Abstract
Juvenile neuronal ceroid lipofuscinosis (JNCL), also known as Batten disease, is a fatal inherited neurodegenerative disorder. The major clinical features of this disease are vision loss, seizures and progressive cognitive and motor decline starting in childhood. Mutations in CLN3 are known to cause the disease, allowing the generation of mouse models that are powerful tools for JNCL research. In this study, we applied behavioural phenotyping protocols to test for early behavioural alterations in Cln3Δex7/8 knock-in mice, a genetic model that harbours the most common disease-causing CLN3 mutation. We found delayed acquisition of developmental milestones, including negative geotaxis, grasping, wire suspension time and postural reflex in both homozygous and heterozygous Cln3Δex7/8 pre-weaning pups. To further investigate the consequences of this neurodevelopmental delay we studied the behaviour of juvenile mice and found that homozygous and heterozygous Cln3Δex7/8 knock-in mice also exhibit deficits in exploratory activity. Moreover, when analysing motor behaviour, we observed severe motor deficits in Cln3Δex7/8 homozygous mice but only a mild impairment in motor coordination and ambulatory gait in Cln3Δex7/8 heterozygous animals. This study reveals previously overlooked behaviour deficits in neonate and young adult Cln3Δex7/8 mice indicating neurodevelopmental delay as a putative novel component of JNCL.
Keywords: CLN3, Batten, Neurodevelopment, Mouse Behaviour, Disease Models, Neuronal Ceroid Lipofuscinosis, Genetics
INTRODUCTION
Juvenile neuronal ceroid lipofuscinosis (JNCL; Batten or Spielmeyer-Vogt disease) is part of a family of fatal lysosomal storage disorders. Progressive visual impairment is typically the first clinical sign of this disease (Consortium, 1995, Kohlschutter et al., 1993), followed by seizures, cognitive decline and motor dysfunction (Bennett & Hofmann, 1999, Raininko et al., 1990). Premature death normally occurs around the third decade of life. These symptoms underscore the severe consequences of the autosomal recessive inheritance of mutations in CLN3. The vast majority of JNCL patients have a common 1.02 kilobase (kb) deletion in CLN3 leading to the loss of exons 7 and 8 (Consortium, 1995). CLN3 encodes a 438 amino acid membrane protein with several proposed functions (Hobert & Dawson, 2007, Holopainen et al., 2001, Koike et al., 2005, Narayan et al., 2006, Osorio et al., 2007, Pearce et al., 1999, Puranam et al., 1999, Ramirez-Montealegre & Pearce, 2005) but our understanding of JNCL pathogenesis is still elusive. Four JNCL mouse models have been developed, namely, the Cln3Δex1–6 knock-out mouse (Mitchison et al., 1999); the Cln3Δex7/8 knock-out (Katz et al., 1999); the Cln3Δex7/8 knock-in (Cotman et al., 2002) and the Cln3LacZ β-galactosidase reporter model (Eliason et al., 2007). The Cln3Δex7/8 knock-in is the only model that recapitulates the most commonly observed 1.02 kb deletion, leading to the loss of exons 7 and 8 (Consortium, 1995, Cotman et al., 2002). Transcript analysis in these mice revealed that in addition to CLN3 mRNA lacking exons 7 and 8, there were additional variant transcripts that lacked exon 5, or retained intron 1, 10, or 11, in combination with the loss of exons 7 and 8 (Cotman et al., 2002). The current sparse knowledge on JNCL pathological mechanism and CLN3 function limits our understanding on the possible relevance that variant CLN3 transcripts might have. Therefore, the Cln3Δex7/8 knock-in mice is a model tool of election for JNCL research. Presently, few efforts have been made in the behavioural characterization of Cln3Δex7/8 knock-in mice and only gait and clasping behaviours have been evaluated in 10–12 months old animals (Cotman et al., 2002). Since symptoms in JNCL emerge in childhood and several studies point to early initiation of JNCL disease process, (Cotman et al., 2002, Herrmann et al., 2008, Kovacs et al., 2006, Lake, 1993), special attention should be placed in characterizing behavioural alterations at early ages. In addition, the heterozygous Cln3Δex7/8 knock-in mice should also be characterized since mild alterations have also been reported in JNCL carriers (Gottlob et al., 1988, Sayit et al., 2002). Therefore, in the present study we have applied behavioural paradigms suitable to study sensorial and motor capabilities in developing Wild type, heterozygous and homozygous Cln3Δex7/8 knock-in pre-weaning pups. Furthermore, we analysed the behaviour of young adult animals using tests to assess major clinical features of JNCL, including, alterations of selected behaviour profiles (open field, elevated plus maze and forced swimming tests), visual function (visual cued version of Morris water maze test) and motor capacity (rotarod test and gate analysis).
MATERIALS AND METHODS
Animals
The strain used in this study was generated in the M. MacDonald laboratory (Cotman et al., 2002). The original strain was backcrossed 16 times to Wild type C57BL/6J mice obtained from Jackson Laboratory. Mice were kept in an animal facility in a 12-hour light: 12-hour dark cycle (light onset at 7:30 AM), with food and water available ad libitum. Male and female heterozygous Cln3Δex7/8 animals were bred. A daily inspection for the presence of new litters in the cages was carried out twice a day and the day of birth was annotated for each litter. After birth, animals were kept in the home cage with their mothers and were then tagged with non-toxic paint (green paste, Ketchum Manufacturing Inc., Canada) in one or two toes per feet at PND 3. Pups were evaluated daily (Wild type, n=25; heterozygous Cln3Δex7/8, n=33; homozygous Cln3Δex7/8, n=29 from 10 litters) in battery of test (approximately 8 min per animal) to evaluate somatic parameters and neurological reflexes until weaning at PND 21. At this point the tip of the tail was cut for DNA extraction and genotyping was performed by a multiplex PCR analysis in a 20 µl volume that included autoclaved ultra-filtered water, PCR buffer (1×), dNTP mixture (200 µM each), primers (for details see Table 1), Taq DNA polymerase (1 U/20 µl) and approximately 50 ng genomic DNA templates. PCR cycling conditions included 35 cycles of 15 s at 94 °C, 30 s at 55 °C, and 45 s at 65 °C, after a 10-min initial period of DNA denaturation and enzyme activation at 94 °C. The amplified fragments had sizes readily distinguishable by electrophoresis through a 2% agarose gel. Groups of eight weeks-old animals from 9 litters that had not been previously tested in behaviour experiments were tested in different days in the open field (Wild type, n=24; heterozygous Cln3Δex7/8, n=27; homozygous Cln3Δex7/8, n=25), elevated plus maze (Wild type, n=28; heterozygous Cln3Δex7/8, n=31; homozygous Cln3Δex7/8, n=27), forced swimming tests (Wild type, n=10; heterozygous Cln3Δex7/8, n=13; homozygous Cln3Δex7/8, n=13). Different groups of eight weeks-old untested animals were used for rotarod, gait analysis and morris water maze tests (Wild type, n=9; heterozygous Cln3Δex7/8, n=12; homozygous Cln3Δex7/8, n=15). The same observer, blinded for animal genotype, evaluated each test performed. Tests were always made in the same circadian period and whenever possible at the same hour of the day. After completing the experiments animals were euthanized by CO2 and decapitation, thus minimizing their suffering. All animal experimentation was conducted in accordance with the European Community Council Directive, 86/609/EEC and NIH guidelines on animal care and experimentation.
