Abstract
Coupled with an aging society, the rising obesity prevalence is likely to increase the future burden of physical disability. We set out to determine whether obesity modified the effects of a physical activity intervention designed to prevent mobility disability in older adults. Older adults at risk for disability (N = 424, age range: 70-88 years) were randomized to a 12 month physical activity (PA) intervention involving moderate intensity aerobic, strength, balance and flexibility exercise (150 min per week) or a successful aging (SA) intervention involving weekly educational workshops. Individuals were stratified by obesity using a body mass index ≥ 30 (n = 179). Mobility function was assessed as usual walking speed over 400 meters and scores on a short physical performance battery (SPPB), which includes short distance walking, balance tests and chair rises. Over 12 months of supervised training, the attendance and total amount of walking time was similar between obese and non-obese subjects and no weight change was observed. Non-obese participants in the PA group had significant increases in 400 meter walking speed (+1.5%), while their counterparts in the SA group declined (−4.3%). In contrast, obese individuals declined regardless of their assigned intervention group (PA: −3.1%; SA: −4.9%). SPPB scores, however, increased following PA in both obese (PA: +13.5%; SA: +2.5%) and non-obese older adults (PA: +18.6%; SA: +6.1%). A moderate intensity PA intervention improves physical function in older adults, but the positive benefits are attenuated with obesity.
INTRODUCTION
Adults heading into late-life are now more obese than their predecessors and the cumulative effects of excess body mass may lead to increased disability rates.1 The combination of an aging society and increase obesity levels make the development of interventions for improving the physical independence of an aging society is an issue of high importance. Current recommendations suggest that low functioning obese older adults should receive weight loss treatments that minimizes muscle and bone loss.2 This recommendation has been empirically supported as obese older adults who follow diet and exercise therapy are able to lose weight while maintaining their muscle mass and improving their physical function.3 Additionally, obese older adults with osteoarthritis can improve physical function by performing walking and resistance exercise, although the improvements are better when exercise is combined with diet-induced weight loss.4, 5 Similar results have been found in non-obese older adults enrolled in a strength plus endurance exercise program without weight loss where aerobic capacity, physical function, balance time and lower extremity strength were improved.6 However, the effects of exercise on physical function without a dietary intervention in obese when compared to non-obese older adults remain equivocal2, 3, and thus it is unclear whether a long duration exercise program without weight loss can improve physical function in moderate to low functioning obese older adults.
We aimed to determine whether obese and non-obese older adults have similar changes in mobility function due to increased levels of moderate intensity physical activity. To address this aim, we performed a stratified data analysis of a pilot clinical trial called the Lifestyle Interventions for the Elderly Pilot Study (LIFE-P). LIFE-P tested the effects of a one-year moderate intensity physical activity program that included walking, strength and balance exercise, although the primary mode was walking.7 Two major outcomes of this trial were the speed needed to complete a 400 meter walk and a score derived from a short duration battery of mobility tasks. Four-hundred and twenty-four low to moderate functioning older adults were enrolled in the study of which 42% were considered obese by current standards (body mass index > 30 kg/m2).
METHODS
The current study is a secondary analysis of the Lifestyle Interventions and Independence for Elders-Pilot (LIFE-P), which was designed to help plan a definitive Phase 3 randomized controlled trial to examine the efficacy of a program of physical activity, compared to attention-control, on the incidence of major mobility disability (www.ClinicalTrials.gov registration #NCT00116194). A complete description of the LIFE-P study design has been reported previously.8 Briefly, participants were followed for an average of 1.2 years and the major findings from LIFE-P were that the structured physical activity (PA) intervention resulted in improved gait speed during a long distance walk and physical function on a battery of physical tasks when compared to a successful aging (SA) health education control group.7
Participant recruitment
Details about specific study inclusion and exclusion criteria have been reported previously.7, 8 Briefly, subjects were eligible for the study if they were between the ages of 70-89 yrs, sedentary (as defined as spending less than 20 min per week in regular structured physical activity), Short Physical Performance Battery score ≤ 9 9, and were able to walk 400 meters within 15 min. Randomization was not stratified by obesity status.
A total of 424 participants were randomized into PA or SA arms at four sites (Cooper Institute, Stanford University, University of Pittsburgh, and Wake Forest University) and followed for at least 12 months. All participants signed an informed consent and the study was approved by the Institutional Review Boards of all participating institutions.
Successful aging intervention
The SA health education control was designed to provide attention and health education. Study participants attended weekly group presentations for the first 26 weeks and then monthly until the end of the trial. Presentations were given on health topics that were relevant to older adults such as nutrition, medication use, foot care, and preventive medicine. All SA participants received basic information about physical activity participation and each class was concluded with upper extremity stretching. Regular telephone contact was made to encourage participation.
