Abstract
OBJECTIVE
Autophagy is a critical cellular system for removal of aggregated proteins and damaged organelles. Although dysregulated autophagy is implicated in the development of heart failure, the role of autophagy in the development of diabetic cardiomyopathy has not been studied. We investigated whether chronic activation of the AMP-activated protein kinase (AMPK) by metformin restores cardiac function and cardiomyocyte autophagy in OVE26 diabetic mice.
RESEARCH DESIGN AND METHODS
OVE26 mice and cardiac-specific AMPK dominant negative transgenic (DN)-AMPK diabetic mice were treated with metformin or vehicle for 4 months, and cardiac autophagy, cardiac functions, and cardiomyocyte apoptosis were monitored.
RESULTS
Compared with control mice, diabetic OVE26 mice exhibited a significant reduction of AMPK activity in parallel with reduced cardiomyocyte autophagy and cardiac dysfunction in vivo and in isolated hearts. Furthermore, diabetic OVE26 mouse hearts exhibited aggregation of chaotically distributed mitochondria between poorly organized myofibrils and increased polyubiquitinated protein and apoptosis. Inhibition of AMPK by overexpression of a cardiac-specific DN-AMPK gene reduced cardiomyocyte autophagy, exacerbated cardiac dysfunctions, and increased mortality in diabetic mice. Finally, chronic metformin therapy significantly enhanced autophagic activity and preserved cardiac functions in diabetic OVE26 mice but not in DN-AMPK diabetic mice.
CONCLUSIONS
Decreased AMPK activity and subsequent reduction in cardiac autophagy are important events in the development of diabetic cardiomyopathy. Chronic AMPK activation by metformin prevents cardiomyopathy by upregulating autophagy activity in diabetic OVE26 mice. Thus, stimulation of AMPK may represent a novel approach to treat diabetic cardiomyopathy.
Autophagy is a physiologic process whereby cytoplasmic components, including long-lived proteins and organelles, are engulfed by a double-membrane structure and targeted for destruction in lysosomes (1). It selectively removes damaged mitochondria as a cytoprotective mechanism for limiting mitochondria-derived oxidative stress and preventing apoptosis (2,3). A low level of constitutive autophagy is important in the heart for maintaining normal cellular function and the quality of proteins and organelles. Defects in this process cause cardiac dysfunction and heart failure, particularly when cellular stress is increased (4). Although autophagy is implicated in various pathologic conditions, including cardiac hypertrophy, cardiomyopathy, and heart failure, there is little information on the pathophysiologic roles of autophagy in the pathogenesis of diabetic cardiomyopathy.
Metformin, one of the most commonly prescribed antidiabetic drugs, improves cardiac function and reduces the incidence of myocardial infarction in type 2 diabetic patients (5,6). The UK Prospective Diabetes Study reported that metformin was more effective than sulfonylureas or insulin in reducing all-cause mortality and diabetes-related end points in diabetic patients, even though these agents decreased HbA1c by comparable magnitudes. These findings suggest that metformin provides cardiovascular protection independent of its hypoglycemic effects (7).
Indeed, metformin ameliorates cardiac dysfunctions induced by global ischemia, without affecting blood glucose in nondiabetic animals (8,9), by activating the AMP-activated protein kinase (AMPK) (10,11). AMPK acts as a sensor of cellular energy status and controls several cellular functions in the cardiovascular system, including protein synthesis (12,13), apoptosis (14–16), and autophagy (17,18) in physiologic and pathologic conditions, such as hemodynamic stress (12,13), myocardial ischemia, and reperfusion injury (16,19,20). However, the roles and molecular mechanisms by which AMPK regulates diabetic cardiomyopathy remain to be established.
Diabetic cardiomyopathy, which develops in diabetic patients in the absence of coronary artery disease or hypertension (21–24), is a major cause of heart failure in diabetic patients. It is characterized by reduced cardiomyocyte contractility, cardiac apoptosis, mitochondrial pathology, and dysfunction (25,26). Despite the importance of this complication, the underlying mechanisms of diabetic cardiomyopathy are still poorly understood. Thus, this study was designed to test whether decreased autophagy is associated with the development of cardiomyopathy in diabetic OVE26 mice and to evaluate whether metformin improves cardiac function by modulating autophagic activity in diabetes.
RESEARCH DESIGN AND METHODS
Animals.
Male OVE26 mice on a Friend virus B (FVB) background and control FVB mice purchased from Jackson Laboratory (Bar Harbor, ME) were used for the experiment at 6 months of age. To study the role of AMPK, one group of OVE26 mice was treated with metformin (200 mg/kg per day, in drinking water) for 4 months. In addition, cardiac-specific transgenic mice overexpressing a dominant negative (DN) α2 subunit (D157A) of AMPK (DN-AMPKα2, a gift of Rong Tian, University of Washington, Seattle, WA), aged 8 weeks old, were rendered diabetic by five consecutive daily injections of streptozotocin (STZ, 50 mg/kg i.p.), whereas control mice were injected with vehicle (citrate buffer, pH 4.5). In separate groups of wild-type (WT) diabetic and DN-AMPKα2 diabetic mice, metformin was administrated in drinking water (200 mg/kg per day) for 4 months.
HbA1c was measured by a commercial cartridge, using the principle of column chromatography, manufactured by Cholestech GDX A1C System (Bio-Rad Laboratories, Hertfordshire, U.K.). Blood glucose and arterial blood pressure were measured as described previously (27,28). Mice with blood glucose >350 mg/dL were considered diabetic. All animal protocols were reviewed and approved by the University of Oklahoma Institute Animal Care and Use Committee.
Materials.
