Abstract
Over the past several decades, much has been revealed about the nature of the host innate immune response to microorganisms, with the identification of pattern recognition receptors (PRRs) and pathogen-associated molecular patterns, which are the conserved microbial motifs sensed by these receptors. It is now apparent that these same PRRs can also be activated by non-microbial signals, many of which are considered as damage-associated molecular patterns. The sterile inflammation that ensues either resolves the initial insult or leads to disease. Here, we review the triggers and receptor pathways that result in sterile inflammation and its impact on human health.
Inflammation is vital for host defence against invasive pathogens. In response to an infection, a cascade of signals leads to the recruitment of inflammatory cells, particularly innate immune cells such as neutrophils and macrophages. These cells, in turn, phagocytose infectious agents and produce additional cytokines and chemokines that lead to the activation of lymphocytes and adaptive immune responses. Similar to the eradication of pathogens, the inflammatory response is also crucial for tissue and wound repair (BOX 1). Inflammation as a result of trauma, ischaemia–reperfusion injury or chemically induced injury typically occurs in the absence of any microorganisms and has therefore been termed ‘sterile inflammation’. Similar to microbially induced inflammation, sterile inflammation is marked by the recruitment of neutrophils and macrophages and the production of pro-inflammatory cytokines and chemokines, notably tumour necrosis factor (TNF) and interleukin-1 (IL-1).
Although inflammation is important in tissue repair and eradication of harmful pathogens, unresolved, chronic inflammation that occurs when the offending agent is not removed or contained can be detrimental to the host. The production of reactive oxygen species (ROS), proteases and growth factors by neutrophils and macrophages results in tissue destruction, as well as fibroblast proliferation, aberrant collagen accumulation and fibrosis. There are several examples of sterile inflammatory diseases. Chronic inhalation of sterile irritants, such as asbestos and silica, can lead to persistent activation of alveolar macrophages and result in pulmonary interstitial fibrosis1. In ischaemia–reperfusion injury, as seen with myocardial infarction and stroke, the restoration of blood flow causes further tissue destruction as a result of neutrophilic infiltration, enhanced production of ROS and inflammatory responses to necrotic cells2. Sterile inflammation has also been implicated in such disease processes as gout and pseudogout, in which the deposition of monosodium urate (MSU) and calcium pyrophosphate dihydrate (CPPD) crystals in the joints results in acute neutrophilic infiltration followed by chronic inflammation, fibrosis and cartilage destruction3. In Alzheimer's disease, neurotoxicity is associated with activated microglial cells adjacent to β-amyloid-containing plaques that generate ROS in addition to pro-inflammatory cytokines4. Sterile inflammation is also an important component of atherosclerosis, as engulfment of cholesterol crystals by macrophages leads to the activation and recruitment of inflammatory cells, endothelial cell dysfunction and plaque formation5. Finally, immune cell infiltration in the absence of microorganisms is also characteristic of tumours, and these cells can influence the growth and progression of cancer6. Thus, understanding the mechanisms of sterile inflammation is important for devising treatment strategies against various human diseases.
As the inflammation induced in response to sterile cell death or injury is similar to that observed during microbial infection, host receptors that mediate the immune response to microorganisms may be involved in the activation of sterile inflammation. In the case of infection, the mechanisms by which the inflammatory response is initiated have been well studied. There are several classes of receptors that are important for sensing microorganisms and for the subsequent induction of pro-inflammatory responses (for a review, see REF. 7). These have been collectively termed pattern recognition receptors (PRRs). These germline-encoded PRRs sense conserved structural moieties that are found in microorganisms and are often called pathogen-associated molecular patterns (PAMPs). Five classes of PRRs have been identified to date: Toll-like receptors (TLRs), which are transmembrane proteins located at the cell surface or in endosomes; NOD-like receptors (NLRs), which are located in the cytoplasm; RIG-I-like receptors (RLRs), which are also located intracellularly and are primarily involved in antiviral responses; C-type lectin receptors (CLRs), which are transmembrane receptors that are characterized by the presence of a carbohydrate-binding domain; and absence in melanoma 2 (AIM2)-like receptors, which are characterized by the presence of a pyrin domain and a DNA-binding HIN domain involved in the detection of intracellular microbial DNA8. Following ligand recognition or cellular disruption, these receptors activate downstream signalling pathways, such as the nuclear factor-κb (NF-κb), mitogen-activated protein kinase (MAPK) and type I interferon pathways, which result in the upregulation of pro-inflammatory cytokines and chemokines that are important in inflammatory and antimicrobial responses.
It is now evident that PRRs also recognize non-infectious material that can cause tissue damage and endogenous molecules that are released during cellular injury (TABLE 1). These endogenous molecules have been termed damage-associated molecular patterns (DAMPs), as these host-derived non-microbial stimuli are released following tissue injury or cell death and have similar functions as PAMPs in terms of their ability to activate pro-inflammatory pathways. Here, we discuss the nature of these instigators of inflammation in the absence of infection, the potential mechanisms by which they are recognized by the host to activate inflammatory pathways and the implications for disease management.
Table 1.
Sterile stimuli
| Sterile inflammatory signal | Putative sensor | Associated pathology | Refs* |
|---|---|---|---|
| Endogenous | |||
| HMGB1 | TLR2, TLR4, TLR9, RAGE and CD24 | Cellular injury and necrosis | 26,93,98,106 |
| HSPs | TLR2, TLR4, CD91, CD24, CD14 and CD40 | Cellular injury and necrosis | 11,25,106,122 |
| S100 proteins | RAGE | Cellular injury and necrosis | 19 |
| SAP130 | CLEC4E | Cellular injury and necrosis | 72 |
| RNA | TLR3 | Cellular injury and necrosis | 39,123 |
| DNA | TLR9 and AIM2 | Cellular injury and necrosis | 40,48–50 |
| Uric acid and MSU crystals | NLRP3 | Gout | 13,55 |
| ATP | NLRP3 | Cellular injury and necrosis | 20,60 |
| Hyaluronan | TLR2, TLR4 and CD44 | Cellular injury and necrosis | 31,32,103 |
| Biglycan | TLR2 and TLR4 | Cellular injury and necrosis | 14,33 |
| Versican | TLR2 | Cellular injury and necrosis | 34 |
| Heparan sulphate | TLR4 | Cellular injury and necrosis | 124 |
| Formyl peptides (mitochondrial) | FPR1 | Cellular injury and necrosis | 125 |
| DNA (mitochondrial) | TLR9 | Cellular injury and necrosis | 125 |
| CPPD crystals | NLRP3 | Pseudogout | 55 |
| β-amyloid | NLRP3, CD36 and RAGE | Alzheimer's disease | 56,94,105 |
| Cholesterol crystals | NLRP3 and CD36 | Atherosclerosis | 59,105 |
| IL-1α | IL-1R | Cellular injury and necrosis | 15,22,41 |
| IL-33 | ST2 | Cellular injury and necrosis | 16,86 |
| Exogenous | |||
| Silica | NLRP3 | Silicosis and pulmonary interstitial fibrosis | 44,57,58 |
| Asbestos | NLRP3 | Asbestosis and pulmonary interstitial fibrosis | 57 |
AIM2, absent in melanoma 2; CLEC4E, C-type lectin 4E; CPPD, calcium pyrophosphate dihydrate; DAMP, damage-associated molecular pattern; FPR1, formyl peptide receptor 1; HMGB1, high-mobility group box 1; HSP, heat shock protein; IL, interleukin; MSU, monosodium urate; IL-1R, IL-1 receptor; NLRP3, NOD-, LRR- and pyrin domain-containing 3; RAGE, receptor for advanced glycation end products; SAP130, spliceosome-associated protein 130; TLR, Toll-like receptor.
