Abstract
OBJECTIVE
Inflammation and dysfunction of the hypothalamus are common features of experimental obesity. However, it is unknown whether obesity and massive loss of body mass can modify the immunologic status or the functional activity of the human brain. Therefore, the aim of this study was to determine the effect of body mass reduction on brain functionality.
RESEARCH DESIGN AND METHODS
In humans, changes in hypothalamic activity after a meal or glucose intake can be detected by functional magnetic resonance imaging (fMRI). Distinct fMRI analytic methods have been developed to explore changes in the brain’s activity in several physiologic and pathologic conditions. We used two analytic methods of fMRI to explore the changes in the brain activity after body mass reduction.
RESULTS
Obese patients present distinct functional activity patterns in selected brain regions compared with lean subjects. On massive loss of body mass, after bariatric surgery, increases in the cerebrospinal fluid (CSF) concentrations of interleukin (IL)-10 and IL-6 are accompanied by changes in fMRI patterns, particularly in the hypothalamus.
CONCLUSIONS
Massive reduction of body mass promotes a partial reversal of hypothalamic dysfunction and increases anti-inflammatory activity in the CSF.
Obesity affects more than 300 million people worldwide (1). It is the main risk factor for type 2 diabetes, atherosclerosis, and hypertension, and therefore plays an important role in the overall mortality of modern societies (2). Increased adiposity results from the progressive loss of the homeostatic control of caloric intake and energy expenditure. In animal models, dysfunctional activity of specialized neurons of the hypothalamus is regarded as an important determinant for the development of obesity (3–6). In both genetic and diet-induced obese rodents, the malfunction of the hypothalamus is a consequence of the activation of local inflammation and eventually apoptosis of selected neuronal populations (3,4,7,8). The inhibition of inflammation by genetic or pharmacologic approaches leads to the reduction of the obese phenotype and correction of the metabolic breakdown, placing hypothalamic inflammation as a potential target for the treatment of obesity (3,4,8–10).
We show that obese patients present distinct functional activity patterns in selected brain regions, compared with lean subjects. On massive loss of body mass, increases in the cerebrospinal fluid (CSF) concentrations of interleukin (IL)-10 and IL-6 are accompanied by changes in functional magnetic resonance imaging (fMRI) patterns, particularly in the hypothalamus.
RESEARCH DESIGN AND METHODS
Thirteen obese patients (11 females) were recruited from the Obesity Clinic at the University of Campinas. All patients were selected for bariatric surgery according to the National Institutes of Health Consensus Statement (11). The surgical technique used was always the Roux-in-Y gastric bypass (12). Patients were submitted to fMRI plus blood and CSF collection at the time of the surgery and after reduction of body mass. Inclusion criteria for patient selection were men and women between 18 and 60 years of age who met the above-mentioned criteria for surgery. Exclusion criteria for patient selection were diabetes, inflammatory or infectious disease, use of psychotropic or anti-inflammatory drugs, and history of substance addiction. In addition, eight lean control subjects were selected among students of the university. Control subjects were submitted to blood collection and fMRI. Control CSF was obtained from patients referred to the university for headache. Criteria for selection of both control groups were the same used for patients, except for a BMI <25. The fMRI studies were performed on a 1.5 GE MRI scanner (GE Healthcare, Waukesha, WI) in 12-h fasting subjects. The method for image acquisition was the same as previously described (13) except that a total of 500 images were acquired in 30 min. d-glucose (50 g) was ingested after 5 min. Leptin, insulin, and adiponectin were determined in sera using ELISA kits from Millipore (Billerica, MA). Tumor necrosis factor-α (TNF-α), IL-1β, IL-10, and IL-6 were determined in sera and CSF using ultra-sensitive ELISA kits from BD Biosciences (Bedford, MA) and Cayman Chemical (Ann Arbor, MI). Biochemical and cellular parameters in the blood and CSF were determined using automated methods from F. Hoffmann-La Roche (Basel, Switzerland) and Beckman Coulter (Brea, CA). Temporal clustering analysis (TCA) and spatial analysis were performed as described (13). The fcMRI analysis was performed as previously described (14,15) except that the seed (virtual label that defines the target area from or toward which connectivity is evaluated) was placed in the hypothalamus. Student t test was used for statistical analysis.