The S-adenosylmethionine peptide was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Antibodies against phospho-AMPK (Thr172), AMPK-α, phospho-mammalian target of rapamycin (mTOR; Ser2448), mTOR, phospho-Raptor (Ser792), Raptor, phospho-4E-BP1 (Thr37/46), phospho-p70 S6 kinase (Thr389), S6K, LC3, Beclin1, and ubiquitin were purchased from Cell Signaling (Danvers, MA). Antibodies against phospho-mTOR (Thr2446) and phospho-Raptor (Ser722) were from Millipore (Billerica, MA). Anti-Raptor antibody was purchased form Abcam (Cambridge, MA). All other chemicals and organic solvents were obtained from Sigma-Aldrich (St. Louis, MO).
Echocardiography.
Transthoracic two-dimensional M-mode echocardiogram and pulsed wave Doppler spectral tracings were obtained using a Sequioa-512 Ultrasound System (Siemens AG, Munich, Germany) with a 15-MHz transducer in mice anesthetized using a mixture of 1.5% isoflurane and 0.5 L/min oxygen. Color Doppler was used to show accurately the mitral valve inflow and to obtain a sharper signal from the early ventricular filling peak velocity (E wave) and late filling velocity (A wave). M-mode tracings were used to measure left ventricular (LV) wall thickness, LV end-systolic diameter (LVESD), and LV end-diastolic diameter (LVEDD). Percentage of fractional shortening was calculated as described previously (29). All examinations were performed by the same personnel.
Langendorff perfusion analysis.
LV function was measured using isolated buffer-perfused heart preparation as described previously (28,30). In brief, isolated hearts were perfused retrograde using normothermic Krebs-Henseleit buffer (118 mol/L NaCl, 25 mol/L NaHCO3, 4.7 mol/L KCl, 1.2 mol/L KH2PO4, 1.2 mol/L MgSO4, 12 mol/L glucose, and 1.9 mol/L CaCl2) at a constant perfusion pressure of 70 mmHg and paced at 7 Hz. The Krebs-Henseleit buffer was gassed continuously with 95% O2 and 5% CO2. A small polyvinyl chloride fluid-filled balloon attached to polyethylene tubing was placed in the LV via the left atrium and connected to a pressure transducer for determination of LV pressures. The balloon was progressively filled in 5-µL increments to generate LV filling and functional curves.
Detection of apoptosis by transferase-mediated dUTP nick end–labeling staining.
To estimate apoptosis, terminal deoxynucleotidyl transferase-mediated dUTP nick end–labeling (TUNEL) staining, followed by DAPI staining, was carried out on 4-µm-thick paraffin–embedded sections using a cell death detection assay kit as specified by the manufacturer’s instructions (Roche Applied Science, Indianapolis, IN). TUNEL-positive nuclei within the cardiomyocytes were counted. The total number of nuclei per unit area of the heart was estimated by counting the number of DAPI-stained nuclei under ultraviolet illumination. The number of apoptotic cardiomyocyte nuclei in 15 fields was averaged, and the data were calculated as the percentage of apoptotic myocyte nuclei/total number of nuclei.
Evaluation of autophagic vacuole by electron microscopy.
Hearts were retrograde perfused using PBS and 2% glutaraldehyde in 0.1 mol/L cacodylate buffer. Postfixation was performed using 2% osmium tetroxide in 0.1 mol/L cacodylate buffer and 1% aqueous uranyl acetate, each for 1 h. Next, the hearts were conventionally prepared for transmission electron microscopy (31). A Hitachi H-7600 Transmission Electron Microscope (Pleasanton, CA) equipped with a digital camera was used to image the sections. Ultrastructural studies were performed to probe for double membrane-bound autophagic vacuoles, a long-established analytic gold standard for autophagy. The alternations of myofibril and mitochondrial ultrastructure were determined from electron micrographs (original magnification ×4,000).
AMPK activity assay.
AMPK activity was measured in ∼20 mg of cardiac tissue, as described previously (10), using the S-adenosylmethionine peptide as a substrate. AMPK activity was expressed as picomoles of phosphate incorporated per milligram of muscle protein subjected to immunoprecipitation per minute.
Immunohistochemistry and Western analysis.
Immunohistochemistry and Western blots were performed using the specific antibodies, as described previously (27,32). The intensities (density × area) of individual bands were measured by densitometry (Model GS-700, Imaging Densitometer; Bio-Rad, Hercules, CA).
Statistical analysis.
Values are presented as mean ± SEM. Differences between experimental groups were determined by one-way or two-way ANOVA, followed by the Bonferroni post hoc test, as appropriate. An unpaired Student t test was performed for single comparisons between groups. P < 0.05 was considered statistically significant.
RESULTS
Inhibition of AMPKα2 reduces myocardium autophagy, aggravates cardiac dysfunctions, and increases mortality of diabetic mice.
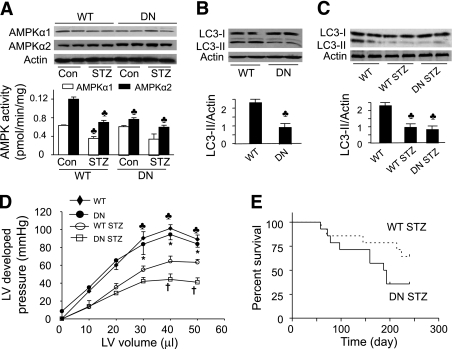
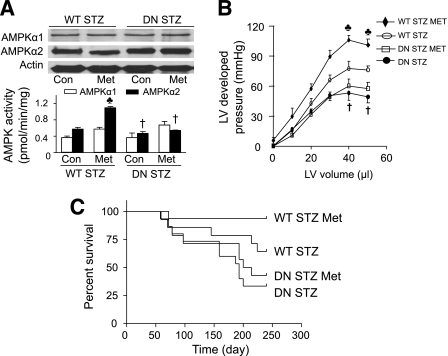
AMPK plays a critical role in glucose deprivation and ischemia-induced autophagy in cardiomyocytes (18). To examine the regulatory role of AMPK in autophagy in the heart, we measured AMPK activity and LC3-II levels in cardiac tissues collected from WT and DN-AMPKα2 mice (33). The expression of AMPKα2 was significantly increased in DN-AMPKα2 transgenic mice relative to WT mice; however, the amount of AMPKα1 was unchanged in the DN-AMPKα2 hearts (Fig. 1A). Overexpression of DN-AMPKα2 reduced AMPKα2 activity but did not affect AMPKα1 activity. STZ-induced diabetes reduced AMPKα1 and AMPKα2 activity in the WT hearts but not in the DN-AMPKα2 hearts (Fig. 1A). Although DN-AMPKα2 transgenic mice have a normal cardiac phenotype, their hearts exhibited lower LC3-II levels (Fig. 1B), indicating a reduced autophagy in the hearts from DN-AMPKα2 transgenic mice.