References may not be all inclusive.
DAMPs: indicators of tissue injury
A common feature of DAMPs is that they are endogenous factors that are normally sequestered intracellularly and are therefore hidden from recognition by the immune system under normal physiological conditions. However, under conditions of cellular stress or injury, these molecules can then be released into the extracellular environment by dying cells and trigger inflammation under sterile conditions. The type of cell death notably affects its immunogenicity and ability to release immunostimulatory DAMPs. However, in general, DAMPs can be construed as signals of a potential danger to the host9 (BOX 2). Necrosis usually occurs under conditions of extreme damage (for example, ischaemia or trauma) when apoptosis fails to occur. An important consequence of necrotic cell death is the loss of plasma membrane integrity, thereby allowing escape of intracellular material from the cell. Prototypical DAMPs derived from necrotic cells include the chromatin-associated protein high-mobility group box 1 (HMGb1)10, heat shock proteins (HSPs)11, and purine metabolites, such as ATP12 and uric acid13 (TABLE 1). In addition to DAMPs from an intracellular source, there are also extracellularly located DAMPs. These are typically released by extracellular matrix degradation during tissue injury. extracellular matrix fragments, such as hyaluronan, heparan sulphate and biglycan, are generated as a result of proteolysis by enzymes released from dying cells or by proteases activated to promote tissue repair and remodelling14. Similarly, in addition to intracellular molecules, intracellular stores of biologically active pro-inflammatory cytokines and chemokines, such as IL-1α15 and IL-33 (REF. 16), may be released by necrotic cells. Although these factors are not conventionally considered as DAMPs, they can mediate sterile inflammatory responses (see below).
DAMPs have been identified by their ability to induce inflammatory responses in vitro and/orin vivo when purified and by the observed reduction in inflammation when they are selectively depleted17. However, in addition to the concern that the stimulatory activity of some DAMPs is attributed to contamination of purified preparations with bacterial products, important questions remain unanswered. It is unclear, for example, whether some of the DAMPs that have been identified based on their ability to stimulate pro-inflammatory cytokine production in vitro have a role in inducing sterile inflammation in vivo, as is the case for HSPs11,18, S100 calcium-binding proteins19 and ATP20. Furthermore, many DAMPs, such as HSPs and HMGb1, seem to interact with several receptors (TABLE 1) and, therefore, the significance of these interactions during sterile inflammation and disease pathogenesis remains to be fully elucidated. Also unknown is the relative importance of individual DAMPs — that is, whether they have redundant roles or whether a predominant DAMP (the expression of which may depend on the inciting event) triggers sterile inflammation. For example, a reduction of uric acid, which is released from dying cells and has adjuvant activity in vivo21, was associated with substantially reduced neutrophil recruitment in the liver after acetaminophen-induced injury in two different mouse models13. by contrast, in particulate-induced sterile inflammation, uric acid depletion had no effect, suggesting that uric acid may be a major pro-inflammatory DAMP that is specifically involved in cell death-related sterile inflammation13. However, uric acid depletion does not completely eliminate acetaminophen-induced liver inflammation or the adjuvant activity of damaged cells, which may reflect residual uric acid following depletion or redundant activities by other DAMPs.
The context of cellular injury leading to sterile inflammation may also be important. In one study, treatment of mice with HMGb1-specific antibodies during acetaminophen-induced liver necrosis ameliorated inflammatory cell recruitment10. However, in a peritoneal model of sterile inflammation, there was no difference between wild-type and HMGb1-deficient necrotic cells in their ability to promote neutrophilic recruitment22. Thus, although substantial progress has been made in identifying potential DAMPs that can elicit inflammatory responses, much remains to be learnt, such as the different biological functions of the various DAMPs during sterile inflammation, which would be important for identifying new therapeutic targets.
Mechanisms of sterile inflammation
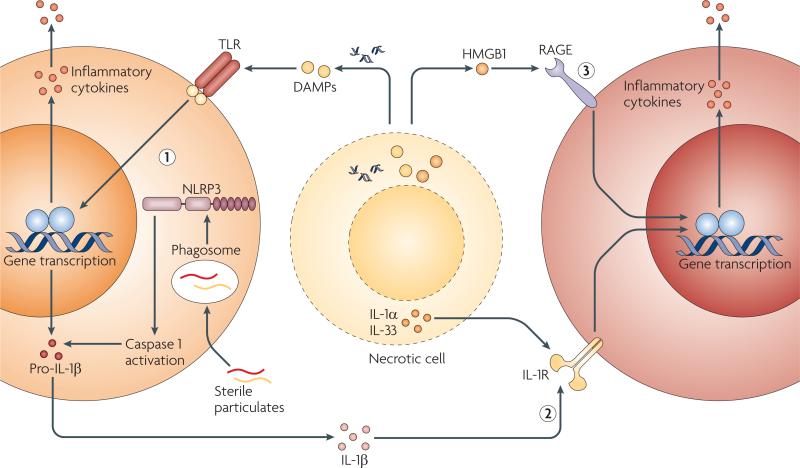
Despite the growing list of sterile immune stimuli, the mechanisms by which these stimuli trigger an inflammatory response are still not fully understood. even though endogenously generated DAMPs are structurally heterogeneous, the outcome of inflammatory responses to these stimuli is generally uniform. Moreover, inflammatory responses during infection are very similar to responses induced by sterile stimuli, including the recruitment of neutrophils and macrophages, the production of inflammatory cytokines and chemokines, and the induction of T cell-mediated adaptive immune responses23. This suggests that both infectious and sterile stimuli may function through common receptors and pathways. based on our current understanding, we propose three, not necessarily mutually exclusive, mechanisms by which sterile endogenous stimuli trigger inflammation: activation of PRRs by mechanisms similar to those used by microorganisms and PAMPs; release of intracellular cytokines and chemokines, such as IL-1α, that activate common pathways downstream of PRRs; and direct activation by receptors that are not typically associated with microbial recognition (FIG. 1).
Figure 1. Mechanisms for inducing sterile inflammation.
Sterile stimuli that include damage-associated molecular patterns (DAMPs), sterile particulates and intracellular cytokines released from necrotic cells can activate the host immune system to induce sterile inflammation through at least three pathways that are not mutually exclusive. DAMPs and sterile particulates can active host pathogen recognition receptors (PRRs), such as the Toll-like receptors (TLRs) and the nucleotide-binding oligomerization domain (NOD)-like receptor NLRP3 (NOD-, LRR- and pyrin domain-containing 3), which are also used by the host to sense microorganisms. Activation of these receptors results in the upregulation of cytokines and chemokines, such as interleukin-1β (IL-1β), which are released to recruit and activate additional inflammatory cells (1). Intracellular cytokines such as IL-1α and IL-33 that are released by damaged, necrotic cells activate signalling pathways downstream of PRRs (2). Endogenous DAMPs signal directly through host receptors that are not typically considered to be PRRs or to be involved in microbial detection (3). HMGB1, high-mobility group box 1; IL-1R, IL-1 receptor; RAGE, receptor for advanced glycation end-products.
Role of PRRs: recognition of endogenous DAMPs by TLRs
There is mounting evidence that TLRs sense endogenous molecules in addition to microbial PAMPs. The possibility that mammalian TLRs recognize non-microbial structures is not unexpected, because the evolutionarily conserved Toll receptor originally identified in Drosophila melanogaster binds to the endogenous ligand Spätzle24. Indeed, several endogenous molecules that are released from necrotic cells or are present in the extracellular matrix have been reported to activate TLRs. These include intracellular proteins, such as HSPs, S100 proteins, uric acid, HMGb1 and endogenous nucleic acids, as well as components of the extracellular matrix, such as hyaluronan, heparan sulphate and proteoglycans17.