RESULTS
Patients were evaluated at the time of the surgery and 238 ± 11 days later, when body mass was reduced by 29 ± 4% (P < 0.05). Although most patients enrolled in the study were women, both male patients presented similar outcomes; therefore, we have no reason to suspect the data shown in this article represent female-specific phenomena. The nutritional habits of the patients were assessed according to the International Collaborative Study of Macro- and Micronutrients and Blood Pressure 24-h dietary recall (16). Total caloric intake decreased from 5,602 ± 3,391 to 803 ± 355 kCal/day (P < 0.05), and the consumption of saturated fats decreased from 33.6 ± 6.1 to 30.3 ± 11.6% of total caloric intake (P < 0.05). Of special interest, the relative consumption of saturated fats by obese patients was greater than that of lean control subjects on enrollment and reduced by 10.5% after surgery, becoming statistically similar to lean subjects. As expected, all the systemic parameters reflecting the metabolic and inflammatory status of the patients improved significantly after massive body mass loss (Table 1), reflecting the known impact of reduction of adiposity and caloric intake on subclinical inflammation and glucose/lipid homeostasis (17).
TABLE 1.
Blood metabolic and inflammatory parameters
| Lean |
Obese before surgery |
Obese after surgery |
|
|---|---|---|---|
| 6 females, 2 males | 11 females, 2 males | ||
| Age (years) | 29.5 ± 4 | 34.0 ± 10 | |
| BMI (kg/m2) | 20.9 ± 2.4 | 39.1 ± 1.9* | 28.1 ± 2.8*§ |
| WC (cm) | 72.2 ± 9.2 | 110.3 ± 9.9* | 89.7 ± 8.7*§ |
| HC (cm) | 91.3 ± 7.3 | 127.0 ± 5.2* | 105.0 ± 7.5*§ |
| Glucose (mg/dL) | 80.6 ± 3.1 | 84.3 ± 6.1 | 77.9 ± 7.3 |
| HbA1c (%) | 4.8 ± 0.2 | 5.2 ± 0.3 | 5.1 ± 0.3 |
| Insulin (pmol/L) | 25.0 ± 10.3 | 68.7 ± 38.1* | 21.5 ± 10.4§ |
| HOMA-IR | 0.7 ± 0.3 | 2.1 ± 1.2* | 0.6 ± 0.2§ |
| Cholesterol (mg/dL) | 189 ± 28 | 153 ± 24 | 141 ± 16 |
| HDL (mg/dL) | 74.5 ± 25.7 | 36.9 ± 5.8* | 52.6 ± 9.1*§ |
| LDL (mg/dL) | 99.5 ± 22.4 | 97.6 ± 26.4 | 73.6 ± 16.1 |
| Triglycerides (mg/dL) | 75.5 ± 38.9 | 94.1 ± 27.1 | 76.6 ± 19.9 |
| CRP (mg/dL) | 0.13 ± 0.02 | 0.91 ± 0.70* | 0.17 ± 0.02§ |
| ESR (mm/1 h) | 14 ± 12 | 26 ± 16* | 16 ± 9§ |
| Adiponectin (μg/mL) | 6.9 ± 1.7 | 2.7 ± 1.8* | 7.8 ± 1.6§ |
| Leptin (ng/mL) | 21.4 ± 6.2 | 36.8 ± 12.0* | 17.0 ± 13.9§ |
| TNF-α (pg/mL) | 5.8 ± 5.5 | 25.6 ± 10.3* | 12.4 ± 9.8§ |
| IL-1β (pg/mL) | 2.0 ± 1.8 | 42.9 ± 26.7* | 13.6 ± 4.3*§ |
| IL-6 (pg/mL) | 4.1 ± 4.2 | 26.3 ± 10.2* | 8.5 ± 6.4§ |
| IL-10 (pg/mL) | 14.0 ± 10.1 | 15.6 ± 16.5 | 15.8 ± 5.5 |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HC, hip circumference; HOMA-IR, homeostasis model assessment of insulin resistance; WC, waist circumference.
*P < 0.05 vs. lean.
§P < 0.05 vs. obese before surgery.
