13Rα1+ T cells in ILNs and spleens from the older male mice was not different from that of older female NOD mice or C57BL/6 control mice (Fig. 7B). An analysis of the pancreatic tissue harvested from the same groups of animals revealed that the level of invasive insulitis was highest among the older female mice, whereas tissues from the older male mice contained more normal islets and far fewer islets with the invasive form of inflammation (Fig. 7C).
Because treatment with p524-538 ameliorates insulitis and type 1 diabetes in young NOD mice, we wanted to discover whether immunization with p524-538 could also alter the expression of IL-13Rα1 on T cells in the PLNs of postinsulitis female NOD mice. We found that number of IL-13Rα1+ T cells in the PLNs of 9-week-old female mice treated with control peptide was significantly lower than that expressed on similar cells in young mice (Fig. 7D) and comparable to untreated 9-week-old mice (Fig. 7D). On the other hand, at 9 weeks of age mice treated with the GAD65 peptide retained a frequency of IL-13Rα1+ T cells in PLNs similar to that of 4-week-old mice (Fig. 7D).
DISCUSSION
Ongoing clinical trials have provided encouraging data suggesting that treatment with the GAD65 protein will be beneficial in prolonging β-cell function in recent-onset human diabetes (21,22). Nevertheless, the molecular mechanisms that govern GAD65-induced regulation of insulitis and β-cell destruction remain unclear. Initially, immune deviation via the enhancement of IL-4–driven Th2 response seemed a likely explanation for the improved outcome observed in neonatal NOD mice successfully treated with GAD65 protein or peptides (10,23,24). This contention was strengthened by data indicating that NOD mice had deficient IL-4 responses, possibly as the result of reduced numbers of IL-4–producing natural killer T cells (25–27) and/or inadequate TCR signaling cascades (28). However, the significance of IL-4 in modulating islet autoimmunity has been challenged in subsequent studies using IL-4–deficient NOD mice (29) or examining the role of natural killer T cells in autoimmunity (30). Although neutralizing antibodies provide strong evidence for IL-10 as an influential cytokine, in many cases the correlation between reduced islet pathology in NOD mice and the appearance of regulatory cytokines such as IL-4 and IL-10 is indirect and does not address the mechanism(s) by which the target cells are engaged. Here, we show that an alternate Th2-like cytokine, IL-13, is able to directly regulate GAD65-specific Th1 cell–mediated autoimmunity. Importantly, IL-13Rα1 expression on T cells may have an impact on the effector functions of β-cell–specific T cells and islet injury during the early stage of type 1 diabetes, a period characterized by benign insulitis (31). It is of particular interest that a distinct epitope on GAD65 is able to incite IL-13–producing cells, either as p524-538 or as a determinant processed from p524-543.
Prolonged administration of rIL-13 prevents or delays the onset of insulitis and diabetes in young NOD mice, and reduces the incidence in those with preexisting insulitis (32). This Th2 cytokine has also been shown to have protective effects in other models of inflammatory autoimmune disease (33,34). However, until recently (35–37), T cells were thought to be devoid of IL-13Rα1 expression and therefore could not be directly regulated by IL-13; the influence of the cytokine on inflammatory autoimmune disease has largely been attributed to its effect on IL-13Rα1+ monocytes and their potential to prevent the expansion of autoreactive T cells (34). Our data, and that of others (36,37), support a role for IL-13–induced signal transduction in Th cells that produces altered gene expression and effector functions. IL-13 can specifically induce phosphorylation of the transcription factor STAT6 in T cells, as well as increase the expression of GATA-3 (37). Our inhibition experiments with the p524-specific T-cell clone GAD35Za clearly showed that IL-13 suppressed native Th1 responses that we observed in NOD mice. The regional enhancement of IL-13Rα1 expression on T cells in the draining nodes of the pancreas was specific to 4-week-old female NOD mice and our 9-week-old male NOD that show resistance to invasive insulitis and diabetes. This age-related loss of IL-13Rα1 receptor expression may contribute to the collapse of islet-specific tolerance, which has been reported to instigate and perpetuate diabetes in the NOD mice (5). Although our report focuses on CD4+ T cells, we also detected IL-13Rα1 expression on CD4-TCR+ cells. It remains to be determined whether treatment with p524 or rIL-13 is able to modulate the activities of islet-specific CD8+ T cells.
