Abstract
We prospectively studied 803 Thai patients admitted to the Bangkok Hospital for Tropical Diseases to assess the safety, tolerability and effectiveness of treatments for strictly defined P. falciparum malaria. Patients were assigned to one of five treatment groups: (i) a 5-day course of intravenous artesunate in a total dose of 600 mg, Group Aiv; (ii) intravenous artesunate as in Group Aiv followed by mefloquine, 25 mg/kg, Group Aiv+M; (iii) a 3-day course of intramuscular artemether in a total dose of 480 mg, Group Aim; (iv) intramuscular artemether as in Group Aim followed by mefloquine, 25 mg/kg, Group Aim+M, and (v) intravenous quinine, 200 mg/kg given in divided doses over seven days followed by oral tetracylcine, 10 mg/kg, for 7 days. When patients could take oral medications, the parenteral antimalarials were administered as oral agents. There were no major adverse effects observed with any of the five treatment regimens. With all regimens, 95 to 100% of the patients survived. Mean parasite clearance times were more rapid with the artemisinin regimens (53 to 62 hours) than with quinine (92 hours). The mean fever clearance times with intravenous artesunate (80 to 82 hours) were about a day shorter than those with intramuscular artemether (108 hours) or intravenous quinine (107 hours). Mefloquine reduced the recrudescence rate from 24 to 5% with intravenous artesunate but from 45 to 20% with intramuscular artemether; recrudescence was 4% with quinine and tetracycline. A dose and duration of therapy greater than those in this study are needed for optimal therapy with intramuscular artemether. Effective therapy for severe falciparum malaria can be provided by either intravenous artesunate followed by mefloquine or by intravenous quinine followed by tetracycline.
INTRODUCTION
A recent meta-analysis of trials comparing artemether with quinine in the treatment of severe falciparum malaria determined that “artemether is at least as effective as quinine in terms of mortality and superior in terms of overall serious adverse events” (Artemether-Quinine Meta-analysis Study Group, 2001). While this conclusion was reassuring, the optimal choice of antimalarial drugs for the treatment of severe falciparum malaria remains problematic, especially in Southeast Asia. In this region, with low malaria transmission, little or no acquired immunity is present and symptomatic malaria may occur at any age. In the past decade, artemisinin derivatives (artemether and artesunate) have become established as the most useful drugs in Southeast Asia but are still not available in all areas. Quinine is generally available, but resistance (Looareesuwan et al, 1990; Pukrittayakamee et al, 1994; Karbwang et al, 1994) and poor response to this drug have been encountered. The meta-analysis included a total of 1,919 patients; 657 of these were from Southeast Asia: 97 from Thailand and 560 from Vietnam) (Artemether-Quinine Meta-analysis Study Group, 2001). We believe that it may be helpful to describe the clinical experience at the Hospital for Tropical Diseases in the use not only of artemether, given intramuscularly, but also of artesunate, given intravenously, and the effects of adding mefloquine after administration of the artemisinin derivatives has been completed. We further describe our experience with intravenous quinine, followed by oral tetracycline. Although these prospective studies of 803 patients with severe falciparum malaria were not randomized, the clinical results may provide useful new information about the relative efficacy, safety and tolerability of these antimalarials and about the risks of recrudescence of falciparum malaria with these regimens.
MATERIALS AND METHODS
Patients
This report describes patients admitted to the Hospital for Tropical Diseases, Bangkok, Thailand, between 1993 and 1996 with severe P. falciparum malaria. The patients were enrolled in investigational drug studies examining the five regimens described below. Each study protocol had been approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Each participant (or for patients with cerebral malaria, their relative or next of kin) gave fully informed consent. The inclusion criteria for these studies were: age 15 years or older, severe falciparum malaria, willingness to give informed consent, body weight of at least 40 kilograms, and agreement to remain in hospital for 28 days. Severe complications of P. falciparum malaria were strictly defined according to World Health Organization (WHO) criteria (WHO, 1990) and included one or more of the following: (i) hyperparasitemia: patients with parasitemia more than 500,000/ml or >2% of erythrocytes infected; (ii) renal failure: serum creatinine concentration >3 mg/ dl (>265 mmol/l), urine output of less than 400 ml in 24 hours, despite rehydration, necessity to receive hemodialysis or any combination of these factors; (iii) severe anemia: hemoglobin <5 g/dl, (iv) hypoglycemia: venous blood glucose <40 mg/dl (<2.2 mmol/l); and (v) cerebral malaria with deep coma: Glasgow coma score <8 and no other evident cause of coma. Patients who were pregnant were excluded, as were those with concomitant P. vivax infection, those with a history of drug hypersensitivity or of taking antimalarials during the fortnight prior to admission.