FIG. 1.
Inhibition of AMPK by overexpression of DN-AMPKα2 reduces autophagy in transgenic mice, aggravates diabetic cardiomyopathy, and increases mortality in STZ mice. A: AMPK expression and activity in cardiac tissues from WT and DN-AMPKα2 (DN) transgenic mice treated with/without STZ were detected as described in research design and methods. ♣P < 0.05 vs. WT control (Con; n = 5–6 in each group). B and C: Western blot analyses of heart homogenates with anti-LC3 antibody. Densitometric analysis of LC3-II levels is shown by the bar graph. Results shown are mean ± SEM. ♣P < 0.05 vs. FVB (n = 4 in each group). D: Langendorff perfusion analysis of the relationship between LV-developed pressure and volume in WT, DN-AMPKα2 (DN), WT STZ, DN STZ mice 6 months after induction of diabetes. ♣P < 0.05, WT vs. WT STZ, or DN STZ; *P < 0.05, DN vs. WT STZ or DN STZ; †P < 0.05 DN STZ vs. WT STZ (n = 4–5 in each group). E: Survival plots for WT STZ and DN STZ animals (n = 14 in each group).
We next evaluated the effect of reduced autophagy on the development of diabetic cardiomyopathy in DN-AMPKα2 transgenic mice, in which AMPK activity is inhibited (Fig. 1A). STZ was used to render 8-week-old WT and DN-AMPKα2 diabetic mice. Six months after diabetes induction, the STZ-injected mice had higher serum glucose levels than their nondiabetic control subjects (WT: 488 ± 25 vs. 119 ± 7 mg/dL, P < 0.001, n = 11; DN-AMPKα2: 493 ± 27 vs. 129 ± 11 mg/dL, P < 0.001, n = 9). Diabetes reduced LC3-II levels in WT mice but did not further decrease LC3-II levels in DN-AMPK mice (Fig. 1C).
LV systolic function was assessed from the maximal LV-developed pressure, measured over a range of volumes. LV-developed pressures were depressed in both diabetic groups after 6 months of diabetes. However, LV-developed pressures in DN-AMPKα2-STZ mice were lower than those of WT STZ mice (Fig. 1D). Diabetes increased mortality in DN-AMPKα2 mice (Fig. 1E), such that only 35.7% survived at 8 months after STZ induction versus 64.2% in WT mice with diabetes (P = 0.0581). These findings suggest that AMPK deficiency in hearts reduced autophagy and cardiac function in diabetic mice.
Reduction of AMPK activity is associated with autophagy defection and cardiomyopathy in OVE26 mice.
STZ is reported to have direct cardiac toxicity (34). To exclude the toxic effect of STZ in hearts, we investigated AMPK activity, autophagy, and cardiac function in OVE26 mice, an established model of type 1 diabetes. As described previously (35), the OVE26 mice exhibited severe hyperglycemia and developed cardiac abnormalities at 5 to 6 months of age. Of note, neither the transgene nor diabetes increased the blood pressure in OVE26 mice compared with FVB mice, whereas the lung wet weight/dry weight ratio was significantly increased (16%) in OVE26 mice (Table 1), suggesting impaired cardiac performance in the diabetic animals. Moreover, chronic administration of metformin diminished diabetes-enhanced fluid retention in the lung.
TABLE 1.
General features of the mice
| Variable | FVB | OVE26 | OVE26/metformin |
|---|---|---|---|
| n | 10 | 8 | 6 |
| Age (month) | 6.2 ± 0.1 | 6.1 ± 0.1 | 5.9 ± 0.3 |
| Blood glucose (mg/dL) | 139.4 ± 11.2 | 420.4 ± 22.7* | 450.8 ± 10.3* |
| HbA1c (%) | 6.2 ± 0.4 | 9.3 ± 0.2* | 8.7 ± 0.3* |
| Plasma insulin (ng/mL) | 2.9 ± 0.6 | 1.2 ± 0.3* | 1.1 ± 0.3* |
| Body weight (g) | 31.0 ± 0.3 | 23.3 ± 1.1* | 25.5 ± 3.1* |
| Heart weight (mg) | 146.5 ± 3.3 | 109.6 ± 5.1* | 119.1 ± 8.9* |
| Heart weight/body weight (mg/g) | 4.8 ± 0.1 | 4.8 ± 0.1 | 4.8 ± 0.3 |
| Blood pressure (mmHg) | 118.0 ± 3.0 | 116.0 ± 6.0 | 125.0 ± 9.0 |
| Lung weight/dry weight ratio (g/g) | 4.7 ± 0.1 | 5.3 ± 0.1* | 4.8 ± 0.1† |
*P < 0.05 vs. FVB;
†P < 0.05 vs. OVE26.