Table 1.
Primers used in multiplex PCR genotyping.
| Primer | Sequence 5’-3’ | Concentration |
|---|---|---|
| Cln3 552F | GAG CTT TGT TCT GGT TGC CTT C | 200 nM |
| Cln3 Ex9R | GCA GTC TCT GCC TCG TTT TCT | 200 nM |
| Cln3 WTF | CAG CAT CTC CTC AGG GCT A | 200 nM |
| Cln3 WTR | CCA ACA TAG AAA GTA GGG TGT GC | 200 nM |
Somatic parameters
Body weight and the ano-genital distance were measured daily from PND 3 to PND 21. In addition, the day of eye- and ear-opening, as well as fur appearance were also evaluated.
Neurological reflexes
Neurodevelopment testing for surface righting, air righting, wire suspension, negative geotaxis and postural reflexes were performed with minor alterations from what had been previously described (Mesquita et al., 2007, Santos et al., 2007). Briefly, animals were separated from their mother at the beginning of each test session and kept with their littermates in a new cage, under soft white light, with towel paper and sawdust from their home cage. The mothers were left in the same room as the pups during separation. Animals were returned to their home cage once testing was finished.
Surface righting reflex
The neonate was placed in the supine position and the time needed to turn over and restore its normal prone position was recorded for a maximum of 30 seconds. Complete acquisition of the reflex was assumed when the animal could rotate 180° around its longitudinal axis.
Air righting reflex
The neonate was held on its back 30 cm above a soft surface before being released. The position in which the animals reach the soft pad was recorded. The reflex was considered to be achieved when neonate landed on the surface with all four paws.
Grasping and wire suspension test
A metal bar was suspended 30 cm above a soft surface. The animal was held and its forepaws were allowed to touch the bar. Complete acquisition of grasping reflex was assumed when the animal was able to grasp the bar with both forepaws. The time the animal was able to hold on the bar using only its forepaws (wire suspension time) was also recorded for a maximum of 30 seconds.
Negative geotaxis
The animal was placed on a grid, tilted 45° to the plane, with its head facing downwards. Animals that could rotate a full 180° and face up within a maximum time of 30 seconds were considered to have acquired this reflex.
Postural reflex
Neonates were placed in a 15 × 15 cm box and shaken left and right, up and down. Animals that could maintain their original position in the box by extending all four limbs were assumed to have acquired this skill.
Open field
Animals were placed in the centre of a 43.2 × 43.2 cm arena with transparent walls (MedAssociates Inc., St. Albans, VT) and were observed for 5 min. The arena was lighted by a 60W bulb suspended above the centre. Activity parameters were automatically collected by the equipment (total distance travelled, speed, resting time, distance travelled and time spent in a predefined 10.8 × 10.8 cm square in the centre of the arena). The number of rears, the time that animals spent exploring vertically were registered by the observer.
Rotarod
Mice were tested in a rotarod apparatus from TSE systems (Hamburg, Germany). The protocol consisted of 3 days of training at the constant speed of 15 r.p.m. for a maximum of 60 seconds in four trials, with a 15-min interval between each trial. At the fourth day animals were tested for each of six velocities (5, 8, 15, 20 and 24 r.p.m.) for a maximum of 60 seconds in two trials, with a 10-min interval between each trial. The latency to fall off the rod was registered.
Elevated plus maze
Mice were placed in an EPM apparatus consisting of two opposite open arms (50.8 × 10.2 cm) and two opposite closed arms (50.8 × 10.2 × 40.6 cm) raised 72.4 cm above the floor (ENV-560, MedAssociates Inc.) and the time spent in each of the arms was measured using a video tracking system (Viewpoint, Champagne au Mont d’Or, France).
Forced swimming test
Learned helplessness, as a measure of susceptibility to depression-related behaviour, was assessed using the forced swimming test. Mice were placed in cylinders (diameter: 37 cm; 55 cm of height) filled with water (25 °C) to a depth where the animals had no solid support for their rear paws. After a 10-minute pre-test session, animals were rested for 24 hours before being subjected to the actual tests, which lasted 5 minutes. At the end of each test session, animals were placed on a heating pad (15 minutes) before being returned to their home cages. Cylinders were filled with fresh water after each trial. A video camera, placed at the top of the cylinder, was used to record test sessions. Recordings were later scored by an investigator blind to the experimental details to determine inactivity (passiveness - defined as time spent either immobile or making righting movements to stay afloat) versus activity periods.
Gait analysis
Eight week-old mouse were placed in an apparatus that consisted of a wooden platform 90 cm long × 9 cm wide with a wooden box (27× 22 cm; 17.5 cm height) at one end of the platform; mice had free access to the box from the platform via a small door. The entire apparatus was elevated 11 cm from the bench and paper (9 × 90 cm long) was placed on the walking platform, the hind paws of the mouse were dipped into non-toxic black paint and the fore paws were painted with red paint. In the day of testing, individual mice were placed on the end of the platform farthest from the box. As each mouse traversed the platform its gait was recorded as finger-paint paw prints. Several parameters of the gait were measured, including length of stride (distance between consecutive same-foot prints), length of step (distance between consecutive alternate-foot prints) and hind and fore paws displacement (distance between left and right hind or fore paws).