Procedures
After clinical evaluation and examination of thick and thin blood smears to establish the diagnosis, blood samples were obtained for baseline hematological and biochemical studies. All patients received antimalarial drug therapy as part of clinical trials. They were assigned to one of five treatment groups:
(1) Group Aiv: patients were administered artesunate (60 mg/vial, diluted in 1 ml of NaHCO3), 2.4 mg/kg given by intravenous injection as a loading dose, and then 1.2 mg/kg by intravenous injection 12-hourly until oral artesunate (50 mg/tablet) could be taken to provide a total dose of 600 mg over 5 days.
(2) Group Aiv+M: patients were treated as in Group Aiv with the addition of two doses of oral mefloquine (25 mg/kg given in 2 divided doses 8 hours apart) given 12 hours after the last dose of artesunate.
(3) Group Aim: patients were administered artemether (80 mg/ampoule) 3.2 mg/kg given by intramuscular injection as a loading dose, then 1.6 mg/kg by intramuscular injection at 12, 24, 48, and 72 hours to provide a total dose of 480 mg. When patients were able to take oral medication, oral artemether (50 mg/capsule) was substituted in the same dose as that administered intramuscularly.
(4) Group Aim+M: patients were treated as in Group Aim with the addition of two doses of oral mefloquine (25 mg/kg given in 2 divided doses 8 hours apart) given 12 hours after the last dose of artesunate.
(5) Group Q+T: patients were administered quinine, 20 mg/kg diluted in 4 mL/kg 0.9% normal saline by intravenous infusion over 4 hours, then 10 mg/kg diluted in 2 ml/kg 0.9% normal saline by intravenous infusion over 2 hours given every 8 hours for a total of 20 doses. When patients were able to take oral medication, oral quinine (10 mg/kg) was substituted in the same dose as that administered intravenously. Oral tetracycline, (10 mg/kg) was then given every 8 hours for 7 days.
Oral temperature, pulse and respiratory rates, and blood pressur were measured every 4 hours until resolution of fever and thereafter every 6 to 12 hours; blood pressure was measured once a day. Patients were closely monitored for signs and symptoms of malaria while in the Intensive Care Unit, examined daily after discharge to the ward the first 7 days after admission and then weekly thereafter. Fever clearance time (FCT) was defined as the time from the start of a patient’s treatment until the oral temperature fell below 37.5∞C and remained at or below this level for 48 hours. Parasite clearance time (PCT) was defined as the time from the start of a patient’s treatment until the patient’s first negative blood film, with the blood film then remaining negative for 24 hours.
All patients were hospitalized for 28 days or until the reappearance of parasites. Patients in whom P. falciparum parasitemia reappeared after initial parasite clearance were considered to have recrudescence. Patients found to be co-infected by asexual forms of P. vivax were treated with the hospital’s standard regimen for vivax malaria: chloroquine (30 mg base/kg once daily for 3 days) and primaquine (15 mg once a day for 14 days). These patients were excluded from the analysis of the 28-day cure rates.
Laboratory studies
Age, sex, temperature (°C), initial liver size and spleen size were recorded. A complete blood count, including hemoglobin, packed cell volume, mean corpuscular volume, white blood cell count, and platelets, were determined by an automated cell counter (Coulter T-890, Hialeah FL, USA). Serum concentrations of total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, creatinine, blood urea nitrogen (BUN) and electrolytes were measured with an automated serum chemistry analyzer (Technicon RA-2000, Miles Inc, Tarrytown NY, USA). Direct and total bilirubin were determined. The indirect bilirubin was derived by subtracting the direct bilirubin from the total bilirubin. These tests were repeated on days 1, 7, 14, 21 and 28. A screening test for glucose-6-phosphate dehydrogenase (G6PD) deficiency was conducted on admission. Thick and thin blood films were stained with Giemsa stain and the parasite counts were determined by counting the number of asexual parasites per 200 white blood cells (thick films) or per 1,000 red blood cells (thin films); blood films were deemed negative if no parasites were seen in 200 oil immersion fields of a thick blood film. Blood transfusion was given if the hemoglobin concentration was less than 5g/dl, or if there was evidence of marked anemia and congestive heart failure.