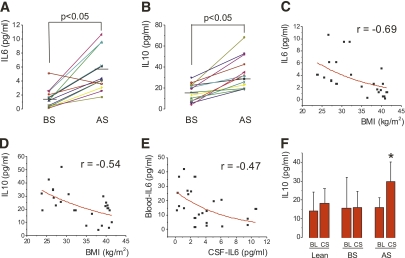
Body mass loss produced no effect on cellular and biochemical parameters in the CSF (Table 2). Two distinct ultra-sensitive ELISA methods were used to evaluate TNF-α and IL-1β in CSF, but these rendered no detectable levels in all samples evaluated. However, the CSF levels of IL-10 increased in all patients, and the CSF levels of IL-6 increased in all except one patient, leading to statistically significant increases of both these cytokines after body mass loss (Fig. 1A and B). Both IL-6 and IL-10 levels in the CSF were inversely correlated with BMI (Fig. 1C and D), and IL-6 levels in the CSF were inversely correlated with IL-6 in the blood (Fig. 1E). Compared with lean subjects, IL-10 levels in the CSF of obese subjects were similar before surgery (15.9 ± 8.6 vs. 18.1 ± 7.8 pg/mL for obese and lean subjects, respectively) and significantly higher after body mass loss (29.8 ± 10.4 pg/mL, P < 0.05). Conversely, IL-6 levels in the CSF of obese patients were significantly lower than in lean control subjects before surgery (1.6 ± 1.3 vs. 6.4 ± 5.3 pg/mL [P < 0.05] for obese and lean subjects, respectively), reaching similar levels after body mass loss (5.7 ± 2.8 pg/mL). In all groups, the CSF levels of IL-10 were similar or higher than the blood levels of this cytokine, suggesting that, at least in part, IL-10 was produced in the central nervous system (Fig. 1F). No significant differences in blood monocyte counts were detected among obese and lean subjects and in obese patients before and after surgery.
TABLE 2.
Cerebrospinal fluid cellular and biochemical parameters
| Lean | Obese before surgery | Obese after surgery | |
|---|---|---|---|
| Glucose (mg/dL) | 59.5 ± 9.6 | 52.9 ± 8.5 | 48.5 ± 4.4 |
| Protein (mg/dL) | 26.3 ± 6.8 | 26.6 ± 11.1 | 23.7 ± 6.8 |
| Leukocytes/mL | 1.6 ± 0.8 | 2.0 ± 1.5 | 2.5 ± 4.3 |
| Erythrocytes/mL | 18 ± 38 | 37 ± 79 | 44 ± 57 |
FIG. 1.
Inflammatory markers in the CSF. Levels of IL-6 (A) and IL-10 (B) were determined in the CSF of obese patients before and after bariatric surgery. Correlation between IL-6 (C) and IL-10 (D) concentrations in the CSF and BMI, and correlation between IL-6 concentrations in the CSF and blood (E) were obtained. The mean (±SD) levels of IL-10 in the blood and CSF were obtained for lean and obese subjects before and after surgery (F). N = 8 for lean subjects; N = 13 for obese subjects. F: *P < 0.05 vs. blood/after surgery. AS, after surgery; BL, blood; BS, before surgery; CS, cerebrospinal fluid.
Two distinct analytic methods were used to evaluate the impact of obesity and body mass loss on the activity of the brain: TCA and functional connectivity MRI (fcMRI). TCA allows for the identification of maximal response to a given stimulus in a combination of signal intensity and spatial extent (13), whereas fcMRI defines temporal correlations between remote neurophysiologic events, which are hemodynamic in nature when evaluated by fMRI (14,18).