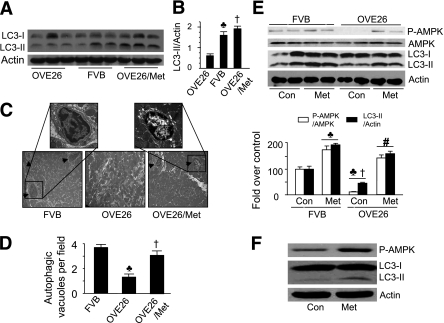
To assess the role of autophagy in diabetic cardiomyopathy, we measured cardiac LC3-II levels with an LC3-specific antibody after 6 months of diabetes. Western blots revealed a decrease in LC3-II levels in the hearts from OVE26 mice versus FVB controls. Remarkably, activation of AMPK with metformin abrogated the reduction of LC3-II levels in diabetic hearts (Fig. 2A and B). Electron micrographic analysis of ventricular tissue double membrane–bound autophagic vesicles confirmed the reduction of autophagy in diabetic hearts. Some autophagosomes could be identified in hearts of FVB controls but were rarely detected in OVE26 mice, suggesting a defect in autophagy. Chronic administration of metformin prevented the decrease in autophagosomes in diabetic hearts (Fig. 2C and D). To determine the acute effect of metformin on autophagy, we treated 6-month-old FVB and OVE26 mice with metformin (200 mg/kg per day) by intraperitoneal injection. At 24 h after the treatment, metformin increased AMPK activity and autophagy capacity in FVB and OVE26 mice (Fig. 2E). In an in vitro experiment, incubation of HL-1 cells, a cardiomyocyte-derived cell line (36–38), with metformin for 16 h increased AMPK phosphorylation and LC3-II levels (Fig. 2F).
FIG. 2.
Metformin (Met) restores autophagy in diabetic hearts. A: Immunoblot analysis of heart homogenates using an anti-LC3 antibody. B: Quantitative analysis of LC3-II levels (n = 6 in each group). ♣P < 0.05, FVB vs. OVE26; †P < 0.05, OVE26/Met vs. OVE26. C: Representative electron micrographs from cardiac tissues of FVB, OVE26, and metformin-treated OVE26 mice. The arrowheads indicate an autophagic vacuole (original magnification of ×1,000). D: Autophagic vacuoles were counted from five to six randomly selected fields. Values represent mean ± SEM (n = 6). ♣P < 0.05 vs. FVB; †P < 0.05 vs. OVE26. Note: There were fewer autophagosomes in OVE26 mice. E: FVB and OVE26 mice were injected with metformin (200 mg/kg per day i.p.) for 24 h, and AMPK phosphorylation and LC3-II levels were detected by Western blotting and quantified to FVB control (Con). ♣P < 0.05 vs. FVB Con; †P < 0.05 vs. FVB Met; #P < 0.05 vs. OVE26 Con (n = 6 in each group). F: Western analyses of phosphorylation of AMPK and LC3-II levels in HL-1 cells treated with metformin (2 mmol/L) for 24 h. (A high-quality digital representation of this figure is available in the online issue.)
Metformin activates AMPK and improves cardiac function in OVE26 mice.
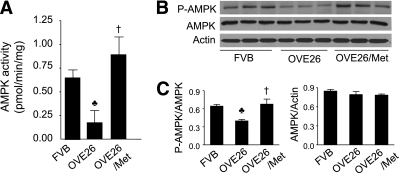
We further determined whether chronic activation of AMPK with metformin improved cardiac function in OVE26 mice. Compared with FVB controls, AMPK activity was dramatically decreased in OVE26 mice (Fig. 3A). Consistent with the downregulation in AMPK activity, diabetes also reduced AMPK phosphorylation at threonine 172 (Fig. 3B and C). Metformin treatment prevented the decrease in AMPK phosphorylation and activity (Fig. 3A–C), alleviated fluid retention in diabetic lungs (Table 1), and completely restored to normal the cardiac dysfunction in OVE26 mice (Fig. 4).
FIG. 3.
Metformin (Met) increases the phosphorylation and activity of AMPK in diabetic hearts. A: AMPK activity in cardiac tissues from FVB, OVE26 mice, and metformin-treated OVE26 mice was detected as described in research design and methods. ♣P < 0.05 vs. FVB; †P < 0.05 vs. OVE26 (n = 5–6 in each group). B: Representative blots of phosphorylation of AMPK at threonine 172 and total AMPK. C: Densitometric analysis of expression of phospho-AMPK and total AMPK. ♣P < 0.05 vs. FVB; †P < 0.05 vs. OVE26 (n = 5–6 in each group).
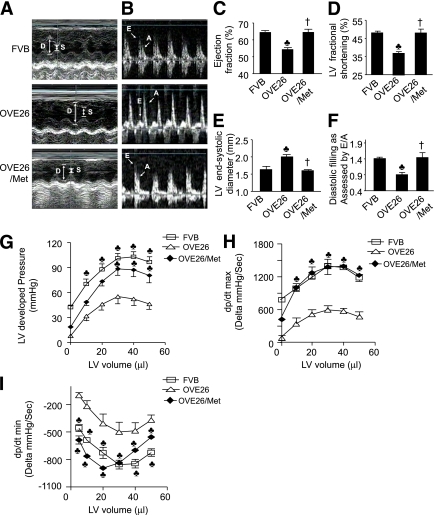
FIG. 4.
Metformin (Met) prevents cardiac dysfunction in diabetic mice. A–F: Echocardiographic assessment of cardiac function as described in research design and methods. All measurements were determined in a short-axis view at the level of the papillary muscles. Representative images of M-mode echocardiography (A) and mitral valvular inflows show E wave and A wave (B). C: Ejection fraction. D: Percentage of fractional shortage as LV contractile function. E: LV end-systolic diameter. F: Diastolic filling as assessed by E/A ratio (E wave: LV early-filling wave; A wave: filling from atrial contraction). Values represent mean ± SEM. FVB, n = 11; OVE26, n = 8; metformin-treated OVE26, n = 6. ♣P < 0.05 vs. FVB; †P < 0.05 vs. OVE26. G: Analysis of LV-developed pressure vs. volume. The developed pressure was depressed in OVE26 mice, which was prevented by metformin. Metformin improved LV dp/dtmax (H) and dp/dtmin (I) in OVE26 mice. Results shown are mean ± SEM. ♣P < 0.05 vs. OVE26. FVB, n = 6; OVE26, n = 5; metformin-treated OVE26 mice, n = 5. (A high-quality color representation of this figure is available in the online issue.)