RESULTS
A total of 803 patients with severe falciparum malaria (Group Aiv: 298, Group Aiv+M: 219, Group Aim: 128, Group Aim+M 76, Gr Q+T 82) was enrolled in the clinical trials summarized here. There were no major adverse effects observed with any of the five treatment regimens. Rigors occurred during the comatose period in all groups. Minor symptoms and signs, which occurred after recovery from coma, such as headache, nausea, vomiting, abdominal pain and tremors, were similar in all groups. During the recovery period, distinguishing adverse reactions to drugs from the post-cerebral malaria syndrome was not possible except for tinnitus, which was more prominent in patients who received quinine (Group Q+T). No episodes of convulsion were observed after treatment with mefloquine in Group Aiv+M or Group Aim+M. All signs and symptoms disappeared by 1 to 2 weeks after recovery and recovery was complete within the 28 day period of hospitalization.
Clinical and laboratory findings in the five therapeutic groups at baseline are summarized in Table 1. Each of the groups was predominantly male, with mean ages in the mid-twenties, and most (70 to 81%) were having their first malarial attack, and geometric mean parasite counts on admission were in the range 224,146/μl to 298,000/μl. Admission laboratory data were generally similar in the five treatment groups.
Table 1.
Baseline characteristics of patients, values are MEAN ± SD.
| Group Aiv (n=298) |
Group Aiv+M (n=219) |
Group Aim (n=128) |
Group Aim+M (n=76) |
Group Q+T (n=82) |
|
|---|---|---|---|---|---|
| Male (%) | 69 | 78 | 80 | 82 | 85 |
| Age (year) | 28 (11) | 28 (12) | 26 (10) | 25 (8) | 26 (9) |
| Min-max | 3-74 | 6-72 | 14-65 | 15-55 | 15-60 |
| Fever | |||||
| Duration before admission (day) | 4.6 (3.0) | 4.9 (2.6) | 4.5 (2.4) | 4.4 (2.1) | 4.5 (2.4) |
| Highest fever before treatment (°C) | 38.2 (1.0) | 38.0 (1.0) | 38.3 (1.0) | 38.2 (0.9) | 38.2 (1.0) |
| Proportion of patients with: | |||||
| Splenomegaly (%) | 13 | 20 | 19 | 9 | 11 |
| Hepatomegaly (%) | 36 | 42 | 43 | 17 | 28 |
| First malaria attack (%) | 76 | 74 | 81 | 70 | 71 |
| Geometric mean parasite (/μl) | 245,910 | 227,166 | 298,778 | 254,439 | 224,146 |
| Max | 3,533,500 | 1,310,400 | 1,780,020 | 1,109,420 | 1,052,050 |
| Min | 18 | 1 | 102 | 510 | 59 |
| Laboratory data | |||||
| Packed cell volume (%) | 34 (8) | 35 (9) | 35 (8) | 37 (7) | 34 (8) |
| WBC count (x103 per μl) | 8.3 (4.8) | 7.9 (4.0) | 7.9 (3.6) | 7.8 (3.5) | 7.8 (4.1) |
| Blood urea (mg/dl) | 38 (34) | 40 (35) | 42 (36) | 34 (29) | 34 (24) |
| Serum creatinine (mg/dl) | 2 (1) | 2 (2) | 2 (2) | 2 (1) | 2 (1) |
| Total bilirubin (U/l) | 6 (7) | 7 (9) | 5 (7) | 6 (9) | 6 (8) |
| Serum AST (U/l) | 142 (236) | 102 (115) | 98 (84) | 103 (101) | 167 (378) |
| Serum ALT (U/l) | 84 (112) | 69 (69) | 58 (51) | 63 (44) | 94 (135) |
| Albumin (mg/dl) | 3 (1) | 3 (1) | 4 (1) | 4 (1) | 4 (1) |
AST = aspartate aminotransferase; ALT = alanine aminotransferase.