Lee et al. (35) show that IL-4 signaling through the IL-4Rα/IL-13Rα1 heterodimer induces apoptosis in neonatal IL-13Rα1–expressing Th1 cells upon antigenic challenge. We found that exposure to IL-13 does not directly reduce the number of autoreactive T cells in vitro, and systemic delivery of the cytokine over several weeks was well tolerated in NOD mice (32), collectively suggesting the induction of anergy or a functional shift in the inflammatory milieu, rather than cell death, as the basis for our findings. Th1-associated IFN-γ and Th17-associated IL-17 can contribute to inflammation, and the lack of either cytokine has been shown to reduce or ameliorate inflammatory autoimmune responses in several murine models of disease (38–40). Upon exposure to either p524-538–specific T cells or rIL-13, we detect a reduction in IFN-γ production by autoreactive p530-543–specific Th1 cells. We found that the T-cell clone GAD35Za was able to inhibit the spontaneous Th1 responses to a dominant GAD65 epitope that has been associated with progression of autoimmune diabetes in wild-type NOD mice (10,41,42). Interestingly, IL-13–deficient mice exhibit increased proliferation of antigen specific CD4+ and CD8+ effector T cells (34), and prophylactic treatment with IL-13 reduces the ability of splenic cells to produce IFN-γ in response to Concanavalin A or anti-CD3. Because IFN-γ is reported to antagonize the generation of IL-17–producing Th17 cells (43), it was possible that suppression of IFN-γ could promote a shift toward a Th17-dominated response. However, we did not find that the IL-13–mediated reduction of IFN-γ resulted in production of IL-17 (data not shown). Furthermore, our previous data show that p524-538 immunization blocks the progression to diabetes in NOD mice, which is not supportive of the conclusion that the treatment skews the milieu toward a Th17 phenotype. Collectively, our data confirm the reports that T cells are able to express IL-13Rα1 (37), but suggest that expression is not restricted to Th17-polarized CD4+ T cells in NOD mice.
Although clone GAD35Za ameliorated diabetes when transferred to young NOD mice (9), disease protection was not elicited upon clone transfer to older NOD with pronounced insulitis, or when cotransferred with diabetogenic splenocytes (data not shown), suggesting that p524-538–specific regulation of autoreactive T cells is most effective in the early stages of pancreatic autoimmunity. This loss of regulatory activity could be related to the temporal loss of IL-13Rα1 expression we observed in the PLNs. Although the antigenic specificity of the IL-13Rα1+ T cells is unknown, our data show that the IL-13–producing T cells (p524-538) and IL-13–responding T cells (β-cell specific) are distinct. Therefore, since β-cell–specific T cells are present in the peripheral blood of patients with type 1 diabetes (44) and of at-risk individuals long before the onset of hyperglycemia (45), as well as being detected in the periphery of diabetes-free first-degree relatives (46), it would be of interest to discover whether differential expression of the IL-13Rα1 on such T cells could serve as a biomarker (47) for progressive β-cell destruction and signal the onset of hyperglycemia. This could be accomplished most efficiently if staining for the receptor is combined with the soluble major histocompatibility complex:peptide technology that helped foster the identification, enumeration, and isolation of β-cell–specific T cells in humans (45,48). A biomarker for progressive immune dysregulation could provide the underpinnings for earlier introduction of GAD65 immunotherapy and the obvious implications for improved benefits.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Juvenile Diabetes Research Foundation International and the University of Toledo (to A.Q.) and the United States Department of Agriculture (to M.F.M.).
No potential conflicts of interest relevant to this article were reported.
S.S.R. and A.Q. performed experiments and wrote the manuscript. M.P. performed experiments. M.F.M. performed experiments and reviewed and edited the manuscript. E.E.S. contributed to discussion and edited the manuscript.
The authors thank Dr. Hermann Grafenstein (University of Toledo, Toledo, Ohio) for his valued input and editorial comments in the preparation of the manuscript and Dr. Atsushi Enomoto (Gunma University, Gunma, Japan) for his technical contributions to this work.
Footnotes
See accompanying commentary, p. 1657.