Morris water maze with visual clues
The Morris water maze consisted of a black tank (diameter: 170cm, depth: 50cm), divided into four quadrants by virtual lines, and filled with water (22°C) to a depth of 31 cm. During testing, a visible platform (12×12cm; identified with a 10 cm2 square flag with 15 cm of height) was placed at a height of 30 cm and extrinsic visual clues were glued to the walls. Data were collected using a video-tracking system (Viewpoint, Champagne au Mont d’Or, France). Animals were tested for 3 consecutive days (4 trials per day, with a maximum of 2 minutes per trial). The visible escape platform was placed in the center of an arbitrarily defined quadrant. Test sessions began with mice being placed, facing the wall of the maze, in a defined start position and finished once the escape platform had been reached. This procedure was continued in a clockwise fashion over the subsequent trials. The time and distance to escape to the platform were recorded. In cases in which the escape platform had not been reached within 2 minutes, the experimenter guided the animal to the platform. In either case, animals were dried and allowed to rest for 30 seconds before being returned to the maze for the remaining test sessions.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
For comparative gene expression studies, total RNA was extracted from the cerebellum isolated from Wild type, heterozygous, and homozygous Cln3Δex7/8 mice using Trizol reagent (Invitrogen, Carlsbad, CA), and treated using the Turbo™ DNA-free kit (Ambion, Austin, TX) to reduce genomic DNA contamination. One µg of RNA was used as template for cDNA synthesis using the High-Capacity cDNA synthesis kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. A total of 3 mice per genotype were used for qRT-PCR analysis. Comparative quantitative PCR was performed using gene specific primers as detailed in table 1, using β-actin for normalization. Amplification was carried out using Power SYBR Green master mix (Applied Biosystems) containing appropriate concentrations of each primer (see table 2) and 2 µl of cDNA in a 96 well plate on a Mx3005p real-time PCR instrument (Stratagene, La Jolla, CA) using the following cycling parameters; 95°C for 10 min, followed by 40 cycles of 95°C for 20s, 60°C for 1 min. Specificity of the amplified product was determined by melt-curve analysis immediately following completion of the final amplification cycle.
Table 2.
Primers used in qRT-PCR.
| Primer | Sequence 5’-3’ | Concentration | |
|---|---|---|---|
| Set 1 | Cln3 ex1F | TGA GAG GGA GGA GAC CGA CTC AGA | 400 nM |
| Cln3 ex3R | CCA AGA TCC AGA AAC CCA CTG CA | 400 nM | |
| Set 2 | Cln3 ex6F | TCT GGT TGC CTT CTC TCA GTC AGT | 300 nM |
| Cln3 ex7/8R | AGA CCA CCA TGA GAT CAC AGC ACT | 300 nM | |
| HSK | ActB ex1F | CTG TCG AGT CGC GTC CAC CC | 600 nM |
| ActB ex2R | CGT CAT CCA TGG CGA ACT GG | 600 nM | |
Statistical analysis
For each component of the test battery for physical maturation and neuronal reflex acquisition the average first day of adult-like responding was statistically analyzed by ANOVA, with Tukey multiple comparison procedure. Further analysis of these data was performed in different PND by comparing the percentage of animals with and without adult-like response against two genotypes (Wild type vs. heterozygous and Wild type vs. homozygous Cln3Δex7/8 mice) by the Fisher´s exact test (FET). Other statistical comparisons were performed, in the case of two groups, through Student´s t-test or, in the case of several groups, by ANOVA, with Tukey multiple comparison procedure. When the homogeneity of variances was not observed, non parametric tests were used, Mann-Whitney test for two groups and Kruskal-Wallis test for several groups, with Bonferroni correction for multiple comparisons. Relative gene expression and statistical analysis of qRT-PCR data was calculated using the REST-XL program (Pfaffl et al., 2002) and expressed as fold-change versus Wild type. Data from male and female animals were pooled together when there was no sex effect and sex vs. genotype interactions as verified by the 2-way ANOVA test. In rotarod experiments homogeneity of variances was not observed (Levene test) and only male animals were used. SPSS version 16 was used to analyze the data and statistical significance was set to p<0.05.
RESULTS
Pre-weaning neurodevelopment abnormalities in Cln3Δex7/8 neonates
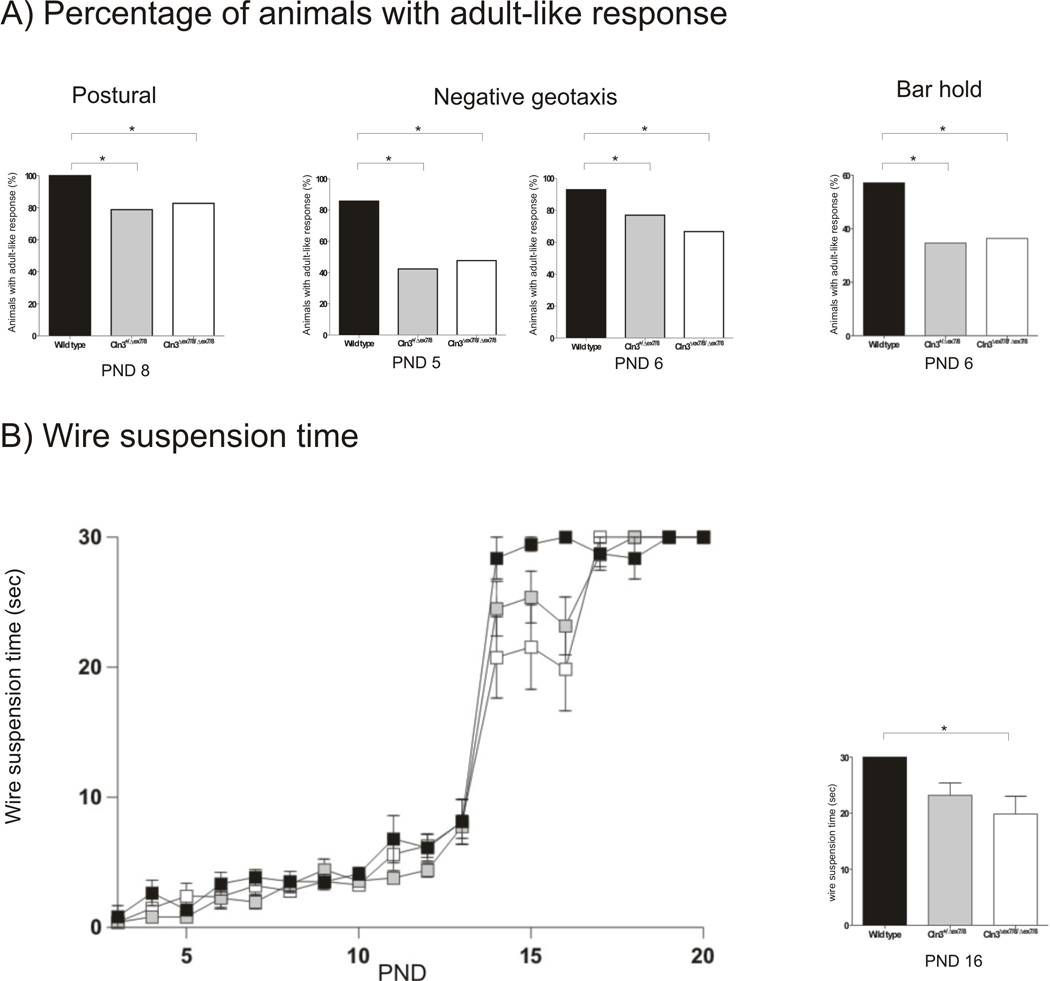