Although several of the purified endogenous molecules, including HSPs, HMGb1 and uric acid, can induce the production of pro-inflammatory cytokines through TLR2 and/or TLR4 in vitro25–27, the relevance of these observations has previously been questioned because of the possibility of microbial contamination: several of these proteins, including HSPs and HMGb1, bind lipopolysaccharide (LPS), making the interpretation of pro-inflammatory responses by these molecules difficult28–30. because mice lacking TLR2 and TLR4 have only a slight reduction in the peritoneal inflammatory response to sterile dead cells in vivo22, a reasonable conclusion is that TLR2–TLR4 signalling is not the main mechanism by which intracellular factors induce inflammatory responses to necrotic cell death. However, this does not exclude a role for TLR signalling in other models of sterile inflammation. For example, there is evidence for a role of TLR2 and/or TLR4 in the induction of cytokine and chemokine production in response to extracellular matrix components, including hyaluronan fragments or soluble proteoglycan components, in vitro and in vivo31–34. Tlr2 and Tlr4 double-knockout mice exhibit impaired transepithelial recruitment of inflammatory cells and decreased survival in response to lung injury31. Deficiency in either TLR2 or TLR4 signalling also reduced atherosclerotic disease in mouse models35–37, and TLR4, specifically, has been shown to mediate inflammatory responses to free fatty acids, which are elevated in obesity and are associated with insulin resistance38. Similarly, TLR3 can sense endogenous RNA derived from necrotic neutrophils, which contributes to the inflammatory response elicited by necrosis in the bowel39. In the liver, which is normally devoid of microbial exposure, TLR9 has been implicated in triggering cytokine production and hepatocyte toxicity in acetaminophen-induced injury40. In this model of sterile inflammation, activation of TLR9 is presumably mediated by genomic DNA released from necrotic hepatocytes40. Collectively, the evidence suggests that endogenous stimuli can induce a sterile inflammatory response through TLRs, although the precise molecules that engage TLRs remain poorly defined. Furthermore, TLRs seem to function redundantly with other signalling pathways, so the contribution of TLRs to the overall sterile inflammatory response remains unclear.
Role of PRRs: generation of IL-1β by inflammasomes
IL-1, which includes both IL-1α and IL-1β, is a key mediator of sterile inflammation that acts through the IL-1 receptor (IL-1R)22,41. IL-1β is a potent pro-inflammatory cytokine that is produced mainly by macrophages and has many biological functions that are important in sterile inflammation, such as the upregulation of those adhesion molecules on endothelial cells that are important for the recruitment of neutrophils and monocytes42 and for the induction of additional pro-inflammatory mediators43. Compared with wild-type mice, IL-1R-deficient mice had decreased neutrophilic infiltration and inflammation in the liver after acetaminophen-induced liver injury22 and in the lungs after exposure to silica44.
IL-1β has been implicated in various non-microbial pro-inflammatory diseases. In atherosclerosis, engulfment of cholesterol by macrophages leads to IL-1β production, which can stimulate the production of platelet-derived growth factor, promotion of smooth muscle cell and fibroblast proliferation, arterial wall thickening and plaque formation5,45. Crystal-induced arthropathies, such as gout, are associated with elevated IL-1β production, which leads to joint inflammation and destruction5. elevated levels of IL-1β are also observed in the pancreatic islet cells from patients with type 2 diabetes46, which is increasingly being recognized as having a strong inflammatory component47. The secretion of IL-1β by inflammatory cells is largely dependent on a multiprotein complex termed the inflammasome, of which the hallmark activity is the activation of caspase 1. Following activation, caspase 1 proteolytically cleaves IL-1β into its biologically active form. Caspase 1 also cleaves the IL-1 family member IL-18 into its active form and, therefore, IL-18 may potentially be involved in sterile responses, but its relevance in sterile inflammation has not been well studied. There are several inflammasomes that have been described to date, and each is named after the specific PRR contained in it. Of these inflammasomes, two have been described that can sense non-microbial molecules: the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome and the AIM2 inflammasome.
The AIM2 inflammasome has been recently shown to recognize cytoplasmic double-stranded DNA (dsDNA) that is not necessarily microbial in origin, resulting in caspase 1 activation and IL-1β secretion48–50. Although distinct from NLRs, AIM2 has a pyrin domain that allows it to interact with the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) to form the inflammasome50. AIM2 has a demonstrated role in immune responses directed against both bacteria51 and DNA viruses52, but it will be important to determine whether there is a physiological role for AIM2 in sterile inflammation, especially in autoimmune diseases such as systemic lupus erythematosus, which is associated with elevated circulating levels of dsDNA.
Our understanding of how IL-1β production is induced during sterile inflammation has been advanced by studies of the NLRP3 inflammasome. NRLP3 was initially identified as an important mediator of chronic inflammation owing to its association with auto-inflammatory disorders (BOX 3). Since then, NLRP3 has been implicated in various sterile inflammatory diseases. NLRP3 signalling has been shown to involve at least two steps, the first involving PRR- or cytokine-dependent transcriptional upregulation of NLRP3 and the second involving activation of the NLRP3 inflammasome, which leads to IL-1β production53,54 (BOX 4). A unique feature of NLRP3 is its ability to sense various structurally diverse stimuli. Therefore, it is posited that NLRP3 does not recognize each stimulus individually, but senses a common downstream event. NLRP3 has been shown to respond to sterile stimuli, including asbestos, silica, MSU and CPPD crystals55, cholesterol crystals and β-amyloid fibrils56. Consistently, in mouse models of sterile injury, such as gout, asbestosis and silicosis, NLRP3-deficient mice exhibited decreased inflammation with reduced levels of tissue infiltration by neutrophils or macrophages55,57,58. Low-density lipoprotein (LDL) receptor-deficient mice, which are prone to developing atherosclerotic lesions, had lower IL-1β levels, smaller atherosclerotic lesions and less neutrophilic infiltration than wild-type mice when lethally irradiated and infused with NLRP3-deficient bone marrow59. NLRP3-deficient mice were also less susceptible to renal ischaemia–reperfusion injuries induced by bilateral renal artery ligation and to acetaminophen-induced hepatotoxicity40,60. A potential role for NLRP3 in promoting elevated levels of IL-1β in type 2 diabetes was also implied by the observation of NLRP3-dependent IL-1β secretion by mouse bone marrow-derived macrophages and dendritic cells in response to islet amyloid polypeptide (IAPP), which is deposited in the pancreas and associated with loss of β-cell function in type 2 diabetes61. Interestingly, the anti-diabetic drug glyburide (Glibenclamide; Roche/Sanofi-Aventis) has been shown to block glucose-mediated secretion of IL-1β by mouse pancreatic islets62, as well as IL-1β production by macrophages in response to IAPP61, and NLRP3-deficient mice were more glucose tolerant than wild-type mice62. However, whether NLRP3 is directly involved in the pathogenesis of type 2 diabetes in humans remains to be determined. Regardless, understanding how NLRP3 senses diverse sterile stimuli is important for understanding the pathogenesis of possibly many sterile inflammatory disorders and for identifying potential therapeutic targets. There are three main pathways that have been proposed to mediate sterile activation of NLRP3 (FIG. 2). These can be categorized as ATP-, lysosomal damage- or ROS-mediated activation.
Figure 2. Proposed pathways for nlRP3 activation.
The activation of the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome has been associated with three separate phenomena. ATP can bind to purinergic receptor P2X7 (P2RX7), which then opens a cation channel and a large pore through pannexin 1 that, in turn, can lead to ionic fluxes, including intracellular K+ depletion, and other events that are poorly understood. Endocytosis of sterile particulates, such as silica, asbestos and cholesterol crystals, results in lysosomal damage and membrane destabilization, leading to activation of the protease cathepsin B. The generation of reactive oxygen species (ROS) during cellular stress or death has been associated with NLRP3 activation, although the role of ROS in NLRP3 activation remains controversial. DAMPs, damage-associated molecular patterns; IL-1β, interleukin-1β.