Physical activity intervention
Participants randomized to the PA intervention performed walking, strength, flexibility, and balance training. The goal for all participants was to walk for 150 minutes at a moderate intensity on 5 or more days of the week, which was approached in three phases. In the adoption phase (weeks 1-8), three supervised center-based physical activity sessions per week were conducted. These sessions were 40-60 minutes in length and used to initiate the walking program and to introduce participants to the strength, stretching, and balance portions of the program in a safe and effective manner. The strength exercises included standing chair squats, toe stands, leg curl, knee extensions and side hip raises with ankle weights. The balance exercises involved a series of dual and signal leg standing movements. Participants were instructed to walk at a rating of perceived exertion (RPE) intensity of 13 (“SOMEWHAT HARD”, range 12-14) and perform strength training at an intensity of 15-16.10 In the transition phase (weeks 9-24), the number of center-based sessions was reduced to two times per week and home-based walking/strengthening/flexibility activities were increased. In the maintenance phase (week 25 to the end), participants were encouraged to perform home-based physical activity a minimum of 5 days per week and one weekly center-based session was offered. The maintenance phase was continued until the final closeout assessment visits.
Measures of adherence
To confirm and objectively validate levels of participation in physical activity, an interview format of the Community Healthy Activities Model Program for Seniors (CHAMPS) physical activity questionnaire was administered at baseline, 6, and 12 months by assessors blinded to the treatment assignment 11. Participants were asked to report weekly frequency and duration of various physical activities over the prior 4-week period.
We were particularly interested in changes in moderate intensity PA in this group and thus we assessed activities > 3.0 metabolic equivalents using the CHAMPS 11. Adherence to the interventions was also measured using attendance to center-based physical activity sessions that was computed as the percentage of attended sessions relative to the total number of possible sessions in each study phase, excluding facility closings (e.g. holidays, weather emergencies, etc.). Additionally, the walking time (in minutes) and RPE at the completion of the walking bout was recorded at each center-based session. The total number of minutes of walking and mean RPE during each intervention phase was stratified by obesity.
Body mass and anthropometry
Body mass was measured with no shoes by a calibrated balance beam scale. Calibration was performed on a monthly basis. Body height was measured on a wall-mounted stadiometer graduated in centimeters. Waist circumference was assessed using a Gulick II tape measure (model 67020) with a spring loaded device to allow precise tension application to the skin. Participants stood with their feet together and the measurement tape was placed at a level midway between the highest point of the iliac crest and lowest part of the costal margin of the mid-axillary line. Measurements were recorded at the end of exhalation.
Physical function outcomes
The primary outcome for this analysis is the speed at which participants completed a 400-meter walk test. During this test, participants were asked to walk 10 laps of a 20-meter course at their usual pace. Participants were allowed to stop and rest if necessary, but without sitting.
We also assessed physical function using the short physical performance battery (SPPB). This test is based on timed measures of standing balance, walking speed, and ability to rise from a chair.9 A summary score (range 0-12) was subsequently calculated by adding the three scores.
Data analysis
Participants were stratified by obesity status using a body mass index cutpoint of greater than or equal to 30 kg/m2. Baseline characteristics between obesity groups within each intervention were compared using analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. Body mass and waist circumference were assessed using ANOVA for between group comparisons at each visit. Because values were non-normally distributed, Wilcoxon’s rank-sum test was used to compare attendance to physical activity sessions, frequency performing moderate physical activity on the CHAMPS, RPE and total minutes walking across groups of interest. Medians (25th and 75th percentiles) are reported for these comparisons. Repeated measures analysis of covariance (ANCOVA) was used to investigate differences in 400 meter gait speed and SPPB scores, with baseline values intervention assignment, visit, obesity status, intervention by visit interaction, intervention by obesity interaction, and obesity by time interaction included in the model. The alpha level was set at 0.05 to determine statistical significance. For these analyses, the interaction terms of obesity by intervention group and obesity by time estimates were investigated to determine whether obesity modified the effect of the intervention over time. When interactions demonstrated clear trends for differences across obesity levels, we further stratified the analysis. In the stratified analysis, baseline values, intervention assignment, visit, and intervention by visit interaction were adjusted in the ANCOVA.
RESULTS
Forty-two percent of individuals enrolled in the LIFE-P study were considered obese (Body mass index ≥ 30 kg/m2). Table 1 provides detailed characteristics of intervention assignment stratified by obesity. In general, obese individuals were similar to non-obese individuals in both intervention assignments with the exception that the obese subgroup was younger, had more prevalent health conditions and walked slower on the 400-meter walk test at baseline, but there were no differences in SPPB. The most common health conditions were hypertension and osteoarthritis, but the presence of these conditions did not differ by obesity status in either intervention assignment. Diabetes was more prevalent in obese than non-obese individuals in the PA (37.2% vs. 18.0%, p = 0.007), but not the SA (20.8% vs. 13.4%, p = 0.088) intervention.
Table 1.