REFERENCES
- 1.Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol 1995;13:179–200 [DOI] [PubMed] [Google Scholar]
- 2.Tian J, Kaufman DL. Antigen-based therapy for the treatment of type 1 diabetes. Diabetes 2009;58:1939–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tisch R, Yang XD, Liblau RS, McDevitt HO. Administering glutamic acid decarboxylase to NOD mice prevents diabetes. J Autoimmun 1994;7:845–850 [DOI] [PubMed] [Google Scholar]
- 4.Cetkovic-Cvrlje M, Gerling IC, Muir A, Atkinson MA, Elliott JF, Leiter EH. Retardation or acceleration of diabetes in NOD/Lt mice mediated by intrathymic administration of candidate beta-cell antigens. Diabetes 1997;46:1975–1982 [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DL, Clare-Salzler M, Tian J, et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 1993;366:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 1993;366:72–75 [DOI] [PubMed] [Google Scholar]
- 7.Tisch R, Wang B, Weaver DJ, et al. Antigen-specific mediated suppression of beta cell autoimmunity by plasmid DNA vaccination. J Immunol 2001;166:2122–2132 [DOI] [PubMed] [Google Scholar]
- 8.Li L, Yi Z, Wang B, Tisch R. Suppression of ongoing T cell-mediated autoimmunity by peptide-MHC class II dimer vaccination. J Immunol 2009;183:4809–4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn A, McInerney B, Reich EP, Kim O, Jensen KP, Sercarz EE. Regulatory and effector CD4 T cells in nonobese diabetic mice recognize overlapping determinants on glutamic acid decarboxylase and use distinct V beta genes. J Immunol 2001;166:2982–2991 [DOI] [PubMed] [Google Scholar]
- 10.Tian J, Atkinson MA, Clare-Salzler M, et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med 1996;183:1561–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson SS, White TC, DeLuca D. Therapeutic alteration of insulin-dependent diabetes mellitus progression by T cell tolerance to glutamic acid decarboxylase 65 peptides in vitro and in vivo. J Immunol 2001;167:569–577 [DOI] [PubMed] [Google Scholar]
- 12.Gerling IC, Atkinson MA, Leiter EH. The thymus as a site for evaluating the potency of candidate beta cell autoantigens in NOD mice. J Autoimmun 1994;7:851–858 [DOI] [PubMed] [Google Scholar]
- 13.Quinn A, McInerney M, Huffman D, et al. T cells to a dominant epitope of GAD65 express a public CDR3 motif. Int Immunol 2006;18:967–979 [DOI] [PubMed] [Google Scholar]
- 14.Dai YD, Jensen KP, Marrero I, Li N, Quinn A, Sercarz EE. N-terminal flanking residues of a diabetes-associated GAD65 determinant are necessary for activation of antigen-specific T cells in diabetes-resistant mice. Eur J Immunol 2008;38:968–976 [DOI] [PubMed] [Google Scholar]
- 15.Busick RY, Aguilera C, Quinn A. Dominant CTL-inducing epitopes on GAD65 are adjacent to or overlap with dominant Th-inducing epitopes. Clin Immunol 2007;122:298–311 [DOI] [PubMed] [Google Scholar]
- 16.Mayo S, Kohler W, Kumar V, Quinn A. Insulin-dependent diabetes loci Idd5 and Idd9 increase sensitivity to experimental autoimmune encephalomyelitis. Clin Immunol 2006;118:219–228 [DOI] [PubMed] [Google Scholar]
- 17.Miloux B, Laurent P, Bonnin O, et al. Cloning of the human IL-13R alpha1 chain and reconstitution with the IL4R alpha of a functional IL-4/IL-13 receptor complex. FEBS Lett 1997;401:163–166 [DOI] [PubMed] [Google Scholar]
- 18.Zurawski SM, Vega F, Jr, Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J 1993;12:2663–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood N, Whitters MJ, Jacobson BA, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med 2003;197:703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer-Crocker RL, Hughes CC, Pober JS. IL-4 and IL-13 activate the JAK2 tyrosine kinase and Stat6 in cultured human vascular endothelial cells through a common pathway that does not involve the gamma c chain. J Clin Invest 1996;98:604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 22.Agardh CD, Lynch KF, Palmér M, Link K, Lernmark A. GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia 2009;52:1363–1368 [DOI] [PubMed] [Google Scholar]
- 23.Rabinovitch A. Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM. Therapeutic intervention by immunostimulation? Diabetes 1994;43:613–621 [DOI] [PubMed] [Google Scholar]
