Abstract
To determine the optimum dose of artemisinin-piperaquine combination therapies for acute uncomplicated Plasmodium falciparum malaria, we examined 7 candidate regimens in 411 patients admitted to the Bangkok Hospital for Tropical Diseases. The studies were performed from May 2005 to October 2005 and November 2005 to June 2006. We compared 3-day courses of artesunate-mefloquine, artemether-lumefantrine (Coartem®) and of dihydroartemisinin-piperaquine (Artekin®) as reference antimalarial treatments, with candidate regimens using 2–3 day courses of artemisinin-piperaquine, Artequick®. Initially, patients receiving each of the regimens had a rapid clinical and parasitological response. All treatments were well tolerated and no serious adverse effects occurred. The 28-day cure rates were <80% for the 2-day treatments with artemisinin-piperaquine at 2.4 mg/kg and 14.4 mg/kg, respectively, in the first study period and artemisinin-piperaquine at 3.2 mg/kg and 16.0 mg/kg, respectively, but >98% for the 3-day regimens. These results suggest that a 3-day course of artemisinin-piperaquine at 3.2 mg/kg and 16.0 mg/kg, respectively, deserve further evaluation as an alternative treatment for multidrug-resistant P. falciparum malaria.
INTRODUCTION
The incidence of malaria has increased 2 to 3-fold over the past 4 decades and nearly half the world’s population now lives in regions endemic for malaria in Asia, Africa and South America (Hartl, 2004; Snow et al, 2005; Guerra et al, 2006). The World Health Organization (WHO) currently recommends artemisinin-based combination therapies (ACTs) as the best first-line treatment for uncomplicated falciparum malaria (WHO, 2006). In Southeast Asia, where P. falciparum is the most drug resistant in the world, a 3-day artesunate-mefloquine treatment (artesunate 12 mg/kg with mefloquine 25 mg/kg) is generally endorsed as the preferred treatment for uncomplicated malarial infection. Despite this recommendation, adoption of artesunate-mefloquine has been limited by high cost (~US$ 6 for a single adult treatment), the frequency of adverse effects associated with mefloquine and the lack of a formulation combining both antimalarials in a single tablet (Smithuis et al, 2006). In addition, reduced efficacy of artesunate-mefloquine has been reported recently from the southeastern border of Thailand (Vijaykadga et al, 2006). Recently dihydroartemisinin-piperaquine (Artekin®) in a fixed-dose preparation of dihydroartemisinin 40 mg and piperaquine 320 mg, Holleykin Pharmaceuticals, Guangzhou, People’s Republic of China, which has been studied in China, Vietnam, Cambodia, Thailand and elsewhere showed an efficacy of over 90% at once a day for 3 days treatment and has a low cost (~ US$ 1 per treatment course). Artemisinin-pipieraquine (Artequick®) which has been recently developed by Artepharm, Guangzhou, People’s Republic of China, showed good results when given in a 2 day treatment course in China and Cambodia (Li GR, personal communication, 2005). We performed a dose-ranging study for artemisinin-piperaquine to determine the efficacy, safety and tolerability of alternative short-course, artemisinin-based combination therapies for acute uncomplicated falciparum malaria. We compared 3-day courses of artesunate-mefloquine, artemetherlumefantrine (Coartem®) and dihydroartemisinin-piperaquine (Artekin®), as reference antimalarial treatments, with candidate regimens using 2–3 day courses of artemisinin-piperaquine (Artequick®).