In vitro, robust activation of the NLRP3 inflammasome in phagocytic cells that were pretreated with microbial products or cytokines such as TNF is dependent on the presence of ATP20. However, the amounts of ATP necessary for NLRP3-mediated caspase 1 activation to be detected in macrophages in vitro are greater than physiological concentrations63 and, therefore, the relevance of this pathway in vivo is uncertain. ATP binds to purinergic receptor P2X7 (P2RX7), which leads to the opening of an ATP-gated cation channel that induces K + efflux and the formation of a large pore mediated by the hemichannel protein pannexin 1 (REFS 64,65). Thus, it has been suggested that NLRP3 senses intracellular K + depletion, which may act as a surrogate marker of cellular injury that is sensed by NLRP3. Consistently, inhibition of K+ efflux by high extracellular K+ concentrations prevents NLRP3-dependent caspase 1 activation in response to ATP, asbestos, silica and MSU crystals58,66. Also, necrotic cells can activate NLRP3, which is partly dependent on actively respiring mitochondria and the physiological release of ATP60. However, IL-1β secretion was only partially dependent on P2RX7, suggesting that the release of ATP may be only one mechanism by which necrotic cells can activate NLRP3. It is probable that additional factors besides ATP have a role60.
P2RX7-dependent pore formation is not a prerequisite for NLRP3 activation by all stimuli. Urate and cholesterol crystals, as well as sterile particulates, for example, can bypass the requirement for ATP and P2RX7 for IL-1β production55,59. For these sterile particulates to activate NLRP3, they must be internalized, as blockade of endocytosis by the actin-depolymerizing drug cytochalasin D inhibited NLRP3-dependent IL-1β production58,59. Importantly, NLRP3-dependent caspase 1 activation was associated with destabilization of the lysosomal membrane and activation of lysosomal proteases (specifically, cathepsin b)44. Lysosomal damage can occur during cellular injury and necrosis, and has also been associated with other sterile activators of NLRP3, such as cholesterol59 and silica crystals44. Therefore, it is possible that divergent upstream danger signals that are sensed by NLRP3 may converge on lysosomal damage and cathepsin b activation. evidence to support this are the observations that artificial lysosomal rupture alone can activate NLRP3 and that pharmacological inhibition or genetic depletion of cathepsin b results in inhibition of caspase 1 activation, although this inhibition was only partial44,56,59. However, cathepsin b-deficient macrophages have no impairment in IL-1β production in response to certain NLRP3 agonists, including LPS plus ATP, or MSU crystals, despite the impairment in their response to silica44,56,59,67. Furthermore, there is only partial inhibition of the neutrophil infiltration and IL-1β production associated with sterile inflammation in response to cholesterol crystals44,56,59,67.
An additional damage signal that has been associated with NLRP3 stimulation and caspase 1 activation is ROS. During sterile inflammation, the production of ROS by neutrophils (for example, through an oxidative burst) is important for the destruction of pathogens but, during excessive cellular stress, high levels of ROS can lead to oxidative stress with ensuing cell death and necrosis. ROS levels can be controlled by neutralization by enzymes with antioxidant activities. Therefore, the detrimental effect of ROS during sterile inflammation depends on the balance between ROS producers and ROS inactivators. Silica58,68, asbestos57 and ATP69 have all been associated with ROS production, and ROS inhibition in vitro resulted in impairment of caspase 1 activation and IL-1β production by these stimuli57,58,69. How ROS production is sensed by NLRP3 is unknown, but it was recently shown in one study that NLRP3 interacts in a ROS-dependent manner with thioredoxin-interacting protein (TXNIP), which dissociates from the antioxidant enzyme thioredoxin during oxidative stress62. In this study, TXNIP-deficient mice had impaired IL-1β production and neutrophil influx after intraperitoneal injection of MSU crystals, and TXNIP-deficient macrophages had decreased IL-1β production in response to a range of known NLRP3 activators, including MSU crystals, silica, and ATP62. Thus, the sensing of ROS may be a unifying mechanism by which NLRP3 senses its various activators. However, the role of TXNIP and ROS in NLRP3 activation has been challenged. In a separate study, no differences in IL-1β secretion were observed between wild-type and TXNIP-deficient bone marrow-derived macrophages in response to several NLRP3 stimuli, including MSU crystals and ATP61. Furthermore, mouse macrophages that are deficient in p22phox (L. Franchi and G.N., unpublished observations) or the Gp91phox subunit of NADPH oxidase cytochrome b, which is a major generator of ROS in the cell, had normal, rather than decreased, levels of caspase 1 activation in response to ATP, silica or MSU crystals44. Thus, the relevance of ROS generation in NLRP3 activation in vivo remains unclear. Collectively, there is still controversy regarding the roles of the individual model pathways for NLRP3 activation, and it remains to be determined whether there is a common mechanism by which numerous heterogeneous stimuli converge on NLRP3.
Role of PRRs: an emerging role for CLRs
CLRs are an increasingly recognized category of PRRs that are important in host defence. This class of PRRs contains a carbohydrate-binding domain that recognizes carbohydrates on viruses, bacteria and fungi. In general, the ligands of these CLRs are unknown70. Stimulation of CLRs typically results in the induction of signalling pathways that, in some cases, can synergize with TLR signalling pathways to upregulate cytokine and chemokine production. In antigen-presenting cells, they are involved in antigen uptake and trafficking for antigen presentation71. Although their primary importance is in host defence against pathogens, CLRs can sense necrotic cell death72. In particular, C-type lectin 4e (CLeC4e; also known as MINCLe) can detect the DAMP spliceosome-associated protein 130 (SAP130), which is a component of a small nuclear ribonucleo protein that can be released by necrotic but not apoptotic cells, resulting in upregulation of pro-inflammatory mediators such as CXC-chemokine ligand 2 (CXCL2) and in neutrophil recruitment73. In a model of high levels of cell death in the thymus by whole-body irradiation, CXCL2 production by thymic macrophages, as well as neutrophil infiltration into the thymus, was significantly suppressed in mice treated with a blocking antibody to CLeC4e73. whether CLeC4e contributes to sterile inflammation-related diseases is unknown. Interestingly, Clec4e mRNA is upregulated in patients with rheumatoid arthritis73,74.
CLeC9A (also known as DNGR1), which is expressed by CD8α+ dendritic cells, is also involved in the recognition of necrotic cells. CLeC9A has been shown to recognize necrotic cells and induce the cross-presentation of dead cell-associated antigens to CD8+ T cells. Therefore, it may be involved in regulating sterile immune responses, although the specific ligand recognized in this case is still unknown73,75. Similar to CLeC4e, the ability of CLeC9A to recognize necrotic cells makes it a potential receptor that is important for sterile inflammatory responses. Determining whether CLeC9A contributes to sterile inflammation in vivo should be possible, as transgenic mice deficient in this receptor have been made75.
Release of intracellular cytokines
The passive release of biologically active cytokines during sterile injury-associated cell death is an important mechanism to alert the immune system of tissue damage and to initiate the healing response. There is evidence that the passive release of cytokines during sterile cell injury has a role in disease. Two cytokines of the IL-1 family, IL-1α and IL-33, are particularly relevant. In the case of IL-1α, its release from injured endothelial cells promotes allogeneic T cell infiltration in a mouse–human chimeric model of artery allograft rejection76. In addition, IL-1α released during hepatocyte necrosis contributes to carcinogen-induced liver tumorigenesis in mice77. Unlike its related family members IL-1β and IL-18, IL-1α is synthesized as a biologically active cytokine in its full-length precursor form and does not require processing for signalling through IL-1R78,79. when cells die by necrosis, such as during injury, this precursor form of IL-1α is released, leading to activation of its cognate receptor and rapid recruitment of inflammatory cells into the surrounding injured tissue22,78. This is in contrast with apoptotic cells, in which IL-1α is sequestered intracellularly78, or with intact cells, in which the secretion of mature IL-1α is partially dependent on caspase 1 activity80–82.