Metformin prevents the cardiomyopathy that otherwise develops in diabetic mice.
Autophagy plays an important role in the heart. Under normal or mild stress conditions, it degrades and recycles cytoplasmic components and selectively removes damaged mitochondria as a cytoprotective mechanism (3). We have investigated the relationship of metformin on cardiac autophagy and the development of diabetic cardiomyopathy. Echocardiographic analyses demonstrated that OVE26 mice developed severe cardiomyopathy at 6 months of age, as evidenced by a significant increase in LVESD (Fig. 4E) and LVEDD (Fig. 4A) and a significant decrease in ejection fraction (Fig. 4C). Metformin treatment restored to normal the ejection fraction (Fig. 4C), LVESD (Fig. 4E), and LVEDD (Fig. 4A) in diabetic mice. Fractional shortening, an estimate of cardiac systolic function, was significantly decreased in OVE26 mice (37 ± 4 vs. 48 ± 4% in FVB mice; Fig. 4D). Metformin therapy completely corrected the impairment in fractional shortening in OVE26 mice (Fig. 4D). To assess LV diastolic function, we measured Doppler flow velocities across the mitral valves during the diastolic phase. The E/A velocity ratio, an estimate of LV diastolic function, was significantly decreased in OVE26 mice, whereas metformin treatment prevented the LV diastolic dysfunctions (Fig. 4B and F).
Langendorff perfusion analyses corroborated the cardioprotection by metformin in diabetic mice. LV systolic and diastolic functions were assessed from the maximal LV-developed pressure, the calculated dp/dtmax (maximal change in pressure per unit time), and the dp/dt minimum (dp/dtmin). OVE26 mice exhibited a significant reduction in LV-developed pressure (Fig. 4G) and dp/dtmax (Fig. 4H). Chronic metformin treatment restored to normal LV-developed pressure and dp/dtmax (Fig. 4G and H). The dp/dtmin measurement is generally considered to reflect LV diastolic function, which is impaired in OVE26 mice, whereas metformin treatment restored cardiac diastolic function (Fig. 4I).
Overexpression of DN-AMPK abolishes the protective effects of metformin.
To evaluate role of AMPK in cardioprotection of metformin, WT and DN-AMPKα2 diabetic mice were treated with metformin for 4 months after diabetes induction. Neither STZ nor metformin affected AMPK expression. Metformin treatment enhanced AMPK activity in WT STZ mice but not in DN STZ mice (Fig. 5A). Chronic treatment with metformin improved cardiac function in STZ-induced diabetic WT mice; however, the salutary effects of metformin on cardiac function were abrogated in DN-AMPKα2-STZ mice (Fig. 5B). In addition, metformin reduced mortality in WT STZ mice, and this effect was prevented in DN STZ mice (Fig. 5C). These results suggest that activation of AMPK is essential for metformin to impart its cardioprotection in diabetes.
FIG. 5.
Metformin (Met) improves cardiac function and survival rate in an AMPK-dependent manner. A: AMPK expression and activity in cardiac tissues from the animals were detected as described in research design and methods. ♣P < 0.05 vs. WT STZ control (Con), †P < 0.05 vs. WT STZ Met (n = 5–6 in each group). B: Langendorff perfusion analysis of the relationship between LV-developed pressure and volume in WT STZ, DN STZ, WT STZ Met, and DN STZ Met mice 6 months after induction of diabetes. ♣P < 0.05, WT STZ Met vs. WT STZ, DN STZ Met, or DN STZ; †P < 0.05 DN STZ vs. WT STZ (n = 4–5 in each group). C: Survival plots for WT STZ, DN STZ, WT STZ Met, and DN STZ Met animals (n = 14–16 in each group).
Metformin prevents the decrease in Beclin1 protein levels in diabetic hearts.
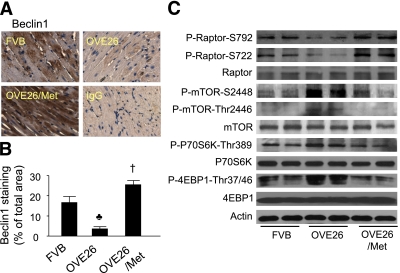
Beclin1 has a key role in autophagy because it is involved in the formation of autophagosomes (39). Beclin1 expression is increased in hibernating myocardium (40) and during reperfusion, where increased autophagy was documented (18). To examine the role of Beclin1 in diabetic cardiomyopathy, we measured and found reduced Beclin1 protein expression in hearts of OVE26 mice at age 6 months (Fig. 6A and B). It is noteworthy that metformin dramatically enhanced Beclin1 protein expression in OVE26 mice (Fig. 6A and B).
FIG. 6.
Metformin (Met) increases the expression of Beclin1 and inhibits mTOR signaling in the heart. A: Immunohistochemical analysis of Beclin1 in the hearts from FVB, OVE26, and metformin-treated OVE26 mice. Nonspecific rabbit IgG was used as a negative control. B: Quantitative analysis of Beclin1-stained areas is shown by the bar graph. Results shown are mean ± SEM. ♣P < 0.05 vs. FVB; †P < 0.05 vs. OVE26 (n = 4 in each group). C: Western analysis of the mTOR signaling pathway in cardiac tissues from FVB, OVE26, and metformin-treated OVE26 mice. (A high-quality digital representation of this figure is available in the online issue.)