MATERIALS AND METHODS
Patients
We prospectively studied patients with acute uncomplicated P. falciparum malaria admitted to the Bangkok Hospital for Tropical Diseases, Bangkok, Thailand. We included all male and female patients presenting with symptoms of malaria who met the inclusion criteria: (1) positive asexual forms of P. falciparum on blood smear, (2) age ≥ 13 years, (3) body weight ≥ 35 kg, (4) ability to take oral medication, and (5) agreement to stay in hospital for at least 28 days. We excluded patients who were pregnant or lactating, who had severe malaria according to WHO criteria (WHO, 2000), who had vomiting not allowing oral medication, who had concomitant systemic disorders or diseases other than malaria requiring therapy, and those with a history of ingestion of other antimalarials in the past 14 days or with sulfonamides (lignin test) or 4-aminoquinolones (Wilson-Edeson test) in the urine. Before enrollment in the study, informed consent for participation was obtained from all patients, or in children, from a parent or guardian. This study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Procedures
Patients were admitted to the hospital for 28 days to assess the safety, efficacy and tolerability of the antimalarial regimens and to exclude reinfection. The Bangkok metropolitan area has no known malaria transmission. The duration of symptoms before presentation, the presence of symptoms on admission, the history of any drug administration, and the findings on physical examination were entered on standard case-record forms. Parasite counts were performed every 12 hours until found to be negative, then daily for 28 days. Parasite density was expressed as the number of parasites per microliter of blood, which was derived from number of parasites per 1,000 red blood cells on a thin film stained with Giemsa stain or calculated from the white blood cell count and the number of parasites per 200 white blood cells on a thick film. Blood films were considered negative if no parasites were seen in 200 oil-immersion microscopic fields. The parasite clearance time (PCT) was measured as the interval from the start of antimalarial treatment until the asexual malaria parasite count fell below detectable levels on a peripheral blood smear. The fever clearance time was measured as the interval from the start of treatment until the oral temperature decreased to 37.5°C and remained below this temperature for the next 48 hours. The cure rate at day 28 (cured patients/evaluable patients × 100%) was defined as the absence of parasite recrudescence during 28 days of follow-up. For any RI, RII, or RIII failure (WHO, 1973), standard antimalarial drugs of the Bangkok Hospital for Tropical Diseases were given. Each drug dose was supervised and was repeated in full if vomiting occurred within 1 hour of administration.
Antimalarial drug treatment
Patients meeting study enrollment criteria were randomized to open-label treatment with one of the three regimens from May to October, 2005 and one of the four regimens from November, 2005 to June, 2006 The details of 7 regimens are showed in Table 1.
Table 1.
Antimalarial drug treatments.
| Study periods | Regimen | Artemisinin derivativesa |
Piperaquine | Mefloquine | Lumefantrine | Time of treatment |
Number of patients |
Note |
|---|---|---|---|---|---|---|---|---|
| May – October 2005 | 1 | ASN: 2.4 mg/kg | 14.4 mg/kg | - | - | 0 and 24 hours | 17 | Artequick1 |
| 2 | ASN:3.2 mg/kg | 16.0 mg/kg | - | - | 0, 12 and 24 hours | 37 | Artequick2 | |
| 3 | AS:4.0 mg/kg | - | 8.0 mg/kg | - | 0, 24 and 48 hours | 27 | ASMQ | |
| November 2005–June 2006 | 1 | ASN 2.5 mg/kg | 15.0 mg/kg | - | - | 0 and 24 hours | 78 | Artequick3 |
| 2 | ASN: 3.2 mg/kg | 16.0 mg/kg | - | - | 0, 24 and 48 hours | 61 | Artequick4 | |
| 3 | DHA:2.0 mg/kg | 15.0 mg/kg | - | - | 0, 24 and 48 hours | 97 | Artekin | |
| 4 | 4ATM:1.6 mg/kg | - | - | 9.6 mg/kg | 0, 12, 24, 36, 48 and 60 hours | 94 | Coartem |
ASN = Artemisinin, AS = Artesunate, DHA = Dihydroartemisinin, ATM = Artemether
Monitoring for adverse events
Adverse events were defined as signs and symptoms that developed or became more severe after antimalarial drug therapy began. Physical examinations were performed regularly and adverse events during the study were recorded with the date and time at which they began and ended. Symptoms were assessed on the basis of non-suggestive questioning by the study investigators; these included gastrointestinal, central nervous system, cardiovascular and dermatological effects, as well as other changes possibly attributable to the study drugs. Routine blood investigations of hematological and biochemical measures and urinalysis were performed prior to beginning treatment on day 0 and repeated on days 1 and 3 and then weekly for the 4 weeks of the study period.
Statistical analysis
Data are presented as means ± standard deviation (SD) or as the geometric mean and range. The normality of the distribution of data was assessed using the Shapiro-Wilks test. One-way analysis of variance was used in comparisons among treatment groups for clinical and laboratory data with a Gaussian distribution and with post hoc adjustment using the Bonferroni test for multiple comparisons. The nonparametric Kruskal-Wallis test with the Dunn’s post-test was used for comparisons among treatment groups for data not following a Gaussian distribution. Comparisons of proportions of categorical data used the χ2 test with Yates’ correction or Fisher’s exact test. All statistical tests were two-tailed and a significance level of 0.05 was used.