Baseline characteristics of the participants according to randomized groups and body weight.*
| Randomized group | Physical activity (n = 213) | Successful aging control group (n =211) | ||||
|---|---|---|---|---|---|---|
| Body weight group | Non-obese (n = 111) |
Obese (n = 102) |
p-value | Non-obese (n = 134) |
Obese (n = 77) |
p-value |
| Age, mean ± SD, y | 77.2 ± 4.2 | 75.7 ± 4.01 | 0.008 | 77.9 ± 4.4 | 75.5 ± 3.7 | < 0.001 |
| Women | 79 (71.2) | 67 (65.7) | 0.389 | 91 (67.9) | 55 (71.4) | 0.594 |
| Other than Caucasian | 25 (22.5) | 28 (27.4) | 0.406 | 27 (20.1) | 29 (37.7) | 0.006 |
| Body mass index, mean ± SD† | 26.1 ± 2.6 | 35.7 ± 5.0 | < 0.001 | 26.3 ± 2.7 | 35.7 ± 4.7 | < 0.001 |
| Education (complete college) | 77 (69.4) | 72 (70.6) | 0.627 | 92 (68.6) | 55 (71.4) | 0.673 |
| Smoking | ||||||
| Current | 5 (5.4) | 2 (1.9) | 0.298 | 7 (5.2) | 0 (0) | 0.041 |
| Smoke > 100 cigarettes in lifetime | 18 (16.2) | 21 (20.6) | 0.410 | 22 (16.4) | 12 (15.6) | 0.874 |
| Fair/poor health self-rated | 22 (19.8) | 17 (16.7) | 0.552 | 28 (20.9) | 19 (24.7) | 0.525 |
| Prevalent health conditions | ||||||
| Myocardial infarction | 12 (10.8) | 12 (11.7) | 0.826 | 10 (7.5) | 5 (6.5) | 0.792 |
| Cancer | 22 (19.8) | 16 (15.7) | 0.431 | 24 (17.9) | 12 (15.6) | 0.386 |
| Hypertension | 72 (64.8) | 76 (74.5) | 0.225 | 89 (66.4) | 56 (72.7) | 0.341 |
| Diabetes | 20 (18.0) | 38 (37.2) | 0.007 | 18 (13.4) | 16 (20.8) | 0.088 |
| Depression | 16 (14.4) | 21 (20.6) | 0.235 | 16 (11.9) | 19 (24.7) | 0.017 |
| Stroke | 2 (1.8) | 6 (5.9) | 0.189 | 9 (6.7) | 3 (3.9) | 0.648 |
| Osteoarthritis | 22 (19.8) | 28 (27.4) | 0.189 | 22 (16.4) | 21 (27.3) | 0.133 |
| Lung disease | 16 (14.4) | 13 (12.7) | 0.723 | 16 (11.9) | 13 (16.9) | 0.315 |
| Congestive heart failure | 6 (5.4) | 5 (4.9) | 0.868 | 6 (4.5) | 7 (9.1) | 0.180 |
| Number of health conditions, mean ± SD | 1.69 ± 1.1 | 2.10 ± 1.3 | 0.015 | 1.56 ± 1.1 | 1.97 ± 1.2 | 0.016 |
| MMSE score | 26.9 ± 2.4 | 27.3 ± 2.4 | 0.221 | 27.4 ± 2.1 | 27.4 ± 2.1 | 0.997 |
| Baseline 400 m gait speed, m/sec | 0.88 ± 0.18 | 0.83 ± 0.17 | 0.066 | 0.88 ± 0.17 | 0.80 ± 0.17 | < 0.001 |
| Baseline SPPB score | 7.6 ± 1.4 | 7.6 ± 1.4 | 0.968 | 7.4 ± 1.3 | 7.5 ± 1.5 | 0.663 |
Non-obese: BMI = 19-29.9 kg/m2
Obese: BMI = 30-55 kg/m2
SPPB: Short Physical Performance Battery
Values are expressed as number (percentage) unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters square
Adherence
Adherence to the PA intervention was assessed through attendance records taken at each phase of the intervention (Table 2). Attendance in the adoption phase was higher for obese subjects, median of 86.9% vs. 79.2% in non-obese individuals. Obese and non-obese individual had similar attendance rates during the transition phase, but obese subjects’ attendance fell 12% lower than non-obese individuals during the optional visit in the maintenance phase. In total, there were no differences in the number of sessions attended for non-obese and obese individuals who reported to 71.4% and 67.0% of the total sessions, respectively.
Table 2.
Summary of adherence to center-based physical activity (n = 213) according to obesity and phase of the study.