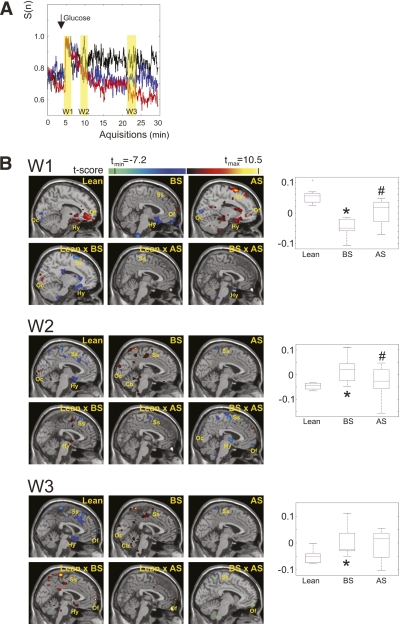
For TCA, a mathematic model converts a multiple-dimension data space into a relationship between the number of voxels, reaching maximum signal intensity, and the time (13). On a given time frame, the spatial mapping allows for the anatomic localization of the activity. We found that a first peak of activity occurred right after glucose intake (Fig. 2A), as previously reported (13). This peak was similar in lean and obese subjects both before and after surgery (Fig. 2A). After approximately 5 min of the glucose stimulus, lean subjects presented a second peak that was comparable to previously reported data (13). However, obese patients did not present such a peak, either before or after surgery (Fig. 2A). In addition, during the remaining 20 min of analysis, the number of voxels reaching maximum activity became progressively different among the groups. The highest activity was presented by lean subjects, whereas obese subjects before surgery presented the lowest activity and obese subjects after surgery presented intermediate activity (Fig. 2A). Spatial analysis was then performed at three distinct time windows, labeled in yellow in Fig. 2A. In the first window (W1), which included the first peak after glucose intake, maximum activity was detected in the hypothalamus and orbitofrontal cortex in all three conditions (lean subjects, obese subjects before surgery, and obese subjects after surgery), in the occipital cortex in lean and obese subjects after surgery, and in the somatosensory cortex in obese subjects both before and after surgery (Fig. 2B, W1). Comparisons between the groups revealed that lean and obese subjects before surgery presented different activities in the hypothalamus (inset graph, Fig. 2B, W1) and occipital and somatosensory cortices; lean and obese subjects after surgery were mostly similar with discrete differences in the somatosensory cortex; and obese subjects before and after surgery presented different activities in the hypothalamus (inset graph, Fig. 2B, W1). In the second window (W2), which included the second peak after glucose intake, lean subjects presented the highest activity in the hypothalamus and somatosensory and occipital cortices; obese subjects before surgery presented the highest activities in the somatosensory and occipital cortices and cerebellum; and obese subjects after surgery presented the highest activity in the somatosensory cortex (Fig. 2B, W2). At W2, the activities in the hypothalamus (inset graph, Fig. 2B, W2) and somatosensory cortex were different between lean and obese subjects before surgery. The comparison between lean and obese subjects after surgery showed a significant difference only in the somatosensory cortex; the comparison between obese subjects before and after surgery showed differences in the hypothalamus (inset graph, Fig. 2B, W2) and occipital, somatosensory, and orbitofrontal cortices. In the third window (W3), lean subjects presented the highest activities in the hypothalamus and orbitofrontal and somatosensory cortices; obese subjects before surgery presented the highest activities in the somatosensory and occipital cortices and in the cerebellum; and obese subjects after surgery presented the highest activity only in the somatosensory cortex (Fig. 2B, W3). Somatosensory and orbitofrontal cortices and the hypothalamus (inset graph, Fig. 2B, W3) presented different activities when lean subjects were compared with obese subjects before surgery. Only the orbitofrontal cortex was different between lean and obese subjects after surgery, and somatosensory and orbitofrontal cortices were different between obese subjects before and after surgery (Fig. 2B, W3).
FIG. 2.
TCA of the human brain after glucose intake. A: Time course of the activation depicted as the means of all analyzed subjects in the respective groups (black, lean; red, obese before surgery; blue, obese after surgery); W1–W3 represent the time windows selected for spatial analysis. B: Spatial mapping of the brain activity at each of the time windows (W1–W3); individual group analyses were performed for lean and obese subjects before and after surgery; comparisons were also performed for all pairs of groups. The inset graphs on the right represent the signal intensity in the region of the hypothalamus for each group. N = 8 for lean subjects; N = 13 for obese subjects. A pixel clustering size of 5 and a t-threshold of |t| >2.1 were chosen to afford a P < 0.01 level of statistical significance of the detected signal changes. This is represented by the different colors, as defined by the normalized color bars. Color bars indicate t value for one-sample t test (lean, BS, and AS) and two-sample t test (lean vs. BS, lean vs. AS, and BS vs. AS). In the insets in B, *P < 0.01 vs. lean and #P < 0.01 vs. before surgery. AS, after surgery; BS, before surgery; Cb, cerebellum; Hy, hypothalamus; Oc, occipital cortex; Of, orbitofrontal cortex; Ss, somatosensory cortex. (A high-quality digital representation of this figure is available in the online issue.)