RESULTS
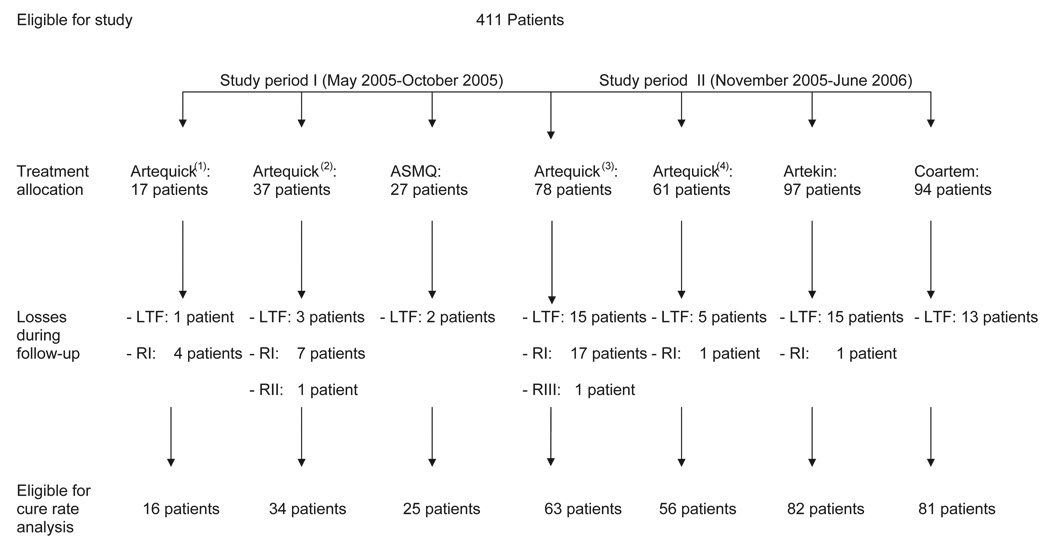
Overall, 287 male and 124 female patients, 13 to 60 years of age, were enrolled in this trial. Treatment allocation and losses during follow-up are shown in Fig 1. During the 28-day follow-up period, 54 patients left the trial for reasons related to family, employment or other social reasons, while 357 (86.9%) completed the study. No significant differences were found among the treatment groups in the distributions of demographics (gender, age, height, weight), clinical data (fever before admission, highest fever before treatment, number of patients with hepatomegaly, with splenomegaly and with first malaria attack) or laboratory data (hematocrit, white blood cell count, platelets, blood urea nitrogen, creatinine, bilirubin, aminotransferases, albumin, alkaline phosphatase and number of patients with G6PD deficiency) at baseline. On admission, patients in all treatment groups exhibited symptoms and signs commonly found with malarial infection, such as headache, dizziness, asthenia, fatigue, fever, nausea, anorexia, vomiting, diarrhea, abdominal pain and myalgia.
Fig 1.
Treatment allocation and losses during follow-up; LTF: lost to follow up; RI: reappearance of P. falciparum during follow-up; RII: early recrudescene; RIII: minimal antimalarial effect.
Initially, patients receiving each of the regimens had a favorable rapid response with the symptomatic manifestations present on admission gradually disappearing during the first days of treatment. No neurological or neuropsychatric manifestations developed during treatment or during the 28-day follow-up period. All patients recovered uneventfully and no severe adverse events were observed.
The fever and parasite clearance times and cure rates for each drug regimen are shown in Table 2. The fever clearance times for the two reference treatments, 3-day courses of artesunate-mefloquine and of artemether-lumefantrine, did not differ significantly, and were similar to that found with the 3-day dihydroartemisinin-piperaquine regimen. The fever clearance times for the two reference treatments were shorter than those found with the 2-day regimens of artemisinin-piperaquine. With respect to parasite clearance times, no significant difference was found between the two reference regimens (3-day courses of artesunate-mefloquine and of artemether-lumefantrine) and any of the alternative candidate regimens. However, the 3-dihydroartemisinin-piperaquine regimen showed the shortest parasite clearance time.
Table 2.