| Body mass group (n = 213) | |||
|---|---|---|---|
| Non-obese (n = 111) |
Obese (n = 102) |
Between group p-value |
|
| Percent attendance (%) | |||
| Adoption phase (weeks 1-8)* | 79.2 (63-91) | 86.9 (75-95) | <0.001 |
| Transition phase (weeks 9-24)** | 74.2 (56-87) | 71.4 (43-89) | 0.412 |
| Maintenance phase (week 25 to end)*** | 60.7 (21-85) | 48.2 (7-78) | 0.050 |
| Total percent attendance | 71.4 (49-82) | 67.0 (41-80) | 0.467 |
| Total walking time recorded (min) | |||
| Adoption phase (weeks 1-8)* | 565 (369-686) | 516 (365-696) | 0.994 |
| Transition phase (weeks 9-24)** | 700 (442-890) | 595 (340-803) | 0.035 |
| Maintenance phase (week 25 to end)*** | 705 (303-1033)‡ | 599 (177-942) ‡ | 0.322 |
| Total walking time recorded (min) | 1910 (1075-2464) | 1506 (902-2403) | 0.142 |
|
Mean rate of perceived exertion (RPE) at
completion of walking session (range 6 – 20) |
|||
| Adoption phase (weeks 1-8)* | 11.9 (11.2-12.4) | 12.3 (11.7-13.1) | 0.005 |
| Transition phase (weeks 9-24)** | 12.0 (11.5-12.6) | 12.6 (11.9-13.2) | <0.001 |
| Maintenance phase (week 25 to end)*** | 12 (11.2-13) ‡ | 12.7 (11.5-13.8) ‡ | 0.061 |
| Mean RPE recorded for all sessions | |||
Values are expressed as medians (25th and 75th percentiles)
3 sessions per week
2 sessions per week
1 session per week (optional)
n = 97 for non-obese, n = 81 for obese
Total walking time and mean RPE was recorded for each intervention phase to determine whether obese individuals responded differently than non-obese individuals in the PA group (Table 2). For the entire 12 mo intervention, non-obese individuals had 21% more total walking activity recorded at the clinic-based sessions than obese individuals (median: 1910 vs. 1506 walking minutes), but this difference was not statistically significant (p = 0.142). Subjects reported RPE levels that matched the goals for the intervention. However, obese individuals reported higher levels of RPE throughout the intervention than non-obese individuals (median: 12.7 vs. 12.0 respectively).
Because subjects randomized to the PA group were encouraged to exercise outside of the clinic-based sessions, the CHAMPS was used to capture both PA performed inside and outside the clinic. Both non-obese and obese individuals responded similarly to the PA intervention through 6 months, which was supervised at the intervention sites (Table 3). However, while non-obese individuals maintained their PA levels, obese subjects demonstrated a striking drop in participation between 6 and 12 months.
Table 3.
Frequency of moderate physical activity (PA) per week spent in moderate PA per week according to randomized groups and obesity status (median (25th – 75th percentile)).
| Randomized group | Physical activity (n = 213) | Successful aging control group (n =211) | ||||
|---|---|---|---|---|---|---|
| Body mass group | Non-obese (n = 111) |
Obese (n = 102) |
Between group p-value |
Non-obese (n = 134) |
Obese (n = 77) |
Between group p-value |
| Minutes of moderate PA per wk | ||||||
| At baseline | 30 (0-165) | 15 (0-135) | 0.282 | 30 (0-135) | 90 (0-120) | 0.649 |
| 6 Months | 165 (30-315) | 135 (30-315) | 0.589 | 30 (0-210) | 30 (0-165) | 0.837 |
| 12 Months | 135 (30-285) | 60 (0-255) | 0.019 | 60 (0-135) | 105 (0-225) | 0.319 |
| Time effect (p-value) | 0.006 | 0.001 | 0.429 | 0.872 | ||
Body mass and anthropometry
Table 4 lists the average change in body mass and waist circumference across obesity groups and intervention assignment. The PA intervention did not induce significant changes in body mass in either non-obese or obese subjects. Non-obese individuals in the SA group lost approximately 1 kg of body mass while obese individuals in the SA group showed no change. Twelve months of physical activity reduced waist circumference by 2 cm in obese with a trend for decrease in non-obese subjects (a 1.4 cm decrease). Non-obese subjects in the SA group lost approximately 2.1 cm in waist circumference.
Table 4.
Body mass and waist circumference according to randomized groups and obesity status (Mean ± SEM).
| Randomized group | Physical activity (n = 213) | Successful aging control group (n =211) | ||||
|---|---|---|---|---|---|---|
| Body mass group | Non-obese (n = 111) |
Obese (n = 102) |
Between group p-value |
Non-obese (n = 134) |
Obese (n = 77) |
Between group p-value |
| Body mass (kg) | ||||||
| At baseline | 70.2 ± 1.1 | 96.3 ± 1.6 | <0.001 | 72.2 ± 1.0 | 97.3 ± 1.8 | <0.001 |
| 6 Months | 69.1 ± 1.4 | 95.0 ± 1.9 | <0.001 | 72.0 ± 1.0 | 95.7 ± 2.3 | <0.001 |
| 12 Months | 69.9 ± 1.1 | 94.4 ± 1.9 | <0.001 | 71.3 ± 1.0 | 96.3 ± 1.9 | <0.001 |
| Time effect (p-value) | 0.160 | 0.288 | <0.001 | 0.471 | ||
| Waist circumference (cm) | ||||||
| At baseline | 92.4 ± 1.0 | 113.5 ± 1.2 | <0.001 | 94.3 ± 0.97 | 112.9 ± 1.4 | <0.001 |
| 6 Months | 91.2 ± 1.2 | 110.5 ± 1.5 | <0.001 | 92.8 ± 0.94 | 111.0 ± 1.6 | <0.001 |
| 12 Months | 91.0 ± 0.98 | 111.6 ± 1.4 | <0.001 | 92.1 ± 0.87 | 111.5 ± 1.3 | <0.001 |
| Time effect (p-value) | 0.086 | 0.028 | <0.001 | 0.356 | ||
Physical function outcomes
400 meter gait speed
Prior to separating the body mass groups we performed an analysis to examine whether obese individuals had differential effects due to the PA intervention. For 400 meter gait speed, the obesity by time interaction was not statistically significant (p = 0.326), but there was evidence an obesity by intervention interaction (p = 0.06). This interaction signified that the intervention effect on 400 meter gait speed had been attenuated in obese participants. Further analysis revealed that non-obese subjects in the PA group had a significant increase in gait speed compared to the SA group (Figure 1A). In non-obese subjects, the adjusted difference between the PA and SA groups was 0.052 m/sec at 6 (p < 0.001) and 0.052 m/sec (p = 0.003) at 12 months. Obese subjects in the PA group, however, had a decline in gait speed, which was similar to that observed in the SA group (Figure 1B). The adjusted difference between the SA and PA intervention assignments was 0.009 m/sec at 6 months (difference between PA & SA: p = 0.544) and 0.014 m/sec at 12 months (p = 0.429) in the obese.
