Abstract
The Otsuka Long Evans Tokushima Fatty (OLETF) rat lacking the CCK-1 receptor is hyperphagic, prefers palatable and high caloric meals, and gradually develops obesity and type-2 diabetes. To determine dopamine levels in this strain, we used in-vivo quantitative (no-net flux) microdialyis at three different ages representing non-diabetic (8 weeks), pre-diabetic (18 weeks), and diabetic (56 weeks) stages in OLETF and age-matched lean LETO controls. Results showed significantly elevated basal dopamine levels in the caudomedial nucleus accumbens of OLETF rats compared to LETO at younger ages (8 weeks: 20.10 ± 5.61 nM vs. 15.85 ± 5.63 nM; 18 weeks: 7.37 ± 3.71 nM vs. 4.75 ± 1.25 nM, Mean ± SD). In contrast, at 56 weeks of age, a profound decline in extracellular dopamine concentrations was seen in both strains with a tendency for a greater effect in OLETF rats (1.78 ± 0.40 nM vs. 2.39 ± 0.42 nM). Further, extracellular fraction, an index for reuptake, was higher in 56-week old OLETF compared to LETO (0.648 ± 0.049 vs. 0.526 ± 0.057). Potassium-stimulated dopamine efflux revealed an increased capacity of vesicular pool in OLETF rats compared to LETO across all age groups with an accentuated strain difference at 56 weeks. These findings demonstrate altered striatal dopamine functions (i.e. increased stimulated release and uptake) in obese OLETF rat. This could be due to the lack of functional CCK-1 receptors, or metabolic and hormonal factors associated with the development of obesity and insulin resistance, or both.
Keywords: obesity, CCK-1 receptor, type-2 diabetes, overeating, no-net flux microdialysis
Introduction
Obesity is a global pandemic that continues to accelerate and increases the risk of multiple medical conditions (14). In addition to genetic and environmental influences (25, 35, 69), food-related factors, such as palatability and energy content also play important role in development of obesity (21, 57, 64, 76). Among other integrative neural substrates, dopamine (DA) has been suggested to be involved in various aspects of eating including intake, food selection, satiety and energy expenditure (5, 43, 46, 66, 72, 77). While much is unclear about DA’s specific role(s) in inducing chronic overeating on high-caloric meals as a plausible antecedent of obesity, there is strong evidence showing that dopaminergic neurotransmission is altered in obese. For example, lower D2 receptor density was found in the striatum of both obese humans and animals (31, 38, 39, 73). Conversely, antipsychotic drugs that block DA D2 receptors increase appetite and weight gain (1). Furthermore, chronic high-fat diet-induced obesity leads to decreased DA turnover in the hypothalamus (45). Additionally, the availability of dopamine transporter (DAT) that regulates synaptic DA concentration by reuptake of the transmitter into presynaptic terminals is higher in mice that are prone compared to those resistant to chronic high-fat diet-induced obesity (39). Collectively, these findings suggest that DA signalling is reduced in obese. In contrast, numerous studies have suggested that obese rats actually have higher hypothalamic DA levels than lean rats (for a review, see (46)). A study by Huang et al. (38) found higher D2 receptor and tyrosine hydroxylase (TH) mRNA expression in the core of the nucleus accumbens (NAC), and ventral tegmental area (VTA), in obesity-prone compared to obesity-resistant mice suggesting that an increased DA signaling may increase susceptibility to overeating and development of obesity. Consistent with this notion, it has been shown that DA is not only required for maintenance of higher body weight in leptin deficient (ob/ob) mice (67) but also is sufficient to restore feeding in cachectic DA knock-out mice (66). The contradictory findings in the literature may be explained by the different dietary regimens and obesity models used, as well as whether the role of DA in either the energy regulatory or reward circuitries was assessed. Nonetheless, since alterations to the synaptic machinery may also reflect a compensatory regulation to altered DA release downstream from obesity and associated metabolic factors, one must be cautious when interpreting relationships between DA functions and obesity based on indirect indices (i.e. receptor and transporter expression). An alternative solution to resolve this intricacy is to measure the actual extrasynaptic DA levels with respect to basal and stimulated release over the course of development of obesity and its metabolic associates.