The mechanism by which necrotic cells and IL-1α induce sterile inflammation remains poorly understood. Using a model of peritonitis triggered by necrotic cells, IL-1α was shown to be important for the recruitment of neutrophils, which was caspase 1 independent, and this response was impaired in mice lacking IL-1R expression by non-myeloid cells22. Subsequently, it was shown that IL-1α released by necrotic cells was crucial for the production of CXCL1, which recruits neutrophils, by non-haematopoietic cells such as mesothelial cells15. However, the mechanism by which necrotic cells induce acute inflammatory responses seems to be more complex in vivo. For example, necrotic dendritic cells, but not necrotic macrophages, heart cells or liver cells, rely on IL-1α to recruit neutrophils, at least in a peritonitis model41. In the case of necrotic liver tissue, IL-1α and IL-1β derived from resident macrophages, but not the necrotic cells themselves, seem to have a crucial role in eliciting the neutrophilic response41. Thus, the release of IL-1α from certain cells, such as dendritic cells, contributes to sterile inflammation, but macrophages seem to be the primary sentinel cells that mediate the sensing of necrotic cells through the production of mature IL-1α and IL-1β41. The mechanism by which resident macro phages produce mature IL-1α and IL-1β in response to necrotic cells to mediate neutrophilic recruitment remains unclear, but it is caspase 1 independent22, suggesting that in the case of IL-1β other proteases may be responsible for cleaving pro-IL-1β to its active form83,84. These studies suggest that the precursor form of IL-1α that is released by necrotic cells contributes to the initial neutrophilic response, but resident macrophages are the main source of mature IL-1α and IL-1β, which are needed for cell death-induced sterile inflammation (FIG. 3). Locally produced IL-1 then mediates neutrophil recruitment within the peritoneal cavity through IL-1R signalling and induction of CXCL1 production (FIG. 3).
Figure 3. Model for IL-1-mediated neutrophil recruitment in response to necrotic cell death.
Necrotic cells release the precursor form of interleukin-1α (IL-1α), which is biologically active and stimulates neighbouring parenchymal cells, through IL-1 receptor (IL-1R), to secrete the chemokine CXC-chemokine ligand 1 (CXCL1). In addition, IL-1α can stimulate resident macrophages to produce additional IL-1α and IL-1β through a caspase 1-independent mechanism that further boosts CXCL1 secretion. In turn, CXCL1 functions through CXC-chemokine receptor 2 (CXCR2) on neutrophils to recruit them to the site of injury. This figure is based on published studies using a peritoneal model of sterile inflammation15,22,41.
Similar to IL-1α, IL-33 is active as a precursor protein. extracellular IL-33 that is released during necrosis also functions as an alarmin to alert cells, such as mast cells and other innate immune cells, to tissue damage85,86. Although it was initially thought that the IL-33 precursor was processed by caspase 1 to produce biologically active IL-33, it is now clear that its processing by the executioner caspases caspase 3 and caspase 7 during apoptosis inactivates IL-33 (REFS 85,86). Thus, IL-33, which is expressed at high levels by endothelial cells and some epithelial cells, is expected to be active when it is released during necrosis, but not apoptosis, which is associated with executioner caspase activation. IL-33 is highly expressed within endothelial cells in the synovium of patients with rheumatoid arthritis87, and IL-33-deficient mice have reduced neutrophil migration in an experimental model of rheumatoid arthritis88, but the actual role of this cytokine in the pathogenesis of rheumatoid arthritis or other sterile inflammatory diseases remains to be determined. Collectively, the evidence suggests that the precursor forms of IL-1α and IL-33 (both of which are biologically active) are preferentially released during necrosis to alert the immune system to cell damage, leading to the initiation of the healing response.
Non-PRR-mediated recognition of DAMPs
In addition to PRRs, DAMPs are recognized by DAMP-specific receptors. The prototypical DAMP-specific receptor is receptor for AGes (RAGe). RAGe is a transmembrane receptor that is expressed by immune cells, endothelial cells, cardiomyocytes and neurons89–91. It detects advanced glycation end-products (AGes) that arise from non-enzymatic glycation and oxidation of proteins and lipids. These products can accumulate under conditions of high oxidant stress and are found at elevated levels in chronic inflammatory disease states such as type 1 diabetes92.
In addition to AGes, RAGe also recognizes HMGb193 and the S100 family members19, which are released during cellular stress and necrotic cell death, and β-amyloid94, the accumulation of which is pathogenic in Alzheimer's disease. Activation of RAGe by its ligands results in the upregulation of several inflammatory signalling pathways, including, but not limited to, NF-κb, phospho inositide 3-kinase, Janus kinase (JAK)–signal transducer and activator of transcription (STAT) and MAPK signalling pathways, which lead to induction of pro-inflammatory cytokines such as TNF19,91,95,96. The mechanism by which RAGe activates these pro-inflammatory signalling pathways is unclear. The protein contains no obvious signalling domains90, although extracellular signal-regulated kinase (eRK) was shown to directly interact with the cytoplasmic tail of RAGe97. In certain cases, RAGe may transduce signals by acting cooperatively with TLRs. Specifically, HMGb1-bound DNA can form a complex with TLR9 and RAGe to induce pro-inflammatory cytokines98, which may be important for promoting inflammation in patients with systemic lupus erythematosus who have elevated circulating levels of DNA-containing immune complexes. The mechanistic detail of RAGe signalling and the importance of its various ligands in disease pathology continue to be areas of investigation.
RAGe has been implicated in both diabetes- and obesity-related atherosclerosis using apolipoprotein e (APOe)-deficient mice, which are susceptible to developing atherosclerosis99,100. Furthermore, blockade of RAGe by using a soluble competitive inhibitor suppressed diabetes-associated atherosclerosis in mouse models92. Apoe–/–Rage–/– mice also exhibited a reduction in atherosclerosis101, as shown by a reduction in both atherosclerotic plaque formation and production of pro-inflammatory mediators within the aorta. In addition, the binding of β-amyloid to RAGe results in activated microglial cells94, and RAGe expression by microglial cells contributed to neuroinflammation in a mouse model of Alzheimer's disease89. whether RAGe has a direct role in the pathogenesis of these diseases in humans and what pathways are specifically activated remain to be elucidated.
Several other non-PRRs have been shown to interact with certain DAMPs, although their precise roles during sterile inflammation in vivo have not been verified. As in the case of TLR9 and RAGe, some of these receptors engage TLRs to form co-receptor complexes, which then induce inflammatory responses through TLR-dependent pathways. Hyaluronan fragments, for example, can signal through CD44, leading to MAPK activation102. Hyaluronan is also directly recognized by TLR2 and TLR4, and this interaction was shown to have a role in mediating inflammatory responses in a mouse model of sterile bleomycin-induced lung injury102. How CD44 signalling contributes to sterile inflammation is unclear, but it has been shown to physically interact with TLR4 in vitro and function as an accessory molecule for TLR4 signalling in response to hyaluronan102,103. Similarly, CD36 is a scavenger receptor that binds oxidized LDL and β-amyloid, which are associated with sterile inflammation related to atherosclerosis and Alzheimer's disease, respectively. CD36 mediates heterodimer formation of TLR4 and TLR6 (REF. 104), and this co-receptor complex upregulates the production of chemokines and cytokines such as pro-IL-1β through the activation of NF-κb. Induction of NF-κb signalling through this co-receptor complex to produce IL-1β may serve as a priming event for subsequent NLRP3 activation105 (the first signal; see BOX 1) or provide additional signals to mediate inflammation in atherosclerosis and Alzheimer's disease.