Metformin inhibits tuberous sclerosis complex-mTOR pathway in diabetic hearts.
Previous studies suggest that AMPK negatively regulates mTOR activity (41) and plays an important role in mediating starvation-induced autophagy (42). Thus, we examined whether metformin inhibits the tuberous sclerosis complex (TSC)-mTOR pathway. Diabetic hearts exhibited activated TSC-mTOR signaling pathway, as reflected by decreased phosphorylation of Raptor at both Ser722 and Ser792, as well as increased phosphorylation of mTOR at both Ser2448 and Thr2446 and its downstream effectors, including 4E-binding protein 1 (4EBP1) and p70 ribosomal protein S6 kinase 1 (p70 S6K1). Activation of AMPK by metformin paralleled the inhibition of TSC-mTOR signaling (Fig. 6C).
Metformin ameliorates ultrastructural abnormalities in diabetic hearts.
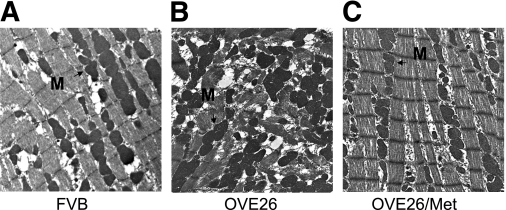
Morphology of LV tissues from FVB, OVE26, and metformin-treated OVE26 mice were analyzed by transmission electron microscopy (Fig. 7). Cardiomyocytes from control FVB mice (Fig. 7A) exhibited normal myocardial fine structure, with myofibrils composed of regular and continuous sarcomeres, and mitochondria formed longitudinal rows between myofibrils. In contrast, heart tissues from OVE26 mice (Fig. 7B) showed the adverse effects of diabetes, as exemplified by the misalignment and aggregation of mitochondria randomly interspersed between disrupted myofibrils. These distorted and chaotic architectures suggest defects in autophagy, the primary mechanism of mitochondrial turnover during normal development and pathologic conditions. Chronic metformin conferred striking protective effects against myocardial injuries inflicted by diabetes (Fig. 7C). There was no detectable difference in the fine structure of mitochondria and myofibrils between mouse hearts from nondiabetic controls and metformin-treated OVE26 mice.
FIG. 7.
Metformin (Met) protects cardiac ultrastructure from chronic diabetic damage. Representative transmission electron micrographs of cardiac tissues from LVs are shown from FVB mice (A), OVE26 mice (B), and OVE26 mice treated with metformin (C). A and C: FVB and metformin-treated OVE26 animals show normal myocardial structure, with myofibrils comprised of regular and continuous sarcomeres. Rows of moderately electron dense mitochondria (M) intervene between myofibrils. B: OVE26 diabetic myocardium shows randomly distributed mitochondria (M) between poorly organized myofibrils (original magnification of ×4,000; n = 6–7 in each group). (A high-quality digital representation of this figure is available in the online issue.)
Metformin attenuates the upregulation of ubiquitinated protein and apoptosis in diabetic hearts.
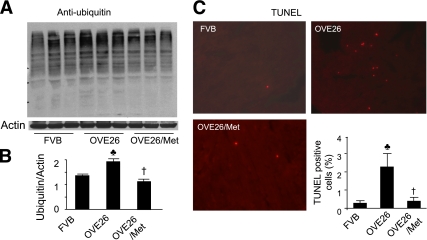
Suppression of autophagy could cause abnormal proteins to accumulate and promote apoptosis. To test this concept in our model, we probed ubiquitin in the cardiac homogenates from FVB, OVE26, and metformin-treated OVE26 mice. We found that polyubiquitinated protein levels were increased in OVE26 mouse hearts. This increase was prevented by metformin administration (Fig. 8A and B). TUNEL staining revealed more apoptotic cells in OVE26 mouse hearts compared with the FVB. Metformin treatment, however, reduced the number of apoptotic cells in diabetic hearts (Fig. 8C).
FIG. 8.
Metformin (Met) administration reduces apoptosis and ubiquitinated proteins in diabetic hearts. A: Immunoblot analysis of ubiquitin in the hearts from FVB, OVE26, and metformin-treated OVE26 mice. B: Densitometric analysis of ubiquitin. ♣P < 0.05 vs. FVB; †P < 0.05 vs. OVE26. C: Representative images of the TUNEL assay in the hearts from FVB, OVE26, and metformin-treated OVE26 mice. The number of TUNEL-positive cells is shown in the bar graph. Values represent mean ± SEM. ♣P < 0.05 vs. FVB; †P < 0.05 vs. OVE26 (n = 4 in each group). (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
The cardioprotective effects of metformin have been considered related to its beneficial actions on lipid metabolism, endothelial function, calcium homeostasis, hypercoagulation, and platelet reactivity (7). In the present investigation, we identified a new mechanism by which metformin prevents the development of diabetic cardiomyopathy. Our findings demonstrate that inhibition of AMPK by the overexpression of a cardiac-specific DN-AMPK gene inhibits cardiomyocyte autophagy, exacerbates cardiac dysfunction, and increases mortality in diabetic mice. Diabetes reduces AMPK activity and cardiomyocyte autophagy, resulting in the accumulation of clustered and damaged mitochondria and polyubiquitinated protein, associated with significantly impaired cardiac functions. More important, chronic activation of AMPK by metformin significantly enhances autophagic activity and prevents diabetic cardiomyopathy in diabetic mice. The cardioprotective effect of metformin is abolished in STZ-induced diabetic mice overexpressing DN-AMPK. These studies implicate inhibition of cardiac autophagy in the pathogenesis of diabetic cardiomyopathy and suggest that metformin confers cardiac protection, at least in part, by stimulating AMPK activity and consequently increasing autophagic activities.