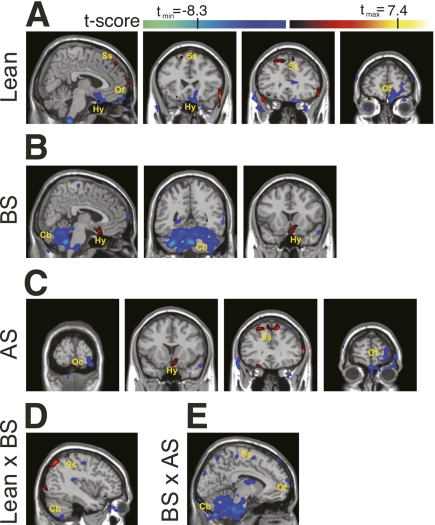
At both resting state and after a stimulus, synchronized fluctuations of blood oxygen levels dependent on function in fMRI signals in remote brain areas reflect physiologic or pathologic patterns in a neuronal network (14,15,18). The measurements of such events, which are the basis of fcMRI, provide maps of connectivity that indicate integration and segregation of brain information. Here, a seed was placed on the hypothalamus to explore the connectivity of this anatomic region with other brain areas. In lean subjects, the hypothalamus presented the highest level of functional connectivity with the orbitofrontal and somatosensory cortices (Fig. 3A). In obese subjects before surgery, functional connectivity was detected between the hypothalamus and the orbitofrontal cortex and cerebellum (Fig. 3B). After surgery, functional connectivity of obese patients was highest between the hypothalamus and the orbitofrontal, somatosensory, and occipital cortices (Fig. 3C). Intergroup comparisons revealed the greatest difference in functional connectivity between lean and obese subjects before surgery, particularly in the regions of the cerebellum and somatosensory cortex (Fig. 3D). Functional connectivity was mostly similar between lean and obese patients after surgery (not shown). Finally, some differences in functional connectivity between obese subjects before and after surgery were detected in the cerebellum, somatosensory, and orbitofrontal cortices (Fig. 3E).
FIG. 3.
Functional connectivity maps. A seed was placed in the hypothalamus, and intragroup (A–C) and intergroup (D and E) analyses were performed. A: Connectivity maps of lean subjects are depicted. B: Connectivity maps of obese patients before surgery are depicted. C: Connectivity maps of obese patients after surgery are depicted. D: Intergroup analysis between lean subjects and obese patients before surgery (lean × BS) is depicted. E: Intergroup analysis between obese patients before surgery and obese patients after surgery (BS × AS) is depicted. Color bars represent t values. For intragroup analysis (A–C), hot colors indicate the common level of correlation and cold colors indicate the common level of anticorrelation with the time course provided by the seed within each group. For intergroup analysis (D and E), hot colors indicate the level of differences between two groups toward lean and obese patients before surgery, respectively, and cold colors indicate the level of differences between two groups toward obese patients before and after surgery, respectively. AS, after surgery; BS, before surgery; Cb, cerebellum; Hy, hypothalamus; Oc, occipital cortex; Of, orbitofrontal cortex; Ss, somatosensory cortex. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
Both TCA and fcMRI analyses showed distinct patterns of functionality between obese and lean subjects. The differences were mostly confined to a few anatomic regions, predominating in the hypothalamus and somatosensory and orbitofrontal cortices. The hypothalamus harbors hormone and nutrient-sensing neurons and provides the integration for the energy intake and expenditure responses (19). Defective leptin and insulin signaling in this region provides the basis for experimental obesity (5,19). The somatosensory and orbitofrontal cortices integrate feeding cues with gustatory activity and facial movements, respectively. The activities of these regions have been shown to be modulated after a meal or glucose ingestion (13,20,21), and data obtained from experimental animals reinforce their role in the control of feeding and energy homeostasis (5,19). The massive loss of body mass obtained after surgery produced obvious changes in the functionality of the brain. By either analytic method, it is clear that changes occurred toward the patterns found in lean subjects. This is particularly evident in the TCA spatial analysis, which showed only minor differences when lean subjects were compared with obese subjects after surgery (Fig. 2B, W1, W2, and W3 – Lean × AS). Another remarkable finding was the increased levels of IL-10 and IL-6 in the CSF of obese subjects after surgery. In a recent study, increased levels of both these cytokines were shown to play a role in the reduction of hypothalamic resistance to leptin after physical activity (22). Although inflammatory cytokines were not detected in the current study, we believe that advancement in measurement methods will allow quantification of these cytokines and a comparison with the findings in animal models, which show reductions in both TNF-α and IL-1β after body mass loss (3,4).