Therapeutic responses.
| Study period I May 2005–October 2005) |
Study period II (November 2005–June 2006) |
p-values | ||||||
|---|---|---|---|---|---|---|---|---|
| Artequick(1 (N = 17) |
Artequick(2) (N = 37) |
ASMQ (N = 27) |
Artequick(3) N = 78) |
Artequick(4) (N = 61) |
Artekin (N = 97) |
Coartem (N = 94) |
||
| No. of patients who dropped out | 1 (6.0%) | 3 (8.8%) | 2 (7.5%) | 15 (19.2%) | 5 (8.2%) | 15 (15.5%) | 13 (13.8%) | NA |
| No. of patients with 28 days follow-up | 16 (94.0%) | 34 (91.2%) | 25 (92.5%) | 63 (80.8%) | 56 (91.8%) | 82 (84.5%) | 81 (86.2%) | NA |
| No. (%) cured at 28 days | 12 (75.0%) | 26 (76.4%) | 25 (100%) | 45 (71.5%) | 55 (98.2%) | 81 (98.8%) | 81 (100%) | <0.001 |
| No. of treatment failures | 4 (25.0%) | 8 (23.6%) | 0 | 18 (28.5%) | 1 (1.8%) | 1 (1.2%) | 0 | NA |
| Fever clearance time (hrs) | ||||||||
| Mean (SD) | 49.0 (16.0) | 45.9 (33.1) | 23.0 (14.8) | 46.4 (31.0) | 31.8 (12.6) | 30.4 (30.0) | 33.5 (23.5) | <0.001 |
| Geometric mean parasite count (/µl) | 8,334 | 8,247 | 7,314 | 6,434 | 6,408 | 7,499 | 10,042 | 0.315 |
| Parasite clearance time (hrs) | ||||||||
| Mean (SD) | 56.1 (19.9) | 62.7 (25.6) | 51.0 (11.6) | 60.1 (35.5) | 51.0 (28.4) | 43.0 (16.7) | 52.2 (34.4) | 0.027 |
| 90% Parasite clearance time (hrs) | ||||||||
| Mean (SD) | 25.6 (6.4) | 23.2 (14.1) | 22.4 (5.9) | 28.2 (12.0) | 21.3 (10.0) | 21.2 (9.2) | 24.0 (8.7) | <0.001 |
| 50% Parasite clearance time (hrs) | ||||||||
| Mean (SD) | 13.4 (5.9) | 13.5 (9.0) | 11.7 (4.6) | 16.4 (9.3) | 10.1 (4.5) | 11.3 (5.7) | 13.1 (7.0) | <0.001 |
Artequick(1): ASN: 2.4 mg/kg + PQP:14.4 mg/kg; at 0 and 24 hours, Artequick(2): ASN: 3.2 mg/kg + PQP:16.0 mg/kg; at 0, 12 and 24 hours, Artequick(3): ASN: 2.5 mg/kg + PQP:15.0 mg/kg; at 0 and 24 hours, Artequick(4): ASN: 3.2 mg/kg + PQP:16.0 mg/kg; at 0, 24 and 48 hours, ASMQ: AS: 4.0 mg/kg + MQ: 8.0 mg/kg; at 0, 24 and 48 hours, Artekin: DHA: 2.0 mg/kg + PQP: 15.0 mg/kg; at 0, 24 and 48 hours, Coartem: ATM: 1.6 mg/kg + LFT: 9.6 mg/kg; at 0, 12, 24, 36, 48 and 60 hours
(ASN = Artemisinin, AS = Artesunate, PQP = Piperaquine, MQ = Mefloquine, DHA = Dihydroartemisinin, ATM = Artemether and LTM= Lumefantrine)
The cure rates for both reference treatments (3-day courses of artesunate-mefloquine and of artemether-lumefantrine) were 100% and did not differ significantly from those of the 3-day alternative treatments, which were >98% for both artemisinin-piperaquine and dihydroartemisinin-piperaquine. The cure rates for the two-day regimens (71.5 to 76.4%) were significantly lower than the preference regimens (p < 0.0001). The average cure rate at day 28 for the 2-day regimen was 73.4% (95% CI was 0.00–0.30, p ≤ 0.01). The number of patients with treatment failure is shown by day of study in Table 3. All treatment failures were of the delayed recrudescence RI type, except for one instance of early recrudescence RII type with the 2-day artemisinin-piperaquine (Artequick(2)) regimen and one case of minimal antimalarial effect (RIII) with the 2-day artemisinin-piperaquine (Artequick(3)) regimen. All patients with treatment failure were administered standard rescue antimalarial chemotherapy and were parasitologically negative on discharge from hospital.
Table 3.