The major finding in this study is that metformin enhances cardiac autophagic activities in diabetic mice, which may prevent the development of diabetic cardiomyopathy. Autophagy is essential in cell growth, development, and homeostasis, where it maintains a balance between the synthesis, degradation, and subsequent recycling of cellular components. It allows recycling of amino acids and removal of damaged organelles to eliminate oxidative stress and promote remodeling for survival (43,44). A low level of constitutive autophagy is cytoprotective by maintaining the quality of proteins and organelles and cell functions in the heart (4). Hartley et al. (45) have demonstrated that chronic hyperglycemia induces aggregation of ubiquitinated proteins in a pancreatic β-cell line. Treatment of pancreatic β-cells with 3-methyladenine, an inhibitor of autophagy, induces the aggregates of ubiquitinated proteins. These observations suggest that autophagy normally removes the misfolded or aggregated proteins induced by diabetes to defend against diabetes-induced cellular damage (45).
In the current study, we demonstrated inhibition of cardiac autophagy by diabetes, as evidenced by fewer autophagosomes, lower LC3-II protein levels, and decreased Beclin-1 protein expression. As a result, polyubiquitinated protein levels were increased in diabetic hearts, which displayed a disorganized sarcomere structure, misalignment, and aggregation of mitochondria, demonstrating the importance of autophagy in organelles turnover. The accumulation of ubiquitinated proteins is known to induce endoplasmic reticulum stress and apoptosis in cardiomyocytes. Moreover, damaged mitochondria release reactive oxygen species (46) and proapoptotic factors such as cytochrome c (2) to trigger apoptosis. Thus, accumulation of abnormal proteins and damaged organelles, such as mitochondria, could directly result in cardiac dysfunction. However, the current study has not established a conclusive link between enhanced autophagy and improved cardiac function in metformin-treated diabetic hearts. Although chronic metformin therapy significantly enhanced autophagic activity and preserved cardiac functions in OVE26 mouse hearts, the changes in autophagy and myocardial function in diabetes, with or without metformin treatment, are still correlative. Further investigation is warranted.
Our observations provide direct experimental support for a protective role of AMPK activation in diabetic cardiomyopathy. First, AMPK activity and phosphorylation on Thr172 were both apparently reduced in diabetic hearts, accompanied by impaired cardiac structure and function. Second, chronic metformin increased AMPK activity and prevented the structural and functional derangements in diabetic cardiomyopathy. Third, myocardial AMPKα2 inhibition was associated with worsened cardiac dysfunctions and increased mortality in diabetic mice. Finally, the cardioprotective actions of metformin were abolished in the mice deficient of AMPKα2. These findings suggest that activation of AMPK is required for metformin to confer its cardioprotective actions in the diabetic cardiomyopathy.
To our knowledge, this is the first report of a decrease in AMPK activity in the heart from diabetic rodents. AMPK has emerged as a key regulator of cellular energy homeostasis in the heart, and inactivation of AMPK would impair energy metabolism and cardiac function. Chronic metformin therapy restores cardiac AMPK activity and improves cardiac function in diabetic OVE26 mice, supporting that dysregulation of AMPK is an important event in the pathogenesis of diabetic cardiomyopathy.
Elucidating the mechanism responsible for the decreased AMPK activity in the diabetic heart may open a new horizon for the treatment and prevention of diabetic cardiomyopathy. Several studies have provided evidence linking the AMPK signaling pathway to autophagy. Compound C, a specific AMPK inhibitor (47) or a DN form of AMPK, inhibits starvation-induced autophagy in various mammalian cells (48). Glucose deprivation induces autophagy via activation of AMPK and inhibition of mTOR in isolated cardiomyocytes (18), whereas autophagy induced by myocardial ischemia is suppressed in transgenic mice overexpressing a DN-AMPK (18). In the current study, we have demonstrated that decreased cardiac AMPK activity is associated with lower Beclin1 levels and defective autophagy, whereas metformin restores Beclin1 expression and autophagic activity. Moreover, diabetes reduces phosphorylation of Raptor, increases phosphorylation of mTOR, and activates the mTOR downstream effectors 4EBP1 and p70S6K1, all of which indicate activation of TSC-mTOR signaling. Activation of AMPK by metformin inhibits the TSC-mTOR pathway and restores cardiac autophagy in OVE26 mice. Collectively, metformin-activated AMPK stimulates autophagic activities in cardiomyocytes by modulating Beclin1 and the TSC-mTOR pathway.
In summary, our findings demonstrate that decreased AMPK activity and the subsequent reduction in cardiac autophagy are central to the development of diabetic cardiomyopathy. Metformin prevents diabetic cardiomyopathy by stimulating AMPK activity and enhancing autophagic capacity. Thus, stimulation of AMPK may represent a novel approach to treat diabetic cardiomyopathy.
ACKNOWLEDGMENTS
This study was supported by funding from the National Institutes of Health grants (HL-079584, HL-080499, HL-074399, HL-089920, and HL-096032 to M.-H.Z. and 1P20-RR-02421501 to Z.X. and M.-H.Z.), the American Heart Association Scientist Development Grant (Z.X.), the Juvenile Diabetes Research Foundation (M.-H.Z.), Oklahoma Center for the Advancement of Science and Technology (M.-H.Z. and Z.X.), the American Diabetes Association (M.-H.Z.), and a grant by the Fraternal Order of Eagles (K.L.). M.-H.Z. is a recipient of the National Established Investigator Award of the American Heart Association.
No potential conflicts of interest relevant to this article were reported.
Z.X. designed and performed the experiments, analyzed data, and prepared the manuscript. K.L. planned the study, researched data, and reviewed and edited the manuscript. B.E., P.L., C.H., B.P., Y.D., H.L., S.R., R.T., and D.K. researched data. M.-H.Z. designed the experiments, reviewed the data, and wrote the manuscript.