To evaluate the possible influence of Cln3Δex7/8 mutation in the appearance of developmental milestones, we performed daily analysis/scoring of physical growth, maturation and acquisition of neurological reflexes in Wild type, homozygous and heterozygous Cln3Δex7/8 knock-in littermates from PND 3 to 21. Both homozygous and heterozygous Cln3Δex7/8 knock-in exhibited delays in the achievement of developmental milestones (Table 3). The greatest delays were observed in the negative geotaxis and grasping reflex. The mean first day of appearance on negative geotaxis reflex was significantly different among the tree genotypes (F(2,71)=3.180, p<0.05) and delayed in approximately 3.0 days for homozygous Cln3Δex7/8 mice and 2.2 days for the heterozygous Cln3Δex7/8 mice. In the grasping behaviour there were also significant differences between the genotypes (F(2,72)=3.024, p<0.05) and the delay was of approximately 2.5 days for the homozygous Cln3Δex7/8 mice and 1.3 days for the heterozygous Cln3Δex7/8 mice. The differences in the mean first day of adult-like response did not reach statistical significance for the other tests performed. Nonetheless, when analysing the percentage of animals with adult-like response at the different PNDs, in addition to finding significant differences in negative geotaxis at PND 5 and 6 (FET, p<0.05) and in grasping at PND 6 (FET, p<0.05) we also found significant differences among genotypes in the percentage of animals with postural reflex at PND 8 (FET, p<0.05) (Figure 1A). Furthermore, the time of wire suspension was also significantly decreased (F(2,50)=3.748, p<0.05) in homozygous Cln3Δex7/8 at PND 16, when compared with Wild type controls (Figure 1 B). These results indicate the presence of a delay in the neurodevelopment of homozygous Cln3Δex7/8 mice that is less severe but also present in heterozygous animals.
Table 3.
Developmental milestones of Wild type, homozygous and heterozygous Cln3Δex7/8 knock-in neonatal mice. Data is presented as mean first day of adult-like response ± standard error (Wild type, n=25; heterozygous Cln3Δex7/8, n=33; homozygous Cln3Δex7/8, n=29).
| Wild type | Cln3 +/Δex7/8 | Cln3 Δex7/8/Δex7/8 | |
|---|---|---|---|
| Air righting reflex, first day | 14.78±0.90 | 15.32±0.92 | 15.87±1.23 |
| Surface righting reflex, first day | 4.08±0.90 | 4.62±1.19 | 4.93±1.46 |
| Eye opening, first day | 12.30±3.34 | 13.33±1.19 | 13.48±1.45 |
| Ear opening, first day | 13.07±0.47 | 13.18±0.51 | 13.00±0.35 |
| Negative geotaxis, first day | 4.50±0.85 | 6.71±2.78 | 7.52±3.38 |
| Postural reflex, first day | 8.13±0.51 | 8.75±2.13 | 8.70±1.96 |
| Grasping, fist day | 5.13±0.58 | 6.40±0.59 | 7.60±0.57 |
Figure 1. Decreased percentage of animals with negative geotaxis, postural reflex and grasping behaviour in homozygous and heterozygous Cln3Δex7/8.
(A) The percentage of homozygous and heterozygous Cln3Δex7/8 mice positively scored was significantly decreased for postural reflex at PND 8, for negative geotaxis at PND 5 and 6 and for grasping behaviour at PND 6. Data are expressed as the percentage of animals achieving adult-like response (Wild type, n=25; heterozygous Cln3Δex7/8, n=33; homozygous Cln3Δex7/8, n=29).
Juvenile Cln3Δex7/8 knock-in mice display reduced exploratory behaviour
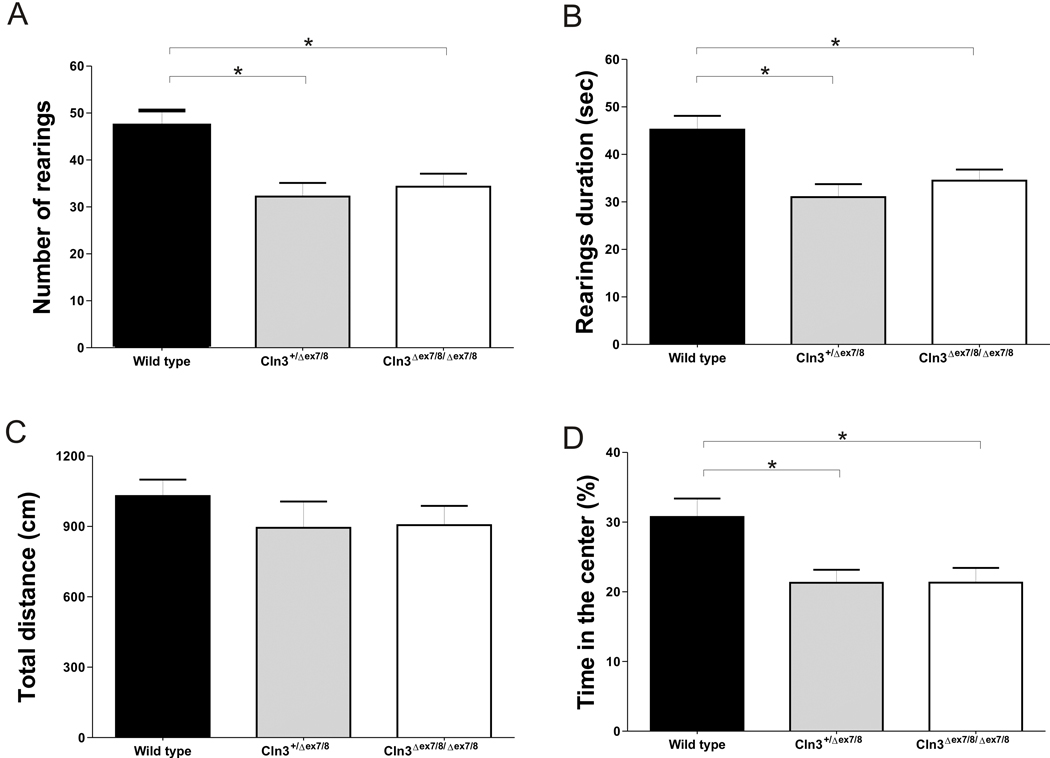
Having observed neurodevelopmental delays in Cln3Δex7/8 mice we sought to evaluate the possible consequences of this anomaly in the behaviour of post-weaning eight weeks-old animals in the open field test. A marked decrease in the exploratory behaviour of homozygous and heterozygous Cln3Δex7/8 mice, expressed by a significant decrease in the number of “rearings” performed (F(2,73)=7.193, p<0.05) (Figure 2 A) and time spent in vertical exploration (F(2,73)=7.300, p<0.05) (Figure 2 B), was found. Spontaneous locomotion was not affected since no genotype-associated differences were found in the total distance travelled (Figure 2 C). Interestingly, there were significant differences in the percentage of time heterozygous or homozygous Cln3Δex7/8 mice (F(2,73)=5.926, p<0.05) spent in the centre of the arena when compared with Wild type animals (Figure 2 D). As this could be indicative of anxiety-like behaviour we then tested the animals in the elevated plus maze test. There were no significant differences in neither the percentage of time spent in the open arms (Table 4 A) nor in the number of open arms entries (not shown), indicating no evidence for anxiety-like behaviour. This result suggests that the decrease in the percentage of time spent in the center of the field by Cln3Δex7/8 knock-in mice is a consequence of the decreased exploratory activity observed in these animals. Finally, the forced swimming test gave no indications of depressive-like behaviour since no differences were observed between the different genotypes in neither the percentage of time spent in immobility status (Table 4 B) nor in the latency to immobility time (not shown).