It has also been shown that DAMP-specific receptors can negatively regulate inflammatory responses. Specifically, CD24, which can bind to both HMGb1 and HSPs, negatively regulates sterile inflammatory responses. CD24-mediated inhibition was associated with increased pro-inflammatory cytokine production by HMGb1 and decreased survival following acetaminophen-induced liver injury106. This may provide one mechanism by which the host immune system can modulate responses to DAMPs and distinguish DAMPs from PAMPs107. whether there are other examples of this phenomenon remains to be determined. Thus, although there are non-PRRs that can sense sterile stimuli, most notably RAGe, whether these receptors have a primary or secondary role (for example through regulation of TLR responses) in sterile inflammation and their relevance in the pathogenesis of human disease remain unknown.
Implications for therapy
Given the crucial role for IL-1 in sterile inflammatory responses, blockade of IL-1R has been tested as a therapeutic target for sterile inflammatory disorders in humans. Promising results have been obtained with the use of IL-1β blockade using anakinra (Kineret; Amgen/Biovitrum), a recombinant IL-1R antagonist. Anakinra was shown to be highly effective in the treatment of patients with gout who could not tolerate or did not respond to previous therapy108. Although the clinical study was small, with only ten patients evaluated, all ten patients treated with anakinra had significant relief from symptoms108. However, the effect of IL-1R antagonism in other inflammatory joint diseases, such as osteoarthritis and rheumatoid arthritis, has not been as promising, with little or no clinical benefit shown in human clinical trials43.
A benefit for anakinra was also shown in a small, randomized trial of 70 patients with type 2 diabetes109. Those treated with anakinra had improved glucose levels and reduced systemic inflammation, as suggested by lower circulating levels of inflammatory markers, such as IL-6 and C-reactive protein. This treatment approach for type 2 diabetes is promising, as the improvement in levels of glucose and inflammatory markers was long-lasting110. However, the clinical study was still small and was not designed to determine optimal dosing, duration of use or long-term outcomes in disease control.
Chronic inflammation is also important for carcinogenesis, as pro-inflammatory cytokines, including IL-1β, can be tumour promoting6,111. Thus, IL-1R may also be a potential target for the treatment of patients with cancer. However, inflammatory responses are equally important for mounting an effective antitumour response against chemotherapy-induced, immunogenic tumour cell death. Dying tumour cells release ATP and, indeed, the ability of dendritic cells to prime tumour-specific T cells required NLRP3 and P2RX7 (REF. 112). Furthermore, chemotherapy-treated tumour cells injected into wild-type mice inhibited tumour development when the mice were rechallenged with tumour cells, but this effect was abrogated in NLRP3-deficient and P2RX7-deficient mice112, suggesting that NLRP3-mediated IL-1β production through the ATP–P2RX7 pathway is important for immune surveillance against tumours. Consistently, a loss-of-function P2RX7 allele was also associated with poorer prognosis (decreased metastasis-free survival) in early-stage breast cancer patients treated with chemotherapy112. Thus, an approach to treating patients with cancer hinges on an improved understanding of how to balance the tumour-suppressive and tumour-promoting functions of IL-1β.
Similarly, as TLRs also mediate sterile inflammation, they could be another potential therapeutic target. Mice deficient in both TLR2 and TLR4 or in myeloid differentiation primary-response protein 88 (MYD88), an adaptor protein for TLR and IL-1R signalling, were protected against bleomycin-induced lung injury related to the generation of hyaluronan fragments, and administration of a hyaluronan-blocking peptide to wild-type mice provided similar, although incomplete, protection31. TLR signalling is also implicated in the pathogenesis of atherosclerotic disease35–37. Furthermore, it was shown that TLR2 signalling by the endogenous extracellular matrix-derived proteoglycan versican can promote tumour metastasis34. However, as TLR signalling is important for tissue repair113,114, caution must also be taken in devising a therapeutic strategy against TLR-mediated sterile inflammation. Indeed, mice deficient in TLR2 and TLR4 expression had increased mortality in response to hypoxia-induced lung injury, which was associated with decreased integrity of the lung epithelium31. TLR signalling during sterile inflammation in the tumour microenvironment also has important clinical implications. TLR4 signalling was found to be important for dendritic cell-mediated cross-presentation of tumour antigens from dying, chemotherapy-treated tumour cells and involved HMGb1 release from the dying cells115. The clinical importance of this finding was suggested by the fact that, in early-stage breast cancer patients who were treated with chemotherapy, lower metastasis-free survival was associated with a TLR4 polymorphism that affects binding of HMGb1 to TLR4 (REF. 115). Thus, as inflammation is important not only in disease pathogenesis but also in tissue repair mechanisms and immune surveillance, challenges remain in identifying optimal targets and appropriate contexts for the treatment of sterile inflammatory disorders.
Conclusion
Much progress has been made in identifying some of the triggers of sterile inflammation. Many questions remain, including the relative importance of the different DAMPs in their biological activity, whether they differentially activate downstream signalling pathways and the molecular basis for their recognition. whether there are additional unidentified endogenous DAMPs that may be implicated in disease is also unknown. Finally, further studies are needed to understand how the different inflammatory signalling pathways (mediated by TLRs, inflammasomes and IL-1R) interact to mediate the sterile inflammatory response and how they may be modulated for the benefit of the host.
Ischaemia–reperfusion injury.
An injury in which the tissue first suffers from hypoxia as a result of severely decreased, or completely arrested, blood flow. Restoration of normal blood flow further enhances inflammation, which exacerbates tissue damage.
Reactive oxygen species.
(ROS). Oxygen radicals that are mainly produced by the mitochondrial respiratory chain. In excess, they can cause intracellular and mitochondrial damage, which promotes cell death.
Box 1 | Inflammation and wound repair.
The acute inflammatory response has an integral role in normal wound healing and tissue repair to eradicate the offending agent, regenerate the parenchyma and heal any sustained damage. In response to injury that disrupts the parenchyma and causes blood vessel damage, the coagulation system is activated, which begins the initial stages of healing with the release of chemical mediators that promote vascular permeability and leukocyte adhesion and recruitment. Activated platelets also produce growth factors such as transforming growth factor-β (TGFβ) and platelet-derived growth factor (PDGF), which activate fibroblasts and act as chemoattractants for leukocytes116. The infiltration of leukocytes — first neutrophils, followed by macrophages — allows the removal of dead cells and cellular debris. More importantly, these cells secrete chemokines and cytokines, such as tumour necrosis factor (TNF) and interleukin-1 (IL-1), that upregulate leukocyte adhesion molecules to increase immune cell recruitment and induce the production of additional growth factors and proteases by macrophages117. The release of proteases including matrix metalloproteases leads to the degradation of the extracellular matrix to allow for tissue remodelling. In addition to IL-1 and TNF, growth factors and inflammatory mediators produced by macrophages, such as fibroblast growth factor (FGF), PDGF, prostaglandins and thrombospondin 1, promote new blood vessel growth, fibroblast proliferation and collagen deposition117. Tissue remodelling is accompanied by parenchymal regeneration or regrowth of the epithelial cell layer with resolution of the healing process. Under conditions in which complete healing does not occur, as in the setting of chronic infection or prolonged exposure to injurious agents, the inflammatory response remains unresolved. Macrophages and neutrophils persist and continue to secrete inflammatory cytokines, proteases and growth factors that lead to inappropriate tissue destruction and scarring or fibrosis.
Myocardial infarction.