Table 2 summarizes the proportions of patients in each of the five groups with major complications of severe malaria and the interventions required in their care (intubation for ventilatory support, transfusion and hemodialysis). Six hundred and eighty-nine patients (689) completed the study, remaining in hospital for the full 28-day period. The proportions of patients with cerebral malaria were greater in those receiving intravenous artesunate (14 to 21% in Group Aiv+M and Group Aiv, respectively) than in the other treatment groups (4, 7 and 10% in Groups Aim+M, Aim and Q+T, respectively). The proportions of patients requiring intubation were also greater in the groups receiving intravenous artesunate, with or without mefloquine, than in the other treatments groups. Group Aim+M had the lowest proportion of patients with anemia who required blood transfusion. With respect to other severe complications, the groups were generally similar in the proportions of patients with azotemia, acute renal failure, hyperparasitemia and jaundice.
Table 2.
Severity and clinical resolution.
| Group Aiv (n=298) |
Group Aiv+M (n=219) |
Group Aim (n=128) |
Group Aim+M (n=76) |
Group Q+T (n=82) |
|
|---|---|---|---|---|---|
| Cerebral malaria (%) | 21 | 14 | 7 | 4 | 10 |
| Glasgow coma score, range | 4-12 | 4-12 | 4-12 | 4-12 | 4-12 |
| Median time to regain consciousness (days) |
2.9 | 3.0 | 3.1 | 3.0 | 3.2 |
| Range (days) | 1-6 | 1-6 | 2-6 | 1-6 | 1-6 |
|
Proportion of patients requiring intubation (%) |
14 | 6 | 2 | 1 | 4 |
| duration of intubation (days) | 1-16 | 1-12 | 2-3 | 3 | 5-6 |
| Anemia (%) | 32 | 26 | 28 | 8 | 22 |
| Proportion of patients needing blood transfusion (%) |
32 | 25 | 28 | 8 | 22 |
| Mean units of blood transfused/patient |
1 | 2.6 | 3.0 | 3.0 | 3.0 |
|
Acute renal failure (%) (required hemodialysis) |
6 | 5 | 3 | 5 | 7 |
| Median number of hemodialyses/patient |
6 | 6 | 7 | 11 | 5 |
| Median duration (days) of hemodialysis/patient |
9 | 9 | 10 | 12 | 8 |
| Azotemiaa (%) | 12 | 12 | 11 | 13 | 13 |
| Hyperparasitemiab (%) | 60 | 53 | 74 | 78 | 67 |
| Jaundice (%) | 69 | 65 | 70 | 71 | 65 |
| Raised serum transaminase enzyme/levelc (%) |
54 | 52 | 56 | 47 | 61 |
Raised creatinine and/or blood urea nitrogen more than 3 times normal levels, which returned to normal after symptomatic treatment.
Parasitemia over 100,000/ml or >2% RBC infected.
Raised over 3 times normal.
The clinical outcomes after treatment are shown in Table 3. The mean parasite clearance times were shorter in the groups receiving artemisinin derivatives (53 to 62 hours) than in the group receiving quinine. The patterns of parasite clearance are shown for each of the five groups in Fig 1; the curves for the artemisinin treatments are almost identical, while that for the group receiving quinine is slower throughout the period of clearance. Despite these differences, the mean fever clearance times for the groups receiving intramuscular artemether and the group receiving intravenous quinine were almost identical (107 to 108 hours). The mean fever clearance times for the groups receiving intravenous artesunate (80 to 82 hours) were more than 25 hours shorter than those of the other treatment groups. The proportions of patients who died ranged from 0 to about 5%. Cure rates were higher (and rates of recrudescence lower) in the treatment groups that received artemisinines with mefloquine or the quinine-tetracyline combination. The proportions of patients in whom P. vivax appeared during the 28 days of observation were lower in the groups receiving intravenous artesunate than in the other treatment groups.
Table 3.
Therapeutic responses, values are MEAN ± SD.
| Group Aiv (n=298) |
Group Aiv+M (n=219) |
Group Aim (n=128) |
Group Aim+M (n=76) |
Group Q+T (n=82) |
|
|---|---|---|---|---|---|
| Proportion of patients with 28 days follow up (%) |
85 | 85 | 89 | 79 | 90 |
| Parasite clearance time (h) | 56 (29) | 53 (21) | 62 (20) | 56 (18) | 92 (32) |
| Min-max | 7-368 | 8-108 | 25-152 | 25-129 | 18-208 |
| Fever clearance time (h) | 80 (77) | 82 (60) | 108 (89) | 108 (115) | 107 (74) |
| Min-max | 4-618 | 4-322 | 12-510 | 8-666 | 8-392 |
| Died (%) | 2 | 0 | 2 | 0 | 5 |
| Cured (%) | 74 | 95 | 53 | 80 | 91 |
| Recrudescence (RI) (%) | 24 | 5 | 45 | 20 | 4 |
| Day at recrudescence (range) | 11-34 | 16-27 | 12-28 | 14-32 | 21-25 |
| P. vivax +ve patients (%) | 9 | 4 | 18 | 24 | 26 |
Fig 1.