To achieve this goal, in the present study, we used the Otsuka Long-Evans Tokushima fatty (OLETF) rat an animal model that gradually develop obesity and type-2 diabetes (42, 49). The OLETF rat has a congenital cholecystokinin (CCK) - 1 receptor deficiency, resulting from a 6847-base pair deletion spanning the promoter region and the first and second exons of the CCK-1 receptor gene (68). CCK-1 receptors are the receptor subtype that mediate CCK’s actions in satiety (48). Consistent with this role, OLETF rats have deficits in the control of meal size. The size of spontaneous meals is almost double that of the LETO controls. Furthermore, OLETF rats have deficits in responding to CCK, and gastric and intestinal preloads (16, 49). In addition to diminished sensitivity to postingestive satiation signals, there has been accumulating evidence demonstrating deficits in central CCK signaling in this strain (6). Recently, we demonstrated that, OLETF rats express increased real and sham intake of normally preferred sucrose solutions (19) and an increased generalized avidity to sweet tastants (28). Thus, similar to humans susceptible to dietary obesity, OLETF rats share multiple characteristics: increased meal-size, reduced satiation, defenseless metabolic response to high caloric diets and an increased sensitivity to food reward (i.e. overeating driven by palatability).
In addition to its use as a model for dietary-induced obesity and diabetes, the OLETF rats offer an opportunity to study CCK modulation of DA. This is based on anatomical and functional evidence demonstrating interactions at multiple levels between the mesencephalic DA and CCK systems (32, 36, 40, 62). Of particular importance, a recent microdialysis study, using net-flux method demonstrated higher cocaine-and amphetamine-induced DA release in the dorsal but not the ventral striatum in OLEFT rats in comparison to LETO rats (23). However, conventional (i.e. net-flux) microdialysis does not provide quantitative measurement of extracellular DA concentration such as tonic DA efflux, actual release as well as quantitative estimates of reuptake (52). Since these variables are relevant to the behavioral phenotype under physiological conditions (i.e. when not challenged pharmacologically with DA mimetics), in the present study we used the no-net flux method of microdialysis. This modified technique provides an estimate of the extracellular neurotransmitter concentration, and a measure of the extraction fraction of the microdialysis probe with respect to time (7). By controlling for probe recovery, this latter parameter can also be used to monitor DA uptake, a functional measure of DAT activity (10, 50, 51). Based on these theoretical considerations and recent studies demonstrating validity of the method (8, 10, 12, 13), we aimed at measuring basal and potassium-stimulated DA release and estimating uptake in OLETF rats compared to age-matched, lean LETO controls. To investigate the relationship between DA and obesity and to control for the effect of escalating insulin resistance, we repeated these assays in separate subsets of rats at three different ages representing non-diabetic, pre-diabetic and diabetic stages (11, 19, 28).
Materials and Methods
Subjects
Male OLETF (n=17) and LETO (n=16) rats were obtained as a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan. Separate sets of rats of both strains at ~8 weeks (5 LETO and 7 OLETF), ~18 weeks (6 LETO and 6 OLETF) and ~56 weeks of age (5 LETO and 4 OLETF) (all within +/− 1wk difference) were used in the experiments. All rats were housed individually in mesh-floored, stainless-steel hanging cages and maintained in a temperature-controlled vivarium on a constant 12:12-h light-dark cycle (lights on at 0700). Tap water and pelleted rat chow (Purina 5001) were available ad libitum throughout experiments. All protocols used were conducted in accordance with the National Institute of Health Guide for the Use of Laboratory Animals (NIH Publications No. 80-23) and approved by The Pennsylvania State University Institutional Animal Care and Use Committee. The authors further attest that all efforts were made to minimize the number of animals used and their suffering.