Figure 2. Decreased exploratory activity in homozygous and heterozygous Cln3Δex7/8 mice.
Eight weeks-old animals were tested in the open field apparatus. Homozygous and heterozygous Cln3Δex7/8 mice have a significant decrease in (A) the number of “rearings” performed and in (B) the total time spent in vertical exploration. (C) The total distance Cln3Δex7/8 mouse travelled during the test is normal while presenting a significant decreased in (D) the percentage of time spent in the centre of the field. Mean values are plotted with SEM (Wild type, n=24; heterozygous Cln3Δex7/8, n=27; homozygous Cln3Δex7/8, n=25).
Table 4.
Behavior of Wild type, homozygous and heterozygousCln3Δex7/8 knock-in eight months old mice in the elevated plus maze (Wild type, n=28; heterozygous Cln3Δex7/8, n=31; homozygous Cln3Δex7/8, n=27) (A), forced swimming (Wild type, n=10; heterozygous Cln3Δex7/8, n=13; homozygous Cln3Δex7/8, n=13) (B) and visual cued water maze tests (Wild type, n=9; heterozygous Cln3Δex7/8, n=12; homozygous Cln3Δex7/8, n=15) (C).
| Wild type | Cln3+/Δex7/8 | Cln3∆ex7/8/Δex7/8 | ||
|---|---|---|---|---|
|
(A) Elevated Plus Maze Time in open arms (%) (mean ± SEM) |
14.43± 2.617 | 13.80±2.125 | 13.25±2.444 | |
|
(B) Forced Swimming Immobility time (%) (mean ± SEM) |
59.48±8.531 | 75.76±2.364 | 65.71±4.192 | |
|
(C) Visual cued water maze Escape latency time (sec) (mean ± SEM) |
||||
| Day 1 | 71.83±6.306 | 82.58±6.345 | 72.48±5.861 | |
| Day 2 | 33.15±5.281 | 35.58±5.048 | 30.71±3.879 | |
| Day 3 | 27.11±5.285 | 20.81±2.832 | 16.20±2.222 | |
Juvenile Cln3Δex7/8 knock-in mice do not have marked visual impairment
Visual impairment is typically the first clinical sign detected in JNCL human patients (Kohlschutter et al., 1993). Therefore, we performed an analysis of visual acuity in eight weeks-old Wild type, heterozygous and homozygous Cln3Δex7/8 animals. For this purpose we used a modified version of the Morris water maze with visual clues to indicate the platform. Testing was performed over 3 days (four trials per day) and our results show no significant genotype-associated differences neither in time needed (Table 4 C) nor in the distance swam to escape to the visible platform (not shown).
Juvenile Cln3Δex7/8 mice show motor coordination impairment and ataxia
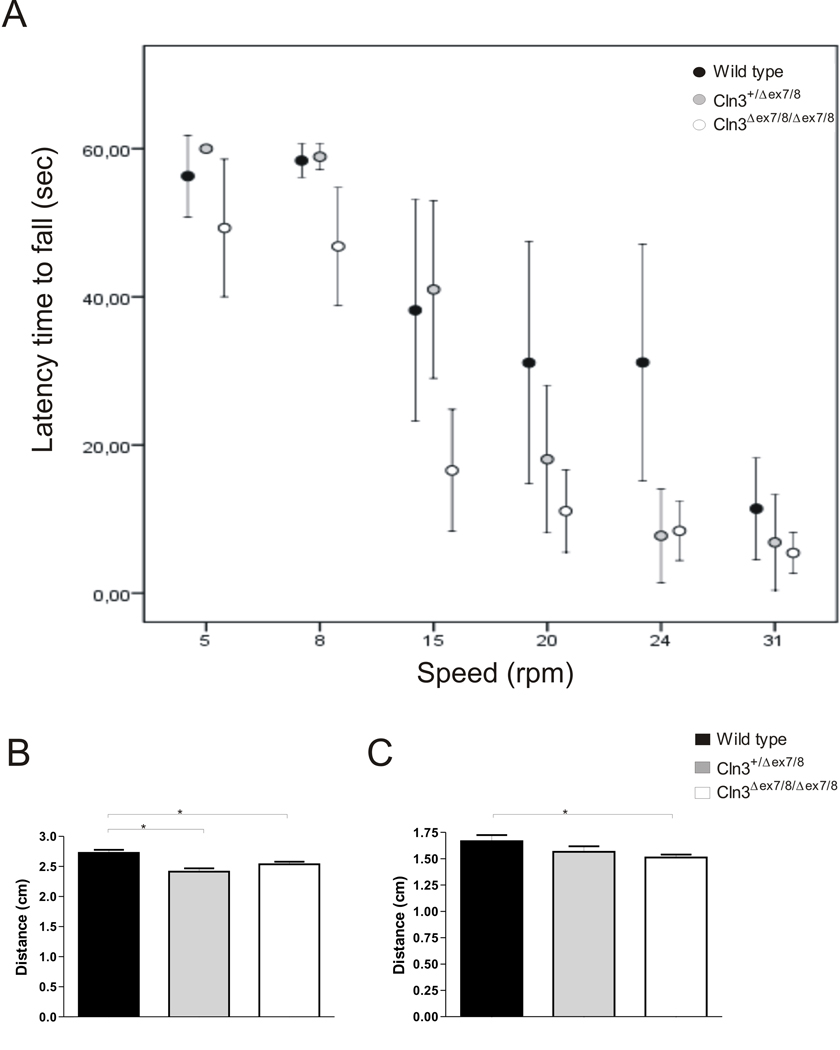
Motor deficits have been established as one of the primary clinical features in JNCL emerging early in disease progression (Raininko et al., 1990). In order to evaluate motor coordination we started by measuring the latency time required by 8 weeks-old Wild type, heterozygous and homozygous Cln3Δex7/8 knock-in to fall from rods rotating at the speed of 5, 8, 15, 20, 24 and 31 r.p.m. (Figure 3 A). Mean latency times were significantly different between groups, at the speeds of 15, 20 and 24 (Kruskal-Wallis test, Χ2(2)=11.379, 9.254, 8.058, respectively, p<0.05). Cln3Δex7/8 homozygous mice present significantly decreased latency times to fall at the speed of 15 (z=−2.246, p<0.05), 20 (z=−2.453, p<0.05) and 24 r.p.m. (z=−.565, p<0.05) when compared with the Wild type controls. Cln3Δex7/8 heterozygous mice only have significantly decreased latency time to fall from the rod when compared with Wild type controls at the speed of 24 r.p.m. (z=−2.380, p<0.05). We also analysed the ambulatory gait of Cln3Δex7/8 mice. Cln3Δex7/8 homozygous mice displayed decreased displacement between hind (q=2.614, p<0.05) (Figure 3 B) and fore paws (q=2.414, p<0.05) (Figure 3 C) when compared with Wild type controls, while presenting no stride length or stride matching differences (not shown), which is indicative of mild ataxia. Cln3Δex7/8 heterozygous only revealed decreased displacement in the hind paws (q=4.106, p<0.05) (Figure 3 B). Collectively, these results indicate motor deficits in eight weeks-old Cln3Δex7/8 homozygous mice and less severe motor deficits in heterozygous Cln3Δex7/8 mice.