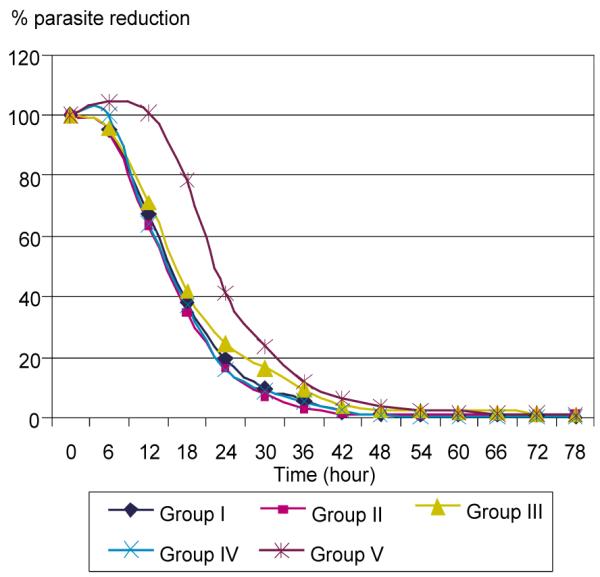
Comparison of percent parasite reduction among 5 regimens.
DISCUSSION
Overall, the proportions of patients who died in these prospective studies of patients with strictly defined severe malaria at the Hospital for Tropical Disease are among the lowest reported in any of the studies included in the recent meta-analyses of artemether (Artemether-Quinine Meta-analysis Study Group, 2001; Pittler and Ernst, 1999). Almost certainly, a major factor responsible is the care provided by the Intensive Care Unit of the hospital. The modern facilities for diagnosis and management make possible the rescue of many patients who would have died in the absence of hemodialysis, ventilatory support, rapid access to transfusion and the extensive experience of the staff in the management of severe malaria and its complications. Because these studies were neither randomized nor blind, valid statistical comparisons of the clinical outcomes of the various treatment groups can not be made. Furthermore, we have no means of taking account of the contributions of differences in malarial strains, host factors and other features of the complex interactions involved in the pathogenesis of severe falciparum malaria. Nonetheless, if these limitations are kept in mind, the clinical experience accumulated in these trials may be instructive. Comparison of the mortality rates found with quinine and those found with the two artemisinin derivatives leads to the same conclusion reached in the meta-analyses (Artemether-Quinine Meta-analysis Study Group, 2001; Pittler and Ernst, 1999). In terms of mortality, treatment with the artemisinines is at least as effective as treatment with quinine, and may be superior.
Similarly, the patterns of parasite clearance found in our trials, shown in Table 3 and Fig 1, closely resemble the overall findings of the meta-analytic study (Artemether-Quinine Meta-analysis Study Group, 2001). Parasite clearance is more rapid with the artemisinin regimens than with quinine, an almost universal observation in comparative trials (White, 1997; Karbwang et al, 1994; Newton et al, 2001; Win et al, 1992; Murphy et al, 1986). Despite the rapidity of parasite clearance, we often observed an increase in parasitemia during the first 24 hours of treatment, or “early rising parasitemia” (Silachamroon et al, 2001). Although this seemingly paradoxical increase in parasite count shortly after the initiation of therapy may cause concerns about resistance to the artemisinines or treatment failure, the increase may be an indicator of a large sequestered parasite mass (White, 1997; Gravenor et al, 1998).