- 24.Tisch R, Wang B, Atkinson MA, Serreze DV, Friedline R. A glutamic acid decarboxylase 65-specific Th2 cell clone immunoregulates autoimmune diabetes in nonobese diabetic mice. J Immunol 2001;166:6925–6936 [DOI] [PubMed] [Google Scholar]
- 25.Lehuen A, Lantz O, Beaudoin L, et al. Overexpression of natural killer T cells protects Valpha14-Jalpha281 transgenic nonobese diabetic mice against diabetes. J Exp Med 1998;188:1831–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. α/β-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med 1998;187:1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulton LD, Smyth MJ, Hawke CG, et al. Cytometric and functional analyses of NK and NKT cell deficiencies in NOD mice. Int Immunol 2001;13:887–896 [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Salojin KV, Delovitch TL. CD28 co-stimulation restores T cell responsiveness in NOD mice by overcoming deficiencies in Rac-1/p38 mitogen-activated protein kinase signaling and IL-2 and IL-4 gene transcription. Int Immunol 2001;13:377–384 [DOI] [PubMed] [Google Scholar]
- 29.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J Immunol 2001;166:1352–1359 [DOI] [PubMed] [Google Scholar]
- 30.Jahng AW, Maricic I, Pedersen B, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med 2001;194:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dilts SM, Lafferty KJ. Autoimmune diabetes: the involvement of benign and malignant autoimmunity. J Autoimmun 1999;12:229–232 [DOI] [PubMed] [Google Scholar]
- 32.Zaccone P, Phillips J, Conget I, et al. Interleukin-13 prevents autoimmune diabetes in NOD mice. Diabetes 1999;48:1522–1528 [DOI] [PubMed] [Google Scholar]
- 33.Young DA, Lowe LD, Booth SS, et al. IL-4, IL-10, IL-13, and TGF-beta from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. J Immunol 2000;164:3563–3572 [DOI] [PubMed] [Google Scholar]
- 34.Cihakova D, Barin JG, Afanasyeva M, et al. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am J Pathol 2008;172:1195–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HH, Hoeman CM, Hardaway JC, et al. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med 2008;205:2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Lee HH, Bell JJ, et al. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity 2004;20:429–440 [DOI] [PubMed] [Google Scholar]
- 37.Newcomb DC, Zhou W, Moore ML, et al. A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol 2009;182:5317–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 2003;171:6173–6177 [DOI] [PubMed] [Google Scholar]
- 39.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol 1998;55:1139–1149 [DOI] [PubMed] [Google Scholar]
- 40.Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med 2008;205:1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian J, Dang H, von Boehmer H, Jaeckel E, Kaufman DL. Transgenically induced GAD tolerance curtails the development of early beta-cell autoreactivities but causes the subsequent development of supernormal autoreactivities to other beta-cell antigens. Diabetes 2009;58:2843–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zekzer D, Wong FS, Ayalon O, et al. GAD-reactive CD4+ Th1 cells induce diabetes in NOD/SCID mice. J Clin Invest 1998;101:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 2009;126:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seyfert-Margolis V, Gisler TD, Asare AL, et al. Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes 2006;55:2588–2594 [DOI] [PubMed] [Google Scholar]
- 45.Oling V, Marttila J, Ilonen J, et al. GAD65- and proinsulin-specific CD4+ T-cells detected by MHC class II tetramers in peripheral blood of type 1 diabetes patients and at-risk subjects. J Autoimmun 2005;25:235–243 [DOI] [PubMed] [Google Scholar]
- 46.Standifer NE, Ouyang Q, Panagiotopoulos C, et al. Identification of Novel HLA-A*0201-restricted epitopes in recent-onset type 1 diabetic subjects and antibody-positive relatives. Diabetes 2006;55:3061–3067 [DOI] [PubMed] [Google Scholar]
- 47.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity 2010;32:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velthuis JH, Unger WW, Abreu JR, et al. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes 2010;59:1721–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]