Figure 1.
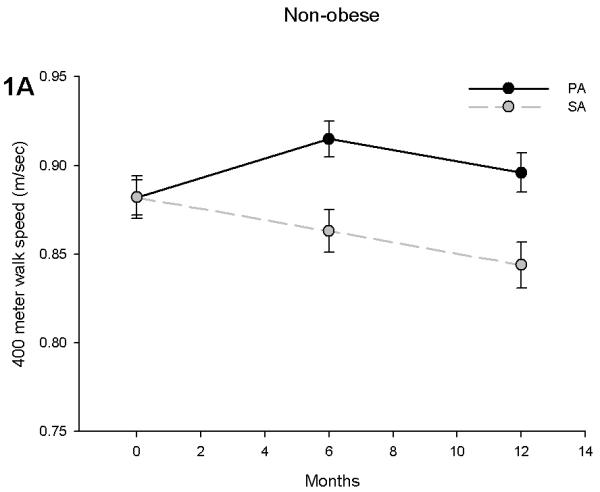
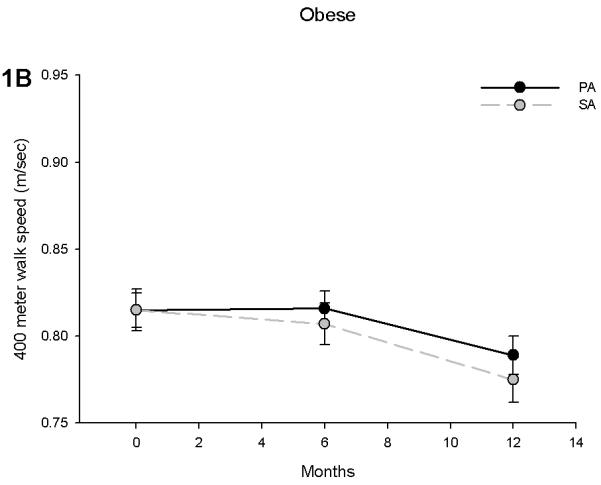
400 meter walk speed in non-obese (A) and obese (B) participants stratified by physical activity (PA) and successful aging (SA) intervention groups. Obese individuals had an attenuated effect of PA on gait speed during a 400 meter walk test. Values are predicted from ANCOVA models that adjust for baseline levels, intervention, time and intervention by time interaction.
Short Physical Performance Battery
SPPB scores improved in non-obese subjects in the PA group by 0.59 points at 6 months (p = 0.015) and 0.56 points at 12 months (p = 0.035) when compared to the SA group (Figure 2A). Obese individuals showed similar trends with the PA group having an adjusted difference with the SA group of 0.98 at 6 months (p < 0.001) and 0.66 at 12 months (p = 0.042) (Figure 2B). However, there was a significant obesity by time interaction (p = 0.032), suggesting that obese individuals had an attenuated increase over time. Although this interaction was likely influenced by an increase in SPPB score in the non-obese in the SA group, further analysis revealed that obese subjects increased their SPPB scores by 13.5% (an SPPB score of 7.53 to 8.55) while their non-obese counterparts had an 18.6% increase (an SPPB score of 7.49 to 8.89) over 6 months of PA. This difference was reduced by the end of the trial (Obese: 11.3% vs. Non-obese: 13.5% increase).
Figure 2.