Figure 3. Evidence for motor deficits in homozygous and heterozygous Cln3Δex7/8 mice.
(A) When tested in the rotarod homozygous eight weeks-old Cln3Δex7/8 mice have a significantly decreased latency to fall off the rod at the speeds of 15, 20 and 24 r.p.m, while heterozygous Cln3Δex7/8 mice only exhibit decreased latency to fall at the speed of 24 r.p.m. (Wild type, n=11; heterozygous Cln3Δex7/8, n=13; homozygous Cln3Δex7/8, n=17). Mean values are plotted with confidence interval at a 95% level. Ambulatory gait was also analysed and homozygous Cln3Δex7/8 mice have a significantly decreased displacement of (B) hind and (C) fore paws while heterozygous Cln3Δex7/8 animals only present a decrease in (B) hind paws displacement (Wild type, n=9; heterozygous Cln3Δex7/8, n=12; homozygous Cln3Δex7/8, n=15). Mean values are plotted with SEM.
Heterozygous Cln3Δex7/8 mice express intermediate levels of Wild type and mutant Cln3 mRNA
Comparative qRT-PCR was used to determine the relative expression of Cln3 mRNA transcripts in Wild type, heterozygous, and homozygous Cln3Δex7/8 mice. Previously, it was shown that Cln3Δex7/8 mice express multiple variant transcripts in response to the deletion of exons 7 and 8 from the genomic sequence (Cotman et al, 2002). Since heterozygote animals have one Wild type allele and one mutant allele, it is assumed that these mice would express partial transcripts lacking exons 7 and 8 (mutant transcript) in addition to the normal full-length transcript. In order to distinguish between full-length Wild type and mutant transcripts, primers were designed to detect either all Cln3 transcripts, or only full-length Wild type transcripts. Primer set 1 was designed to amplify a region within exon 1 through 3 that is upstream of the deleted region, and thus is common to both Wild type and mutant Cln3 transcripts. Primer set 2 amplifies a region within exon 6 through exon 8, and thus would detect only full-length Cln3 transcripts since mutant transcripts lack exons 7 and 8. Using primer set 1, which detects both full-length and mutant Cln3 transcripts, we observed no significant differences in Cln3 transcript levels in heterozygote mice in comparison to Wild type, but a 3.6-fold decrease (p<0.05) in transcript levels in the homozygous mice. Since no changes in the overall level of Cln3 was detected in heterozygotes, we sought to determine the relative contribution of full-length transcripts to the overall transcript level by using primer set 2 which is directed at the deleted region of Cln3 such that mutant transcripts lacking exon 7 and 8 are not detected. Using this approach, we observed a 1.8-fold decrease (p<0.05) in full-length transcripts in heterozygotes, and no detectable transcripts in homozygote animals. These results suggest that in heterozygote mice, approximately half of the transcripts present are full-length, with the remainder being mutant variant transcripts lacking exons 7 and 8.