Despite the greater speed of parasite removal, mean fever clearance times with the artemether treatments were almost identical to those found with quinine, again reproducing the summary findings of the meta-analytic study (Artemether-Quinine Meta-analysis Study Group, 2001). By contrast, the intravenous artesunate regimens reduced the fever clearance time by at least a day with respect to the intramuscular artemether and quinine treatments (ie, from 107-108 hours to 80-82 hours). A previous study in Vietnamese adults with severe or complicated malaria reported a similar finding: the median time of defervescence was 48 hours [95% confidence interval (CI) 38-58 hours] with intramuscular artemether and 30 hours (95% CI 26-34 hours) with intravenous artesunate, although the difference was not significant (p=0.13) (Ha et al, 1997). We also emphasize that the total dose and duration of intravenous artesunate treatment, 600 mg over 5 days, were greater and longer than those of intramuscular artemether, 480 mg over 3 days. These differences should be considered in assessing the more rapid defervescence with intravenous artesunate.
As shown in Table 3, the regimen with intramuscular artemether alone had a rate of recrudescence (45%) that was almost twice that of intravenous artesunate (24%). This difference is almost certainly related to the duration of therapy in these patients, most of whom probably had no antimalarial immunity. To achieve maximum cure rates, blood levels of an antimalarial drug must exceed the minimum parasiticidal concentration for at least four asexual cycles, or more than 6 days (White, 1997). Accordingly, neither artemisinin regimen as monotherapy was very effective. Adding mefloquine at a dose of 25 mg base/kg body weight (Simpson et al, 2000) still left the 3-day regimen with intramuscular artemether with a high rate of recrudescence, but in combination with the 5 days of intravenous artesunate, the rate reduced to 5%. The 7-day regimen of quinine, followed by 7 days of tetracycline produced a recrudescence of 4%; this combination therapy has been effective in Thailand for more than 20 years (Looareesuwan et al, 1992a,b,c; Pukrittayakamee et al, 2000).
We first evaluated sequential treatment with artesunate and mefloquine a decade ago (Looareesuwan et al, 1992d; Looareesuwan et al, 1997a,b) in the treatment of recrudescent falciparum malaria and have found this regimen uniformly effective. Continued experience with this regimen and the concept of artemisinin-based combination therapy lead us to consider this therapy one of the most effective for the treatment of multidrug resistant falciparum malaria in Thailand and elsewhere in Southeast Asia. The evidence also suggests that both a higher dose (total dose of 600 mg) and longer duration (5 days) of artemether are needed, and that use in combination with mefloquine or other antimalarial is recommended (Bunnag et al, 1991; 1992). Finally, the experience presented here also illustrates that the combination of quinine with tetracyline remains an effective antimalarial regimen that can be used in circumstances where artesunate is not available.
ACKNOWLEDGEMENTS
We would like to acknowledge the staff of the Hospital for Tropical Diseases, especially the nurses and other personnel in the Intensive Care Unit for their help and support. This study was supported in part by the National Science and Technology Development Agency (NSTDA), Thailand, by a Mahidol University grant and by the National Institutes of Health, USA, Grant Number R01 AI51310.
REFERENCES
- Artemether-Quinine Meta-analysis Study Group A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:637–50. doi: 10.1016/s0035-9203(01)90104-x. [DOI] [PubMed] [Google Scholar]
- Bunnag D, Karbwang J, Harinasuta T. Artemether in the treatment of multiple drug resistant falciparum malaria. Southeast Asian J Trop Med Public Health. 1992;23:762–7. [PubMed] [Google Scholar]
- Bunnag D, Viravan C, Looareesuwan S, Karbwang J, Harinasuta T. Clinical trial of artesunate and artemether on multidrug resistant falciparum malaria in Thailand. A preliminary report. Southeast Asian J Trop Med Public Health. 1991;22:380–5. [PubMed] [Google Scholar]
- Gravenor MB, van Hensbroek MB, Kwiatkowski D. Estimating sequestered parasite population dynamics in cerebral malaria. Proc Natl Acad Sci USA. 1998;95:7620–4. doi: 10.1073/pnas.95.13.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha V, Nguyen NH, Tran TB, et al. Severe and complicated malaria treated with artemisinin, artesunate or artemether in Viet Nam. Trans R Soc Trop Med Hyg. 1997;91:465–7. doi: 10.1016/s0035-9203(97)90287-x. [DOI] [PubMed] [Google Scholar]