An episode of acute cardiac ischaemia that leads to death of heart muscle cells. It is usually caused by a thrombotic atherosclerotic plaque.
Atherosclerosis.
A chronic disorder of the arterial wall characterized by endothelial cell damage that gradually induces deposits of cholesterol, cellular debris, calcium and other substances. These deposits finally lead to plaque formation and arterial stiffness.
Necrosis.
A form of cell death that frequently results from toxic injury, hypoxia or stress. Necrosis involves the loss of cell integrity and the release of cell contents into the interstitium. This form of cell death usually occurs together with inflammation. Depending on the context, the self antigens that are released by necrosis can become immunogenic.
Apoptosis.
A common form of cell death that is defined by specific morphological changes and by the involvement of caspases. The morphological features include chromatin condensation, plasma membrane blebbing and DNA fragmentation into segments of ~180 base pairs. eventually, the cell breaks up into many membrane-bound ‘apoptotic bodies’, which are phagocytosed by neighbouring cells.
High-mobility group box 1.
(HMGB1; also known as amphoterin). A nuclear protein that binds DNA in a non-sequence-specific manner and modulates transcription and chromatin remodelling by bending DNA and facilitating the binding of transcription factors and nucleosomes, respectively.
Adjuvant.
A substance that stimulates the immune system to enhance the immunogenicity of antigens or vaccines and enhance antigen-specific antibody production.
Box 2 | Immunogenic cell death.
Sterile stimuli — specifically, damage-associated molecular patterns (DAMPs) — are generally intracellular factors that are normally hidden from recognition by the host immune system, and this ‘hiding’ effectively prevents pathological inflammation and autoimmunity. Sterile inflammation occurs when DAMPs are released into the extracellular environment. This occurs mostly when a cell undergoes necrotic, as opposed to apoptotic, cell death. Indeed, necrotic cells are normally immunostimulatory and lead to inflammatory cell infiltration and cytokine production10,18. Apoptosis involves an orchestrated caspase signalling cascade that ultimately leads to cell rounding and shrinkage (pyknosis), chromatin condensation, non-random DNA fragmentation or laddering, plasma membrane blebbing and nuclear fragmentation (karyorrhexis). The apoptotic bodies that form can be cleared effectively by phagocytes. This death process is therefore self-contained, and immunogenic endogenous molecules are not released to a significant extent into the extracellular environment.
By contrast, necrotic cell death involves cellular and organelle swelling (oncosis) and, most importantly, rupture of the plasma membrane, resulting in the release of intracellular molecules that can elicit an inflammatory response. Reactive oxygen species production, lysosomal membrane destabilization, activation of proteases (including cathepsins) and ionic flux changes are all associated with necrosis and can activate sterile inflammatory pathways in addition to the release of DAMPs118. Necrosis predominates in conditions such as toxin- or ischaemia-induced injury. Although typically not associated with immunogenicity and inflammation, apoptosis can become inflammatory under conditions in which there is delayed clearance of apoptotic cells, as can occur with high levels of apoptosis, resulting in secondary necrosis with loss of plasma membrane integrity119. Signalling through death receptors that classically lead to apoptosis, such as FAS (also known as CD95), has also been associated with inflammatory responses, although whether this is actually correlated with the extent of apoptotic cell death is unclear120.
Inflammasome.
A multiprotein complex that contains a pattern recognition receptor (PRR), typically a member of the NOD-like receptor (NlR) family, that, on sensing its cognate agonist, oligomerizes and recruits the adaptor protein ASC (apoptosis-related speck-like protein containing a CARD) through protein domain interactions. ASC can recruit caspase 1 through its CARD, thereby linking the PRR to caspase 1 activation and interleukin-1 production. There are currently four characterized inflammasomes, named by the PRRs that form them: the NRlP1 (NOD-, lRR- and pyrin domain-containing 1), NlRP3, NlRC4 (NOD-, lRR- and CARD-containing 4) and absence in melanoma 2 (AIM2) inflammasomes.
Box 3 | Role of NLRP3 in autoinflammatory syndromes.
Autoinflammatory syndromes are a group of rare monogenic inherited disorders that are characterized by episodic occurrences of fever, sterile inflammation and other more variable inflammatory manifestations in the absence of clinical and laboratory markers of autoimmunity or infection. These syndromes include familial cold autoinflammatory syndrome, Muckle–Wells syndrome and neonatal-onset multisystemic inflammatory disease (NOMID; also known as CINCA syndrome) and are caused by missense mutations in the nucleotide-binding oligomerization domain (NOD)-like receptor family member NLRP3 (NOD-, LRR- and pyrin domain-containing 3; also known as cryopyrin). Clinical features of these dominantly inherited disorders, which are commonly referred as cryopyrin-associated periodic syndromes, include recurring episodes of fever, urticarial skin rash and arthropathy. These disease-asssociated missense NLRP3 mutations result in enhanced activation of caspase 1 and secretion of interleukin-1β (IL-1β) by causing constitutive activation of the NLRP3 inflammasome. Notably, treatment of these patients with an IL-1 receptor antagonist or IL-1β-specific blocking antibody reverses clinical symptoms, which suggests a cause–effect relationship between IL-1β production and the development of disease.
Box 4 | NLRP3 activation.
The assembly and activation of the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome results in the cleavage of pro-caspase 1 to its active form, which in turn, cleaves pro-interleukin-1β (pro-IL-1β) and pro-IL-18 into their mature, biologically active forms. In vitro studies have led to a model of NLRP3-mediated IL-1β production that requires two separate signals. The first signal is the nuclear factor-κB (NF-κB)-dependent transcription of pro-IL-1β and NLRP3, either through the activation of Toll-like receptors (TLRs) or nucleotide-binding oligomerization domain 2 (NOD2), and therefore, in vitro, this signal can be provided by various TLR or NOD2 agonists, such as lipopolysaccharide20,53,121. In the case of sterile inflammation, certain cytokines that induce NF-κB, such as tumour necrosis factor or IL-1, can provide the first signal for NLRP3 activation54. In addition, during sterile inflammation, endogenous molecules that signal through TLRs, such as low-density lipoprotein, may be the first signal to prime the activation of the NLRP3 inflammasome, resulting in cooperative signalling through separate pathways14,40,61,105. The second signal is provided by stimuli that specifically activate NLRP3 and leads to caspase 1 activation. In vitro, this second signal is typically provided by the addition of ATP or certain bacterial toxins, which results in pore formation and K+ depletion that may be sensed by NLRP3, leading to caspase 1 activation and pro-IL-1β cleavage. As intracellular pro-IL-1β levels are usually low, the first signal has been considered to be a ‘priming’ event to allow for subsequent NLRP3 activation and IL-1β release, which otherwise could not occur. The two step model would allow for an additional level of regulation of caspase 1 activation.
NADPH oxidase.
An enzyme system that consists of several cytoplasmic and membrane-bound subunits. The complex is assembled in activated phagocytic cells mainly on phagolysosomal membranes. NADPH oxidase uses electrons from NADPH to reduce molecular oxygen to form superoxide anions. Superoxide anions are enzymatically converted to hydrogen peroxide, which is converted by myeloperoxidase to hypochloric acid, a highly toxic and microbicidal agent.