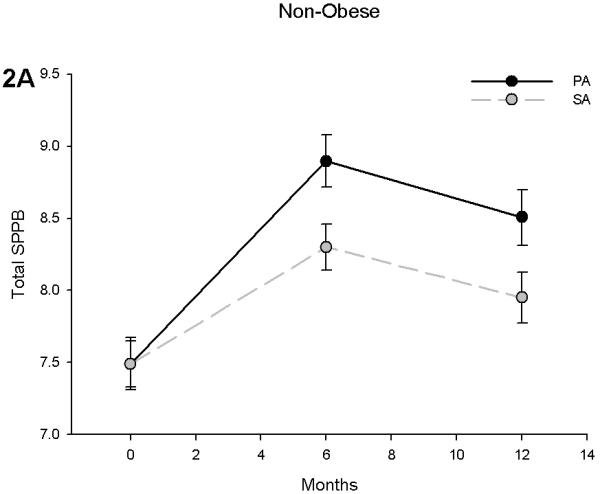
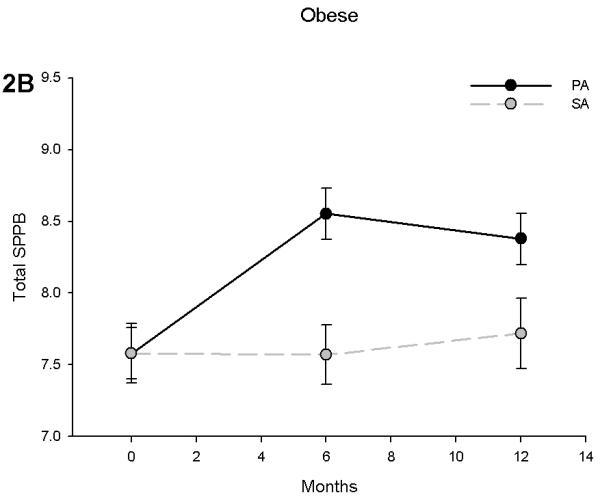
Short Physical Performance Battery (SPPB) scores in non-obese (A) and obese (B) participants stratified by physical activity and successful aging intervention groups. Values are predicted from ANCOVA models that adjust for baseline levels, intervention, time and intervention by time interactio
DISCUSSION
Obesity is an important factor in determining the rate of physical disability among older adults.1, 12, 13 Considering that improved health care has extended the life of obese individuals14, it is becoming clear that the aging obese population will have a significant contribution to future physical disability rates. We sought to determine whether moderate intensity physical activity can improve physical function in moderate to low functioning older adults and whether the effects are modified by obesity. The major findings of this study are that moderate intensity physical activity was: 1) successfully implemented in low to moderate functioning obese and non-obese older adults, 2) obese individuals were less able to sustain the intervention when supervision was reduced, 3) as demonstrated by the ratings of perceived exertion, obese individuals were exerting themselves to an adequate level to induce physiological benefits, 4) obese subjects did not improve their speed while walking long-distances, and 5) obesity blunted the positive effects of physical activity on SPPB scores. The results highlight that increased physical activity without weight loss in obese older adults can promote improvements in short-duration mobility tasks of daily life as measured with the SPPB. However, these benefits do not appear to transfer to long-distance mobility tasks such as walking 400 meters.
Exercise studies on physical function in obese older adults have typically included weight loss interventions3 and the effect of exercise alone on physical function in obese older adults is not completely understood. These data help to fill an important gap in the literature by suggesting that low to moderate functioning obese older adults have relatively good adherence and exert themselves during the supervised sessions and that this intervention can induce adaptations to short duration daily tasks such as those performed in the SPPB. However, obese individuals had a substantially attenuated 400 meter walk performance when compared to non-obese older adults in the PA group. To put these changes in perspective, the non-obese individuals had a 5 times greater improvement in long-distance walking speed than obese individuals in the PA group. Because studies that intervene with weight loss plus exercise have had beneficial effects on long-distance walking ability4, we believe that weight loss may be an important component to optimize the benefits of moderate intensity physical activity in obese older adults. Other components that could improve these outcomes include different exercise programs that use more vigorous activity, and adding more supervision and individualized training programs due to underlying comorbidities that may deter long-term adherence to PA.
Both the 400-meter walk and SPPB tests are valuable tools for assessing risks of physical disability in the elderly. While these two tests are correlated, they capture different dimensions of physical ability and successful performance depends on different physical reserve capacities. The PA intervention included strength training performed on a chair (i.e. chair squatting), which may have strongly influenced subjects ability to rise from a chair quickly as assessed in the SPPB.15 Therefore, the strength training aspect of the intervention may have improved muscle power in obese individuals, which would help to increase performance on short duration tasks of the SPPB more effectively. It is also possible that complications related to diabetes may have hindered improvements due to PA alone, but both diabetics and non-diabetics had identical results. Another factor that could partly explain the attenuated effect in obese older adults is due to the adherence to the PA intervention that seemed to be lower in the obese subjects. It remains unclear why obese individuals have reduced adherence, but one factor may be the heightened RPE level indicating that the exercise was more strenuous and difficult than the non-obese group. However, considering that the accumulated walk time during supervised PA sessions through 6 months of the trial was only 154 min less in the obese, which is equivalent to 6.4 minutes per week, we feel that the obese subjects undergoing PA had ample opportunity to demonstrate improved ability to walk 400 meters. Therefore, high body mass may preclude improvements in long distance walking ability due to a physical activity intervention.