DISCUSSION
The use of the Cln3lacZ reporter mouse model has revealed that Cln3 is expressed in the brain during embryonic and early postnatal stages (Eliason et al., 2007) further supporting early initiation of JNCL disease process (Cotman et al., 2002, Herrmann et al., 2008, Kovacs et al., 2006, Lake, 1993). In addition, it has been suggested that mutated polypeptides resulting from expression of Cln3Δex7/8, the most common JNCL mutation, may have biological function (Kitzmuller et al., 2008). Therefore, we studied the early postnatal behaviour of heterozygous and homozygous Cln3Δex7/8 knock-in mice. In homozygous Cln3Δex7/8 mice we detected a significant delay in the acquisition of negative geotaxis and grasping reflexes and also a decrease in the percentage of animals with postural reflex at PND 8 and in the wire suspension time at PND 16. Interestingly, we also detected alterations in heterozygous Cln3Δex7/8 mice including a less pronounced delay in the achievement of negative geotaxis and grasping reflex and a decrease in the percentage of animals with postural reflex at PND 8. These tasks evaluate vestibular and motor functions (Dierssen et al., 2002, Lake, 1993) and our results suggest that Cln3Δex7/8 induces a delay in the maturation of brainstem and cerebellar structures in homozygous but also heterozygous Cln3Δex7/8 mice. This is the earliest description of behavioural abnormalities in JNCL mouse models indicating delayed neurodevelopment as a putative novel component of JNCL disease. Adult behaviour phenotypes can be influenced by the deficit in one or more functional domains during neurodevelopment. In fact, it is possible that the detected developmental alterations are in the basis of the reduced exploratory activity encountered both in homozygous and heterozygous Cln3Δex7/8 young adult mice. When focusing the behavioural characterization in tests that assess the major and earlier clinical features of JNCL we found no evidence for visual impairment in young adult Cln3Δex7/8 mice. Visual loss is, commonly, the earliest symptom detected in patients and has been associated with retinal degeneration (Goebel, 1977) but the use of the visual cued version of Morris water maze pointed to the absence of marked visual impairment in Cln3Δex7/8 mice at juvenile age. This result is in accordance with previous reports showing minimal photoreceptor loss (Cotman et al., 2002, Seigel et al., 2002), normal electroretinograms (Seigel et al., 2002), and alterations in pupillary reflexes only in month-old animals (Katz et al., 2008). Motor deficits are another major clinical feature of JNCL with early onset, which is mainly attributed to cerebellar degeneration (Raininko et al., 1990). In accordance with data from other JNCL mouse models (Eliason et al., 2007, Kovacs et al., 2006), eight weeks-old homozygous Cln3Δex7/8 mice exhibit a marked motor impairment that is evident in the rotarod test. Interestingly, Cln3Δex7/8 heterozygous animals show a milder phenotype only detectable with increasing difficulty of the test. Additionally, previous studies also showed that 10–12 month old homozygous Cln3Δex7/8 mice exhibit alterations in ambulatory gait (Cotman et al., 2002). Herein, we demonstrate that gait alterations are already present in eight weeks-old homozygous Cln3Δex7/8 mice and are, again, present and milder in the heterozygous Cln3Δex7/8 mice. As the rotarod and gait analysis are highly influenced by cerebellar function, these behavioural results suggest that, as expected, homozygous Cln3Δex7/8 mice might undergo more pronounced cerebellar degeneration. The demonstration of behaviour deficits in heterozygous Cln3Δex7/8 mice is a novel finding of the present work since previous studies with the various JNCL murine models had never compared Wild type, heterozygous and homozygous animals (Cotman et al., 2002, Eliason et al., 2007, Katz et al., 2008, Mitchison et al., 1999). It has been reported that Cln3Δex7/8 knock-in mice exhibit earlier and more severe neurological disease (Cotman et al., 2002) when compared to Cln3LacZ and Cln3Δex1–6 mouse models. This observation could be suggestive that variant CLN3 transcripts might contribute to the pathologic alterations. Nonetheless, the studies that support a more severe phenotype in Cln3Δex7/8 knock-in might be influenced by strain background effects and genetic modifiers since different strains were used (Cotman et al., 2002, Eliason et al., 2007, Katz et al., 2008, Mitchison et al., 1999). This hypothesis is supported by the comparison of Cln3Δex7/8 knock-in mice with the Cln3Δex7/8 described by Katz et al. In these transgenic strains exons 7 and 8 are deleted, albeit using differing strategies and targeted regions both would result in a predicted truncated protein without exons 7 and 8. The Cln3Δex7/8 (Katz) model exhibited a later onset of pathological changes (Katz et al., 2008, Katz et al., 1999) in a time-frame more consistent with the Cln3Δex1–6 and Cln3LacZ mice. This illustrates the need to perform a comprehensive characterization of the available murine JNCL models on a standard genetic background to allow proper comparison. We analysed the transcripts present in Cln3Δex7/8 mice and found intermediate levels of Wild type and truncated Cln3 transcripts in heterozygous animals. A recent study suggests that the truncated CLN3 protein is unlikely to be expressed (Chan et al., 2008). Therefore, it is likely that Wild type CLN3 levels in heterozygous Cln3Δex7/8 animals are insufficient to maintain the required CLN3 activity resulting in the anomalies found. As previously suggested a threshold for CLN3 activity might exists before the onset of neurological disease (Cotman et al., 2002). Nevertheless, at this point we cannot fully discard a possible influence of alternative Cln3 transcripts on the observed phenotype and further studies at the biochemical and molecular levels are necessary to increase our understanding on this aspect. Irrespective of the underlying mechanism, it is interesting to note that existing studies in human patients have documented brain structural abnormalities and functional ophthalmological changes in JNCL carriers (Gottlob et al., 1988, Sayit et al., 2002) suggesting that heterozygous human carriers may also exhibit mild neurological alterations.
ACKNOWLEDGEMENTS
We thank Ana R. Mesquita and Anabela S. Fernandes from the Neuroscience Research Domain of the Life and Health Sciences Research Institute (ICVS), University of Minho for the excellent technical assistance and troubleshooting during this study. We are also grateful to Daniel S. Osório from the Facultée Médecine - Pitiée-Salpérièe, UPMC Paris VI for helpful discussion. This work was funded by Fundação para a Ciêcia e Tecnologia (FCT) project PTDC/SAU-NEU/70161/2006, NIH R01NS044310 and the Beat Batten Foundation. NSO was supported by the PhD grant 15318 from FCT.
REFERENCES
- Bennett MJ, Hofmann SL. The neuronal ceroid-lipofuscinoses (Batten disease): a new class of lysosomal storage diseases. J Inherit Metab Dis. 1999;22:535–544. doi: 10.1023/a:1005564509027. [DOI] [PubMed] [Google Scholar]