- Karbwang J, Bangchang KN, Thanavibul A, Wattanakoon Y, Harinasuta T. Quinine toxicity when given with doxycycline and mefloquine. Southeast Asian J Trop Med Public Health. 1994;25:397–400. [PubMed] [Google Scholar]
- Karbwang J, Na-Bangchang K, Thanavibul A, Bunnag D, Chongsuphajaisiddhi T, Harinasuta T. Comparison of oral artesunate and quinine plus tetracycline in acute uncomplicated falciparum malaria. Bull WHO. 1994;72:233–8. [PMC free article] [PubMed] [Google Scholar]
- Looareesuwan S, Charoenpan P, Ho M, et al. Fatal Plasmodium falciparum malaria after an inadequate response to quinine treatment. J Infect Dis. 1990;161:577–80. doi: 10.1093/infdis/161.3.577. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Harinasuta T, Chongsuphajaisiddhi T. Drug resistant malaria, with special reference to Thailand. Southeast Asian J Trop Med Public Health. 1992b;23:621–34. [PubMed] [Google Scholar]
- Looareesuwan S, Kyle DE, Viravan C, et al. Treatment of patients with recrudescent falciparum malaria with a sequential combination of artesunate and mefloquine. Am J Trop Med Hyg. 1992c;47:794–9. doi: 10.4269/ajtmh.1992.47.794. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Viravan C, Vaanijanonta S, et al. Randomized trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria. Lancet. 1992d;339:821–4. doi: 10.1016/0140-6736(92)90276-9. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Wilairatana P, Molunto W, Chalermrut K, Olliaro P, Andrial M. A comparative clinical trial of sequential treatments of severe malaria with artesunate suppository followed by mefloquine in Thailand. Am J Trop Med Hyg. 1997a;57:348–53. doi: 10.4269/ajtmh.1997.57.348. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Wilairatana P, Vanijanonta S, Kyle D, Webster K. Efficacy of quinine-tetracycline for acute uncomplicated falciparum malaria in Thailand. Lancet. 1992a;339:369. doi: 10.1016/0140-6736(92)91690-a. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Wilairatana P, Viravan C, Vanijanonta S, Pitisutthithum P, Kyle DE. Open randomized trial of oral artemether alone and a sequential combination with mefloquine for acute uncomplicated falciparum malaria. Am J Trop Med Hyg. 1997b;56:613–7. doi: 10.4269/ajtmh.1997.56.613. [DOI] [PubMed] [Google Scholar]
- Murphy S, English M, Waruiru C, et al. An open randomizes trial of artemether versus quinine in the treatment of cerebral malaria in African children. Trans R Soc Trop Med Hyg. 1986;90:298–301. doi: 10.1016/s0035-9203(96)90260-6. [DOI] [PubMed] [Google Scholar]
- Newton PN, Chierakul W, Ruangveerayuth R, et al. A comparison of artesunate alone with combined artesunate and quinine in the parenteral treatment of acute falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:519–23. doi: 10.1016/s0035-9203(01)90025-2. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Ernst E. Artemether for severe malaria: a metaanalysis of randomized clinical trials. Clin Infect Dis. 1999;28:597–601. doi: 10.1086/515148. [DOI] [PubMed] [Google Scholar]
- Pukrittayakamee S, Chantra A, Vanifanonta S, Clemens R, Looareesuwan S, White NJ. Therapeutic responses to quinine and clindamycin in multidrug-resistant falciparum malaria. Antimicrob Agents Chemother. 2000;44:2395–8. doi: 10.1128/aac.44.9.2395-2398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukrittayakamee S, Supanaranond W, Looareesuwan S, Vanijanonta S, White NJ. Quinine in severe falciparum malaria: evidence of declining efficacy in Thailand. Trans R Soc Trop Med Hyg. 1994;88:324–7. doi: 10.1016/0035-9203(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Silachamroon U, Phumratanaprapin W, Krudsood S, et al. Frequency of early rising parasitemia in falciparum malaria treated with artemisinin derivatives. Southeast Asian J Trop Med Public Health. 2001;32:50–6. [PubMed] [Google Scholar]
- Simpson JA, Watkins ER, Price RN, Aarons L, Kyle DE, White NJ. Mefloquine pharmacokinetic-pharmacodynamic models: implications for dosing and resistance. Antimicrob Agents Chemother. 2000;44:3414–24. doi: 10.1128/aac.44.12.3414-3424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–22. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win K, Than M, Thwe Y. Comparison of combinations of parenteral arteminisin derivatives plus oral mefloquine with intravenous quinine plus oral tetracycline for treating cerebral malaria. Bull World Health Organ. 1992;70:777–82. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84(suppl 2):S1/1. [PubMed] [Google Scholar]