The human brain activity in response to glucose ingestion is modified by obesity, and body mass loss reestablishes some of the parameters. We cannot be sure if a longer period of time elapsed from the surgery or whether the complete restoration of body mass to levels similar to those of lean control subjects would completely correct the dysfunction. As observed in experimental obesity, neuronal apoptosis in the hypothalamus can affect distinct cellular subpopulations, leading to a defective response to hormonal and nutritional inputs (7). Should a similar phenomenon occur in humans, a complete restoration of the functional activity may not be achieved. Nevertheless, the increase in the anti-inflammatory activity detected in the CSF of obese patients after the loss of body mass is remarkable. In addition to the well-known anti-inflammatory activity of IL-10, the inhibition of neuronal degeneration through the reduction of apoptosis has been reported (23). Thus, we can hypothesize that body mass loss or other conditions that increase IL-10 in the brain may reduce neuronal damage resulting in the restoration of functionality. Moreover, these findings place IL-10 and perhaps IL-6 as attractive therapeutic agents for obesity, as recently suggested (22).
In conclusion, reduction of body mass in obese humans increases the anti-inflammatory activity in the CSF and partially corrects the dysfunctional activity in response to glucose in selected brain areas. These data suggest that obesity and body mass loss affect the human brain in a manner similar to the animal models for this disease.
ACKNOWLEDGMENTS
This work was supported by grants from the Fundação de Amparo a Pesquisa do Estado de Sao Paulo. The Laboratory of Cell Signaling belongs to the Instituto Nacional de Ciência e Tecnologia–Obesidade e Diabetes. F.R.S.P., P.T.F., L.M.L., and F.C. are affiliated with the Cooperação Interinstitucional de Apoio a Pesquisas sobre o Cerebro.
No potential conflicts of interest relevant to this article were reported.
S.v.d.S.-L. performed patient care and collected clinical and nutritional data. F.R.S.P. performed neuroimaging studies. D.E.C. performed patient care and collected clinical and nutritional data. P.T.F. performed neuroimaging studies. A.R.C. and C.R.G. collected and evaluated data. E.A.C., J.C.P., and B.G. performed surgery and the metabolic studies. L.M.L. and F.C. performed neuroimaging studies. L.A.V. conceived, organized, and wrote the article.
The authors thank Dr. N. Conran, University of Campinas, for editing the English grammar.
REFERENCES
- 1.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437 [DOI] [PubMed] [Google Scholar]
- 2.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887 [DOI] [PubMed] [Google Scholar]
- 3.De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005;146:4192–4199 [DOI] [PubMed] [Google Scholar]
- 4.Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 2009;29:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velloso LA, Araújo EP, de Souza CT. Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation 2008;15:189–193 [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Hotamisligil GS. Stressing the brain, fattening the body. Cell 2008;135:20–22 [DOI] [PubMed] [Google Scholar]
- 7.Moraes JC, Coope A, Morari J, et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS ONE 2009;4:e5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 2010;151:4109–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 1992;55(Suppl.):615S–619S [DOI] [PubMed] [Google Scholar]
- 12.de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 2009;19:313–320 [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Gao JH, Liu HL, Fox PT. The temporal response of the brain after eating revealed by functional MRI. Nature 2000;405:1058–1062 [DOI] [PubMed] [Google Scholar]
- 14.Pereira FR, Alessio A, Sercheli MS, et al. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci 2010;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed 1997;10:165–170 [DOI] [PubMed] [Google Scholar]
- 16.Robertson C, Conway R, Dennis B, Yarnell J, Stamler J, Elliott P. Attainment of precision in implementation of 24 h dietary recalls: INTERMAP UK. Br J Nutr 2005;94:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289:1799–1804 [DOI] [PubMed] [Google Scholar]
- 18.Colonnese MT, Phillips MA, Constantine-Paton M, Kaila K, Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci 2008;11:72–79 [DOI] [PubMed] [Google Scholar]
- 19.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 2006;443:289–295 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, Liu Y, Mahankali S, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999;48:1801–1806 [DOI] [PubMed] [Google Scholar]
- 21.Führer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–950 [DOI] [PubMed] [Google Scholar]
- 22.Ropelle ER, Flores MB, Cintra DE, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol 2010;8:e1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Bachis A, Colangelo AM, Vicini S, et al. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci 2001;21:3104–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]