Acknowledgements
We apologize to our colleagues whose work was not cited or was cited through others’ review articles because of space limitations. Work in the authors’ laboratories is supported by US National Institutes of Health grants CA133185 (G.C.), and DK61707, AR051790, AI06331, AR059688 and DK091191 (G.N.).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Gabriel Nuñez's homepage: http://www.pathology.med.umich.edu/faculty/Nunez/index.html
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998;157:1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 2.Cotran RS, Kumar V, Robbins S. In: Robbins Pathologic Basis of Disease. Schoen FJ, editor. W. B. Saunders Company; Philadelphia: 1994. pp. 6–11. [Google Scholar]
- 3.Cotran RS, Kumar V, Robbins S. In: Robbins Pathologic Basis of Disease. Schoen FJ, editor. W. B. Saunders Company; Philadelphia: 1994. pp. 1255–1259. [Google Scholar]
- 4.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer's disease. Nature Rev. Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nature Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 10.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 11.Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J. Immunol. 2005;175:2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- 12.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babelova A, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via Toll-like and P2X receptors. J. Biol. Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1α released from dying cells. J. Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [This paper shows that the passive release of IL-1α from necrotic cells, in particular necrotic dendritic cells, is important for the recruitment of neutrophils in the sterile inflammatory response through the production of CXCL1 by cells responsive to IL-1α.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kono H, Rock KL. How dying cells alert the immune system to danger. Nature Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann MA, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 20.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 22.Chen CJ, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature Med. 2007;13:851–856. doi: 10.1038/nm1603. [This paper demonstrates a crucial role for IL-1α in sterile inflammation and, in particular, neutrophil recruitment induced by necrotic cells.] [DOI] [PubMed] [Google Scholar]
- 23.Mbitikon-Kobo FM, et al. Characterization of a CD44/CD122int memory CD8 T cell subset generated under sterile inflammatory conditions. J. Immunol. 2009;182:3846–3854. doi: 10.4049/jimmunol.0802438. [DOI] [PubMed] [Google Scholar]
- 24.Weber AN, et al. Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nature Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 25.Vabulas RM, et al. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 26.Yu M, et al. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 27.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52:2936–2946. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 28.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor α release by murine macrophages. J. Biol. Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 29.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J. Leukoc. Biol. 2007;81:49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- 30.Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-α production in human monocytes. J. Immunol. 2008;180:5067–5074. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- 31.Jiang D, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [This paper shows the dual role of TLRs in mediating sterile inflammation in response to hyaluronan fragments released during injury and in promoting tissue repair.] [DOI] [PubMed] [Google Scholar]
- 32.Scheibner KA, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer L, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelsen KS, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl Acad. Sci. USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjorkbacka H, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nature Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 38.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavassani KA, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imaeda AB, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J. Immunol. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [This study shows the crucial role for macrophages in mediating the inflammatory response to sterile cell death, such as by IL-1α production.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Feuerstein GZ, Gu JL, Lysko PG, Yue TL. Interleukin-1β induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis. 1995;115:89–98. doi: 10.1016/0021-9150(94)05503-b. [DOI] [PubMed] [Google Scholar]
- 43.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nature Rev. Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 44.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [This study was pivotal in providing a model of NLRP3 activation that involves lysosomal damage and cathepsin B activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raines EW, Dower SK, Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989;243:393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- 46.Boni-Schnetzler M, et al. Increased interleukin (IL)-1β messenger ribonucleic acid expression in β-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J. Clin. Endocrinol. Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burckstummer T, et al. An orthogonal proteomicgenomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauernfeind FG, et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [References 53 and 54 provide evidence that the first signal, or priming event, necessary for activation of the NLRP3 inflammasome involves upregulation of NLRP3 expression by NF-κB through the action of TLRs or pro-inflammatory cytokines such as TNF.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [This study is one of the first to identify an endogenous, non-microbial signal for NLPR3 inflammasome activation that can lead to a non-infectious inflammatory disease (in this case, gout).] [DOI] [PubMed] [Google Scholar]
- 56.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nature Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [This study led to the model of NLRP3 activation that is dependent on the sensing of ROS, and demonstrated a role for NLRP3 in asbestosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cassel SL, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl Acad. Sci. USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iyer SS, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl Acad. Sci. USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nature Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 63.el-Moatassim C, Dubyak GR. A novel pathway for the activation of phospholipase D by P2z purinergic receptors in BAC1.2F5 macrophages. J. Biol. Chem. 1992;267:23664–23673. [PubMed] [Google Scholar]
- 64.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Petrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 67.Dostert C, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS ONE. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003;34:1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 69.Cruz CM, et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nature Rev. Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nature Rev. Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 72.Yamasaki S, et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nature Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 73.Cambi A, Figdor C. Necrosis: C-type lectins sense cell death. Curr. Biol. 2009;19:R375–R378. doi: 10.1016/j.cub.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura N, et al. Isolation and expression profiling of genes upregulated in bone marrow-derived mononuclear cells of rheumatoid arthritis patients. DNA Res. 2006;13:169–183. doi: 10.1093/dnares/dsl006. [DOI] [PubMed] [Google Scholar]
- 75.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [This paper showed a role for CLEC9A in regulating immune responses to sterile cell death, specifically through the cross-presentation of dead cell-associated antigens.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao DA, et al. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J. Exp. Med. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakurai T, et al. Hepatocyte necrosis induced by oxidative stress and IL-1α release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen I, et al. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc. Natl Acad. Sci. USA. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dinarello CA. IL-1: discoveries, controversies and future directions. Eur. J. Immunol. 2010;40:599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 80.Li P, et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 81.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 82.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 83.Fantuzzi G, et al. Response to local inflammation of IL-1β-converting enzyme-deficient mice. J. Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 84.Mayer-Barber KD, et al. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl Acad. Sci. USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carriere V, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl Acad. Sci. USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verri WA, Jr, et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 2010;69:1697–1703. doi: 10.1136/ard.2009.122655. [DOI] [PubMed] [Google Scholar]
- 89.Fang F, et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. FASEB J. 2009;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 91.Shang L, et al. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3β signaling pathways. PLoS ONE. 2010;5:e10092. doi: 10.1371/journal.pone.0010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bucciarelli LG, et al. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell. Mol. Life Sci. 2002;59:1117–1128. doi: 10.1007/s00018-002-8491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hori O, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 94.Yan SD, et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 95.Huang JS, et al. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J. Cell Biochem. 2001;81:102–113. doi: 10.1002/1097-4644(20010401)81:1<102::aid-jcb1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 96.Dukic-Stefanovic S, Schinzel R, Riederer P, Munch G. AGES in brain ageing: AGE-inhibitors as neuroprotective and anti-dementia drugs? Biogerontology. 2001;2:19–34. doi: 10.1023/a:1010052800347. [DOI] [PubMed] [Google Scholar]
- 97.Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 2003;550:107–113. doi: 10.1016/s0014-5793(03)00846-9. [DOI] [PubMed] [Google Scholar]
- 98.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 99.Ueno H, et al. Receptor for advanced glycation end-products (RAGE) regulation of adiposity and adiponectin is associated with atherogenesis in apoE-deficient mouse. Atherosclerosis. 2010;211:431–436. doi: 10.1016/j.atherosclerosis.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 100.Soro-Paavonen A, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harja E, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J. Clin. Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 103.Taylor KR, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 104.Hoebe K, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 105.Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Y, Chen GY, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30:557–561. doi: 10.1016/j.it.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res. Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 110.Larsen CM, et al. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pantschenko AG, et al. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int. J. Oncol. 2003;23:269–284. [PubMed] [Google Scholar]
- 112.Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nature Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 113.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 114.Brown SL, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J. Clin. Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [This paper showed the importance of TLR4 signalling in response to DAMPs derived from tumour cell death after chemotherapy or radiation treatment during the induction of host immune responses that are important for inhibiting tumour growth.] [DOI] [PubMed] [Google Scholar]
- 116.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 117.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- 118.Kroemer G, et al. Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Silva MT, do Vale A, dos Santos NM. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 2008;13:463–482. doi: 10.1007/s10495-008-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miwa K, et al. Caspase 1-independent IL-1β release and inflammation induced by the apoptosis inducer Fas ligand. Nature Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 121.Marina-Garcia N, et al. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J. Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 122.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 123.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 124.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Q, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]