The fact that many low functioning older adults are obese permitted an adequate sample size to conduct an appropriate secondary data analysis. This study is limited, however, as it was not statistically powered to detect differences among obese and non-obese individuals. Consequently, we are aware of the potential misinterpretation that can occur while performing subgroup analyses in a clinical trial not designed to investigate the effects PA by obesity status. This strategy is known to lead to false-positive results or statistically significant subgroup differences when none exist.16 Coupled with analyses from the original report of LIFE-P, a total of 6 subgroup analyses have been performed to date. Thus the false positive rate is 1 out of every 4 hypothesis tests (26%). Therefore, because this analysis was conducted to examine the heterogeneity of a PA intervention for treating mobility limitation it should not be over-interpreted. However, it should be noted that these results are consistent with epidemiologic data showing that obesity blunts the effect of physical activity on the prevention of mobility limitation in similarly aged individuals.17 Further research with appropriate design methods is needed to confirm these findings.
The strengths of this study are close monitoring of PA levels using data recorded at PA sessions and questionnaires that helped to determine that obese and non-obese older adults both comply and adhere to a PA intervention. In interpreting these findings, this study partially supports the current recommendations in that exercise alone improves some aspects of physical function among obese older adults, but it highlights that these improvements are less in obese older adults.
Acknowledgements
The Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study was funded by a grant from the National Institutes of Health/National Institute on Aging (U01 AG22376) and supported in part by the Intramural Research Program, National Institute on Aging, NIH.
The University of Florida Administrative Center and Dr. Manini was, in part, supported by the Claude D. Pepper Center P30AG028740
The Wake Forest University Field Center was, in part, supported by the Claude D. Pepper Older American Independence Center #1 P30 AG21332.
The Pittsburgh Field Center was partially supported by the Pittsburgh Claude D. Pepper Center P30 AG024827.
Dr. Fielding’s contribution is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-4-401. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Dr. Pahor’s contribution is partially supported by the Geriatric Research, Education and Clinical Center (GRECC), Malcom Randall Veteran’s Affairs Medical Center, North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging.
This study was funded by a grant from the National Institute on Aging (NIA). The NIA scientists had substantial involvement in the study design, data collection, analysis, interpretation and manuscript preparation.
*. LIFE STUDY INVESTIGATORS
Contribution Key
a – Conception and design
b – Analysis and interpretation of the data
c – Drafting of the article
d – Critical revision of the article for important intellectual content
e – Final approval of the article
f – Provision of study materials or patients
g – Statistical expertise
h – Obtaining of funding
i – Administrative, technical, or logistic support
j – Collection and assembly of data
Cooper Institute, Dallas, TX (Field Center)
Steven N. Blair, PED – Field Center Principal Investigator (b,d,e,i)
Timothy Church, MD, PhD, MPH – Field Center Co-Principal Investigator (j,e,a)
Jamile A. Ashmore, PhD (i)
Judy Dubreuil, MS (i,j)
Georita Frierson, PhD (i)
Alexander N. Jordan, MS (j)
Gina Morss, MA (i,j)
Ruben Q. Rodarte, MS (i,j)
Jason M. Wallace, MPH (i,j)
National Institute on Aging
Jack M. Guralnik, MD, PhD, Co-Principal Investigator of the Study (a,h,j)
Evan C. Hadley, MD (a,i)
Sergei Romashkan, MD, PhD (i)
Stanford University, Palo Alto, CA (Field Center)
Abby C. King, PhD – Field Center Principal Investigator (f)
William L. Haskell, PhD – Field Center Co-Principal Investigator (a,f,i,j)
Leslie A. Pruitt, PhD (j)
Kari Abbott-Pilolla, MS (f,j)
Stephen Fortmann, MD (i)
Carolyn Prosak, RD (j)
Kristin Wallace, MPH (f,j)
Karen Bolen, MS (i,j)
Tufts University
Roger Fielding, PhD (a,b,c,d,e)
Miriam Nelson, PhD (a,f)
University of California, Los Angeles,Los Angeles, CA
Robert M. Kaplan, PhD, MA (a,i)
VA San Diego Health Care System University of California, San Diego, San Diego, CA
Erik J. Groessl, PhD (a)
University of Florida, Gainesville, FL (Administrative Coordinating Center)
Marco Pahor, MD – Principal Investigator of the Study (a,b,c,d,e,f,h,i,j)
Todd Manini, PhD (a, b, c, d, e)
Michael Perri, PhD (d, e)
Connie Caudle (i)
Lauren Crump, MPH (i)
Sarah Hayden (i)
Latonia Holmes (i)
Cinzia Maraldi, MD (i)
Crystal Quirin (i)
University of Pittsburgh, Pittsburgh, PA (Field Center)
Anne B. Newman, MD, MPH – Field Center Principal Investigator (a,b,d)
Stephanie Studenski, MD, MPH – Field Center Co-Principal Investigator (a,b,d,e,j)
Bret H. Goodpaster, PhD, MS (a,b, )
Erin K. Aiken, BS (j)
Steve Anthony, MS (j)
Nancy W. Glynn, PhD (a,i,j)
Judith Kadosh, BSN, RN (i,j)
Piera Kost, BA (i,j)