The authors thank Drs. Eugene Patterson and Xichun Yu, Department of Medicine, the University of Oklahoma Health Sciences Center, for technical support.
REFERENCES
- 1.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004;6:463–477 [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson AB, Gottlieb RA. Mechanisms of apoptosis in the heart. J Clin Immunol 2003;23:447–459 [DOI] [PubMed] [Google Scholar]
- 3.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 2007;462:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007;13:619–624 [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 6.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 2002;137:25–33 [DOI] [PubMed] [Google Scholar]
- 8.Verma S, McNeill JH. Metformin improves cardiac function in isolated streptozotocin-diabetic rat hearts. Am J Physiol 1994;266:H714–H719 [DOI] [PubMed] [Google Scholar]
- 9.Legtenberg RJ, Houston RJ, Oeseburg B, Smits P. Metformin improves cardiac functional recovery after ischemia in rats. Horm Metab Res 2002;34:182–185 [DOI] [PubMed] [Google Scholar]
- 10.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 2008;117:952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Z, Dong Y, Zhang J, Scholz R, Neumann D, Zou MH. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol Cell Biol 2009;29:3582–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 2004;10:1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation 2001;104:1664–1669 [DOI] [PubMed] [Google Scholar]
- 14.Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J 2006;395:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickson-Bick DL, Buja LM, McMillin JB. Palmitate-mediated alterations in the fatty acid metabolism of rat neonatal cardiac myocytes. J Mol Cell Cardiol 2000;32:511–519 [DOI] [PubMed] [Google Scholar]
- 16.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 2005;11:1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijer AJ, Codogno P. AMP-activated protein kinase and autophagy. Autophagy 2007;3:238–240 [DOI] [PubMed] [Google Scholar]
- 18.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 2007;100:914–922 [DOI] [PubMed] [Google Scholar]
- 19.Calvert JW, Gundewar S, Jha S, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 2008;57:696–705 [DOI] [PubMed] [Google Scholar]
- 20.Russell RR, 3rd, Li J, Coven DL, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 2004;114:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis GS. Diabetic cardiomyopathy: fact or fiction? Heart 2001;85:247–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picano E. Diabetic cardiomyopathy: the importance of being earliest. J Am Coll Cardiol 2003;42:454–457 [DOI] [PubMed] [Google Scholar]
- 23.Avogaro A, Vigili de Kreutzenberg S, Negut C, Tiengo A, Scognamiglio R. Diabetic cardiomyopathy: a metabolic perspective. Am J Cardiol 2004;93:13A–16A [DOI] [PubMed] [Google Scholar]
- 24.Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: a short review. Mol Cell Biochem 2004;261:187–191 [DOI] [PubMed] [Google Scholar]
- 25.Shen X, Zheng S, Thongboonkerd V, et al. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab 2004;287:E896–E905 [DOI] [PubMed] [Google Scholar]
- 26.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223 [DOI] [PubMed] [Google Scholar]
- 27.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes 2008;57:3222–3230 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension 2004;44:826–831 [DOI] [PubMed] [Google Scholar]
- 29.Finsen AV, Christensen G, Sjaastad I. Echocardiographic parameters discriminating myocardial infarction with pulmonary congestion from myocardial infarction without congestion in the mouse. J Appl Physiol 2005;98:680–689 [DOI] [PubMed] [Google Scholar]
- 30.Eberli FR, Sam F, Ngoy S, Apstein CS, Colucci WS. Left-ventricular structural and functional remodeling in the mouse after myocardial infarction: assessment with the isovolumetrically-contracting Langendorff heart. J Mol Cell Cardiol 1998;30:1443–1447 [DOI] [PubMed] [Google Scholar]
- 31.Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest 2007;117:326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He C, Choi HC, Xie Z. Enhanced tyrosine nitration of prostacyclin synthase is associated with increased inflammation in atherosclerotic carotid arteries from type 2 diabetic patients. Am J Pathol 2010;176:2542–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing Y, Musi N, Fujii N, et al. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem 2003;278:28372–28377 [DOI] [PubMed] [Google Scholar]
- 34.Salem KA, Kosanovic M, Qureshi A, Ljubisavljevic M, Howarth FC. The direct effects of streptozotocin and alloxan on contractile function in rat heart. Pharmacol Res 2009;59:235–241 [DOI] [PubMed] [Google Scholar]
- 35.Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes 2002;51:174–181 [DOI] [PubMed] [Google Scholar]
- 36.Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the HL-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. FEBS J 2007;274:3184–3197 [DOI] [PubMed] [Google Scholar]
- 37.Claycomb WC, Lanson NA, Jr, Stallworth BS, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 1998;95:2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol 2004;286:H823–H829 [DOI] [PubMed] [Google Scholar]
- 39.Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999;402:672–676 [DOI] [PubMed] [Google Scholar]
- 40.Yan L, Vatner DE, Kim SJ, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A 2005;102:13807–13812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003;115:577–590 [DOI] [PubMed] [Google Scholar]
- 42.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol 2005;6:439–448 [DOI] [PubMed] [Google Scholar]
- 43.Stromhaug PE, Klionsky DJ. Approaching the molecular mechanism of autophagy. Traffic 2001;2:524–531 [DOI] [PubMed] [Google Scholar]
- 44.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000;290:1717–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes 2007;56:930–939 [DOI] [PubMed] [Google Scholar]
- 46.Song Y, Du Y, Prabhu SD, Epstein PN. Diabetic cardiomyopathy in OVE26 mice shows mitochondrial ROS production and divergence between in vivo and in vitro contractility. Rev Diabet Stud 2007;4:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meley D, Bauvy C, Houben-Weerts JH, et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem 2006;281:34870–34879 [DOI] [PubMed] [Google Scholar]