Number of patients experiencing treatment failure by days.
| Day(s) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 7 | 13 | 14 | 16 | 18 | 19 | 20 | 21 | 22 | 23 | 26 | 28 |
| Artequick (1) | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Artequick (2)b | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1 |
| ASMQ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Artequick (3)a | 0 | 1 | 5 | 1 | 1 | 1 | 2 | 0 | 1 | 2 | 2 | 1 |
| Artequick (4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Artekin | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coartem | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
One case of minimal antimalarial effect (RIII)
One case of early recrudescence (RII)
DISCUSSION
Our exploratory study of alternative artemisinin-based combination therapies (ACTs) compared a series of five candidate treatments with reference regimens of 3-day courses of artesunate-mefloquine and of artemether-lumefantrine (Coartem®). Artemisinin compounds are potent and promptly acting antimalarials that quickly clear parasitemia and rapidly resolve symptoms (WHO, 2006). The artemisinins can reduce the P. falciparum biomass by ~10,000-fold in a single 48-hour asexual cycle of the blood stage infection (White, 2004) and are also active against gametocytes (Chotivanich et al, 2006), potentially reducing the transmissibility of malaria. Artemisinin derivatives have proven to be generally safe and well tolerated and have now been used to treat millions of patients over the past three decades (Davis et al, 2005b). The only antimalarial agents in use that have not elicited the emergence of clinical resistance, the artemisinins remain active against multi-drug resistant falciparum malaria. The elimination half-lives of the derivatives in clinical use are short – minutes to hours. As monotherapy, at least 7 days of treatment with artemisinins are needed; shorter courses are associated with recrudescence rates of 25% or higher. To improve efficacy, shorten the duration of therapy, and counter the threat of the emergence of resistance to the artemisinins, the WHO now recommends combination therapy with other safe, well-tolerated antimalarial(s) with independent modes of action (WHO, 2006).
Each of the five alternative regimens that we examined combined an artemisinin derivative with piperaquine as a component. Piperaquine is a bisquinoline antimalarial first synthesized in the 1960s and later used extensively in China and Southeast Asia for both prophylaxis and treatment (Davis et al, 2005a). Piperaquine is a highly lipid soluble drug with a large volume of distribution at a steady state/bioavailability and a long elimination half life. The mean terminal elimination half life (t1/2) is long (543 hours) (Davis et al, 2005a). These properties, together with its tolerability, efficacy and low cost have identified piperaquine as an excellent potential partner drug in ACT. In our studies, piperaquine was combined with either artemisinin or dihyroartemisinin, both poorly water-soluble forms.
Since compliance with antimalarial therapy would be enhanced by shorter and simpler regimens, we examined 2-day treatments combining artemisinin with piperaquine. Drug administration was completed within 24 hours. Unfortunately, with each of the 2-day regimens, roughly one-quarter of the patients treated developed treatment failure with late recrudescence during the 28 days of hospital observation. In these regimens, the artemisinin component was present during only a single asexual parasite life-cycle, apparently providing an insufficient reduction in the number of parasites to prevent frequent recrudescence. The better efficacy of artesunate-mefloquine may not be due to the half-life of mefloquine, since piperaquine has a similar half-life, but may due to the suboptimal doses of piperaquine and artemisinin which were only 3.2 mg/kg.
In contrast, both candidate 3-day regimens, with artemisinin-piperaquine (Artequick®) and dihydroartemisinin-piperaquine (Artekin®), gave cure rates >98% and were as well tolerated as the reference treatments. Our findings with the dihydroartemisinin-piperaquine combination (2 mg/kg/day of dihydroartemisinin and 15 mg/kg/day of piperaquine given by oral route in 3 doses over 48 hours) recapitulate our earlier experience (Tangpukdee et al, 2005) which found that this formulation was as effective and well-tolerated as artesunate-mefloquine. Several other studies have further demonstrated the effectiveness of this form of ACT (Denis et al, 2002; Karunajeewa et al 2003; Smithuis et al, 2006). Production of the dihydroartemisinin-piperaquine combination as Artekin has recently been upgraded to meet the international GMP standard, required for European registration; Phase 3 trials are now in progress. (Ashley and White, 2005). In our present study, we obtained equivalent results with the artemisininpiperaquine (Artequick®) combination.
In conclusion, we found that 2-day courses of artemisinin with piperaquine were associated with late recrudescence within the 28 days of hospital observation in about onequarter of the patients treated. In contrast, both candidate 3-day regimens, with artemisinin-piperaquine (Artequick®) and dihydroartemisinin-piperaquine (Artekin®), gave cure rates >98% and were as well tolerated as the reference treatments. Both combinations represent potential alternatives to artesunate-mefloquine and artemether-lumefantrine and deserve further evaluation, including better pharmacokinetic characterizations of these combinations, additional studies in children and pregnant women, and carefully conducted field trials.
Table 4.