- Consortium. Isolation of a novel gene underlying Batten disease, CLN3. The International Batten Disease Consortium. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Vrbanac V, Lebel LA, Lee RL, Johnson KA, Donahue LR, Teed AM, Antonellis K, Bronson RT, Lerner TJ, MacDonald ME. Cln3(Deltaex7/8) knock-in mice with the common JNCL mutation exhibit progressive neurologic disease that begins before birth. Hum Mol Genet. 2002;11:2709–2721. doi: 10.1093/hmg/11.22.2709. [DOI] [PubMed] [Google Scholar]
- Chan CH, Mitchison HM, Pearce DA. Transcript and in silico analysis of CLN3 in juvenile neuronal ceroid lipofuscinosis and associated mouse models. Hum Mol Genet. 2008;11:2709–2721. doi: 10.1093/hmg/ddn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierssen M, Fotaki V, Martinez de Lagran M, Gratacos M, Arbones M, Fillat C, Estivill X. Neurobehavioral development of two mouse lines commonly used in transgenic studies. Pharmacol Biochem Behav. 2002;73:19–25. doi: 10.1016/s0091-3057(02)00792-x. [DOI] [PubMed] [Google Scholar]
- Eliason SL, Stein CS, Mao Q, Tecedor L, Ding SL, Gaines DM, Davidson BL. A knock-in reporter model of Batten disease. J Neurosci. 2007;27:9826–9834. doi: 10.1523/JNEUROSCI.1710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel HH. Ultrastructure of neuronal ceroid-lipofuscinoses. Fortschr Med. 1977;95:2432–2436. [PubMed] [Google Scholar]
- Gottlob I, Leipert KP, Kohlschutter A, Goebel HH. Electrophysiological findings of neuronal ceroid lipofuscinosis in heterozygotes. Graefes Arch Clin Exp Ophthalmol. 1988;226:516–521. doi: 10.1007/BF02169198. [DOI] [PubMed] [Google Scholar]
- Herrmann P, Druckrey-Fiskaaen C, Kouznetsova E, Heinitz K, Bigl M, Cotman SL, Schliebs R. Developmental impairments of select neurotransmitter systems in brains of Cln3(Deltaex7/8) knock-in mice, an animal model of juvenile neuronal ceroid lipofuscinosis. J Neurosci Res. 2008;6:1857–1870. doi: 10.1002/jnr.21630. [DOI] [PubMed] [Google Scholar]
- Hobert JA, Dawson G. A novel role of the Batten disease gene CLN3: association with BMP synthesis. Biochem Biophys Res Commun. 2007;358:111–116. doi: 10.1016/j.bbrc.2007.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen JM, Saarikoski J, Kinnunen PK, Jarvela I. Elevated lysosomal pH in neuronal ceroid lipofuscinoses (NCLs) Eur J Biochem. 2001;268:5851–5856. doi: 10.1046/j.0014-2956.2001.02530.x. [DOI] [PubMed] [Google Scholar]
- Katz ML, Johnson GS, Tullis GE, Lei B. Phenotypic characterization of a mouse model of juvenile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2008;29:242–253. doi: 10.1016/j.nbd.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Shibuya H, Liu PC, Kaur S, Gao CL, Johnson GS. A mouse gene knockout model for juvenile ceroid-lipofuscinosis (Batten disease) J Neurosci Res. 1999;57:551–556. [PubMed] [Google Scholar]
- Kitzmuller C, Haines RL, Codlin S, Cutler DF, Mole SE. A function retained by the common mutant CLN3 protein is responsible for the late onset of juvenile neuronal ceroid lipofuscinosis. Hum Mol Genet. 2008;17:303–312. doi: 10.1093/hmg/ddm306. [DOI] [PubMed] [Google Scholar]
- Kohlschutter A, Gardiner RM, Goebel HH. Human forms of neuronal ceroid-lipofuscinosis (Batten disease): consensus on diagnostic criteria, Hamburg 1992. J Inherit Metab Dis. 1993;16:241–244. doi: 10.1007/BF00710254. [DOI] [PubMed] [Google Scholar]
- Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, Gotow T, Peters C, von Figura K, Mizushima N, Saftig P, Uchiyama Y. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease) Am J Pathol. 2005;167:1713–1728. doi: 10.1016/S0002-9440(10)61253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AD, Weimer JM, Pearce DA. Selectively increased sensitivity of cerebellar granule cells to AMPA receptor-mediated excitotoxicity in a mouse model of Batten disease. Neurobiol Dis. 2006;22:575–585. doi: 10.1016/j.nbd.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Lake BD. Morphological approaches to the prenatal diagnosis of late-infantile and juvenile Batten disease. J Inherit Metab Dis. 1993;16:345–348. doi: 10.1007/BF00710280. [DOI] [PubMed] [Google Scholar]
- Mesquita AR, Pego JM, Summavielle T, Maciel P, Almeida OF, Sousa N. Neurodevelopment milestone abnormalities in rats exposed to stress in early life. Neuroscience. 2007;147:1022–1033. doi: 10.1016/j.neuroscience.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Mitchison HM, Bernard DJ, Greene ND, Cooper JD, Junaid MA, Pullarkat RK, de Vos N, Breuning MH, Owens JW, Mobley WC, Gardiner RM, Lake BD, Taschner PE, Nussbaum RL. Targeted disruption of the Cln3 gene provides a mouse model for Batten disease. Neurobiol Dis. 1999;6:321–334. doi: 10.1006/nbdi.1999.0267. [DOI] [PubMed] [Google Scholar]
- Narayan SB, Rakheja D, Tan L, Pastor JV, Bennett MJ. CLN3P, the Batten's disease protein, is a novel palmitoyl-protein Delta-9 desaturase. Ann Neurol. 2006;60:570–577. doi: 10.1002/ana.20975. [DOI] [PubMed] [Google Scholar]
- Osorio NS, Carvalho A, Almeida AJ, Padilla-Lopez S, Leao C, Laranjinha J, Ludovico P, Pearce DA, Rodrigues F. Nitric oxide signaling is disrupted in the yeast model for Batten disease. Mol Biol Cell. 2007;18:2755–2767. doi: 10.1091/mbc.E06-11-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce DA, Ferea T, Nosel SA, Das B, Sherman F. Action of BTN1, the yeast orthologue of the gene mutated in Batten disease. Nat Genet. 1999;22:55–58. doi: 10.1038/8861. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranam KL, Guo WX, Qian WH, Nikbakht K, Boustany RM. CLN3 defines a novel antiapoptotic pathway operative in neurodegeneration and mediated by ceramide. Mol Genet Metab. 1999;66:294–308. doi: 10.1006/mgme.1999.2834. [DOI] [PubMed] [Google Scholar]
- Raininko R, Santavuori P, Heiskala H, Sainio K, Palo J. CT findings in neuronal ceroid lipofuscinoses. Neuropediatrics. 1990;21:95–101. doi: 10.1055/s-2008-1071470. [DOI] [PubMed] [Google Scholar]
- Ramirez-Montealegre D, Pearce DA. Defective lysosomal arginine transport in juvenile Batten disease. Hum Mol Genet. 2005;14:3759–3773. doi: 10.1093/hmg/ddi406. [DOI] [PubMed] [Google Scholar]
- Santos M, Silva-Fernandes A, Oliveira P, Sousa N, Maciel P. Evidence for abnormal early development in a mouse model of Rett syndrome. Genes Brain Behav. 2007;6:277–286. doi: 10.1111/j.1601-183X.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Sayit E, Yorulmaz I, Bekis R, Kaya G, Gumuser FG, Dirik E, Durak H. Comparison of brain perfusion SPECT and MRI findings in children with neuronal ceroid-lipofuscinosis and in their families. Ann Nucl Med. 2002;16:201–206. doi: 10.1007/BF02996301. [DOI] [PubMed] [Google Scholar]
- Seigel GM, Lotery A, Kummer A, Bernard DJ, Greene ND, Turmaine M, Derksen T, Nussbaum RL, Davidson B, Wagner J, Mitchison HM. Retinal pathology and function in a Cln3 knockout mouse model of juvenile Neuronal Ceroid Lipofuscinosis (batten disease) Mol Cell Neurosci. 2002;19:515–527. doi: 10.1006/mcne.2001.1099. [DOI] [PubMed] [Google Scholar]