Mark Newman, MS (j)
Christopher A. Taylor, BS (i,j)
Pam Vincent, CMA (j)
Wake Forest University, Winston-Salem, NC Field Center
Stephen B. Kritchevsky, PhD – Field Center Principal Investigator (a,b,d,e,f,i,j)
Peter Brubaker, PhD (e,f,i,j)
Jamehl Demons, MD ( j)
Curt Furberg, MD, PhD (a,b,c,d,e)
Jeffrey A. Katula, PhD, MA (b,f,i,j)
Anthony Marsh, PhD (e,i,j)
Barbara J. Nicklas, PhD (e,h,i,j)
Jeff D. Williamson, MD, MHS (i,j)
Rose Fries, LPM (j)
Kimberly Kennedy (i,j)
Karin Murphy, BS, MT (ASCP) (j)
Shruti Nagaria, MS (e,f,i,j)
Katie Wickley-Krupel, MS (f,i,j)
Data Management, Analysis and Quality Control Center (DMAQC)
Michael E. Miller, PhD – DMAQC Principal Investigator (g,h,i,j)
Mark Espeland, PhD – DMAQC Co- Principal
Investigator (a,b,c,d,e,g,j)
Fang-Chi Hsu, PhD (b,g)
Walter J. Rejeski, PhD (a,b,d,e)
Don P. Babcock, Jr., PE (i,j)
Lorraine Costanza (i)
Lea N. Harvin (i,j)
Lisa Kaltenbach, MS (g)
Wesley A. Roberson (j)
Julia Rushing, MS (j)
Scott Rushing (j)
Michael P. Walkup, MS (b,g,j)
Wei Lang, PhD (g)
Yale University
Thomas M. Gill, MD (a,e)
Contributor Information
T.M. Manini, Department of Aging and Geriatric Research, College of Medicine, University of Florida, Gainesville FL
A.B. Newman, Departments of Epidemiology and Medicine, University of Pittsburgh, Pittsburgh PA
R. Fielding, Nutrition, Exercise Physiology, and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University
S.N. Blair, Arnold School of Public Health, University of South Carolina, Columbia, SC
M.G. Perri, Department of Clinical and Health Psychology, College of Public Heath and Health Professions, University of Florida, Gainesville, FL
S.D. Anton, Department of Aging and Geriatric Research, College of Medicine, University of Florida, Gainesville FL
B.C. Goodpaster, Division of Endocrinology and Metabolism, University of Pittsburgh, Medical Center, Pittsburgh, PA
J. Katula, Departments of Health and Exercise Science, Wake Forest University, Winston-Salem, NC.
J. Rejeski, Departments of Health and Exercise Science, Wake Forest University, Winston-Salem, NC.
A.C. King, Department of Health Research & Policy, and Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine, Stanford, CA
S.B. Kritchevsky, Section on Gerontology and Geriatric Medicine, J. Paul Sticht Center on Aging, Wake Forest University School of Medicine, Winston-Salem, North Carolina
F.-C Hsu, Section on Gerontology and Geriatric Medicine, J. Paul Sticht Center on Aging, Wake Forest University School of Medicine, Winston-Salem, North Carolina.
M. Pahor, Department of Aging and Geriatric Research, College of Medicine, University of Florida, Gainesville FL
Literature cited
- 1.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988-2004. Jama. 2007;298:2020–7. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 2.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–63. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 3.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–6. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 4.Messier SP, Miller GD, Morgan TP, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet and Activity Promotion Trial (ADAPT) Arthritis and Rheumatism. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 5.Messier SP, Royer TD, Craven TE, O’Toole ML, Burns R, Ettinger WH., Jr. Long-term exercise and its effect on balance in older, osteoarthritic adults: results from the Fitness, Arthritis, and Seniors Trial (FAST) J Am.Geriatr.Soc. 2000;48:131–138. doi: 10.1111/j.1532-5415.2000.tb03903.x. [DOI] [PubMed] [Google Scholar]
- 6.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–8. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 7.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–65. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 8.Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: design and methods. Contemp Clin Trials. 2005;26:141–54. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 10.Borg G. Perceived exertion and pain scales. Human Kinetics; Champaign IL: 1988. [Google Scholar]
- 11.Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J.Gerontol.A Biol.Sci.Med Sci. 2001;56:M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds SL, Saito Y, Crimmins EM. The impact of obesity on active life expectancy in older American men and women. Gerontologist. 2005;45:438–44. doi: 10.1093/geront/45.4.438. [DOI] [PubMed] [Google Scholar]
- 13.Sturm R, Ringel JS, Andreyeva T. Increasing obesity rates and disability trends. Health Aff (Millwood) 2004;23:199–205. doi: 10.1377/hlthaff.23.2.199. [DOI] [PubMed] [Google Scholar]
- 14.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. Jama. 2005;293:1868–74. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 15.Manini T, Marko M, VanArnam T, et al. Efficacy of resistance and task-specific exercise in older adults who modify tasks of everyday life. J Gerontol A Biol Sci Med Sci. 2007;62:616–23. doi: 10.1093/gerona/62.6.616. [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 17.Koster A, Patel KV, Visser M, et al. Joint effects of adiposity and physical activity on incident mobility limitation in older adults. J Am Geriatr Soc. 2008;56:636–43. doi: 10.1111/j.1532-5415.2007.01632.x. [DOI] [PubMed] [Google Scholar]