Potential adverse effects after treatment in patients enrolled in the study comparing treatment 7 groups.
| Artequick(1) | Artequick(2) | ASMQ | Artequick(3) | Artequick(4) | Artekin | Coartem | |
|---|---|---|---|---|---|---|---|
| Headache | 5 (31) | 12 (35) | 3 (12) | 20 (32) | 14 (25) | 8 (10) | 5 (6) |
| Weakness | 4 (25) | 7 (20) | 2 (8) | 17 (27) | 5 (9) | 9 (11) | 7 (9) |
| Dizziness | 4 (25) | 7 (20) | 3 (12) | 19 (30) | 4 (7) | 7 (8) | 5 (6) |
| Anorexia | 1 (6) | 5 (15) | 1 (4) | 5 (8) | 2 (3) | 4 (5) | 4 (5) |
| Nausea | 1 (6) | 5 (15) | 3 (12) | 8 (13) | 2 (3) | 4 (5) | 3 (4) |
| Vomiting | 0 | 2 (6) | 2 (8) | 1 (1) | 0 | 4 (5) | 0 |
| Insomnia | 2 (12) | 4 (12) | 1 (4) | 7 (12) | 4 (7) | 9 (11) | 3 (4) |
| Abdominal pain | 4 (25) | 4 (12) | 1 (4) | 9 (14) | 5 (9) | 8 (10) | 5 (6) |
| Diarrhea | 2 (12) | 1 (3) | 0 | 4 (6) | 2 (3) | 3 (4) | 1 (1) |
Data are number (%) of patients.
ACKNOWLEDGEMENTS
We thank the nurses of the Bangkok Hospital for Tropical Diseases for their excellent care of the patients. This study was supported by a Mahidol University Research Grant.
REFERENCES
- Ashley EA, White NJ. Artemisinin-based combinations. Curr Opin Infect Dis. 2005;18:531–536. doi: 10.1097/01.qco.0000186848.46417.6c. [DOI] [PubMed] [Google Scholar]
- Chotivanich K, Sattabongkot J, Udomsangpetch R, et al. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob Agents Chemother. 2006;50:1927–1930. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TM, Hung TY, Sim IK, et al. Piperaquine: a resurgent antimalarial drug. Drugs. 2005a;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- Davis TM, Karunajeewa HA, Ilett KF. Artemisinin-based combination therapies for uncomplicated malaria. Med J Aust. 2005b;182:181–185. doi: 10.5694/j.1326-5377.2005.tb06650.x. [DOI] [PubMed] [Google Scholar]
- Denis MB, Devis TM, Hewitt S, et al. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin®) in Cambodian children and adults with uncomplicated falciparum malaria. CID. 2002;35:1469–1476. doi: 10.1086/344647. [DOI] [PubMed] [Google Scholar]
- Guerra CA, Snow RW, Hay SI. Mapping the global extent of malaria in 2005. Trends Parasitol. 2006;22:353–358. doi: 10.1016/j.pt.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl DL. The origin of malaria: mixed messages from genetic diversity. Nat Rev Microbiol. 2004;2:15–22. doi: 10.1038/nrmicro795. [DOI] [PubMed] [Google Scholar]
- Karunajeewa H, Lim C, Hung TY, et al. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin®) in Cambodian children and adults with malaria. Br J Clin Pharmacol. 2003;57:93–99. doi: 10.1046/j.1365-2125.2003.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithuis F, Kyaw MK, Phe O, et al. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: an open-label randomised comparison. Lancet. 2006;367:2075–2085. doi: 10.1016/S0140-6736(06)68931-9. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangpukdee N, Krudsood S, Thanachartwet W, et al. An open randomized clinical trial of Artekin vs artesunate-mefloquine in the treatment of acute uncomplicated falciparum malaria. Southeast Asian J Trop Med Public Health. 2005;36:1085, 1091. [PubMed] [Google Scholar]
- Vijaykadga S, Rojanawatsirivej C, Cholpol S, Phoungmanee D, Nakavej A, Wongsrichanalai C. In vivo sensitivity monitoring of mefloquinemonotherapy and artesunate-mefloquine combinations for the treatment of uncomplicated falciparum malaria in Thailand in 2003. Trop Med Int Health. 2006;11:211–219. doi: 10.1111/j.1365-3156.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Chemotherapy of malaria and resistance to antimalarials. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1973;529:1–121. [PubMed]
- World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:1–90. [PubMed]
- World Health Organization. Geneva: WHO Press; 2006. Guidelines for the treatment of malaria. [Google Scholar]