Surgery and Microdialysis
On the day of experiment, rats were anesthetized with chloral hydrate (Sigma-Aldrich, USA), dissolved in physiological saline (loading dose 400 mg/kg i.p.; supplemented with 80 mg/kg i.p. every 2 hours) and placed on a heating pad (37°C ) (Stoelting, Co., Wood Dale, IL). Each rat was fixed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA and two microdialysis probes (CMA 12 Elite, 2 mm membrane length, 20kDa, CMA Microdialysis AB, Sweden) were implanted into right and left NAcc. The probe implants were targeted at the posterior medial extent of the accumbens shell/core region. Accordingly, the stereotaxic coordinates for the 8 wks age group were: AP 1.2 mm (from the bregma), ML 1.1 mm, and DV −7.5 mm (from the skull); and for 18 and 56 wks age groups were: AP 1.3 mm, ML 1.1 mm, and DV −7.5 mm (54). Probes were kept continually perfused with artificial cerebrospinal fluid (aCSF; containing 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 2.0 mM Na2HPO4, ascorbic acid 0.25 mM, set at pH=7.4), at a flow rate of 1.0 µL/min. Before used, the aCSF solution was filtered through a 0.22 µm filter.
Quantitative no-net-flux microdialysis
Briefly, no-net-flux microdialysis assesses the ability of the target brain structure to supply endogenous DA to a microdialysis probe and remove exogenous DA applied by the probe. When the concentration of DA perfused through the probe is equal to the extracellular concentration, there will be no net diffusion of the DA molecule into or out of the probe. In practice, a mathematical approximation is used to get an extracellular concentration of neurotransmitter. This method allows performing a correct evaluation of actual content of DA without excessive over- or underestimation of its extraneuronal concentration.
Theoretical considerations as well as practical observations of no-net-flux method show that extraction fraction index, the slope of a regression line, is a useful tool for non-direct dopamine uptake evaluation (10, 51). Thus, an angle between regression line and X-axis reflects an activity of dopamine transporter (DAT). As had been suggested, relatively small changes in extraction fraction index correspond to significant changes in up-take (9).
Accordingly, in our design, after a 90 min equilibration period probes were perfused in a random order with aCSF containing DA in the following concentrations (DAin): 0, 1.0, 2.0, 4.0 and 8.0 pg/ µL (~ 0, 5, 10, 20 and 40 nM, respectively) with 60 min of equilibration period for each concentration. During this period, samples were collected in 10 min intervals‥
Potassium-stimulated DA release
After completion of no-net flux measurements with the rat still in anesthesia, aCSF with no DA was perfused for 60 min to establish a stable baseline. Then, the normal aCSF was replaced by switching to a stream of a modified aCSF solution containing 100 mM KCl using a low dead-volume liquid switch (Univentor Ltd, Malta). The high potassium aCSF solution was made to iso-osmotic to normal aCSF by modifying Na+ content (to 48.3 mM), and was perfused over 20-min period, providing two equal samples within this interval. Following this period (“stimulation”), perfusion buffer was changed back to normal aCSF, and four consecutive 10-min samples were collected (“wash-out”). The internal capacity of the inlet tubing was 1.8 µm. In this arrangement, there was a 1.8 min delay between the onset of high potassium aCSF infusion and its appearance in the outflow from the microdialysis probe.
Dopamine and Metabolite Assays
Once collected, all microdialysis samples were stored at −80°C and analyzed together within 18 hours following sampling. Dopamine, and 3,-4 dihydroxyphenylacetic acid (DOPAC) were determined by reverse-phase HPLC coupled with coulometric detection. The chromatographic system consisted of a refrigerated autosampler (Model 542, ESA Biosciences, Inc., Chelmsford, MA), a solvent delivery isocratic system (Model 582, ESA Biosciences, Inc., Chelmsford, MA) and a coulometric detector (CoulArray, Model 5600A; ESA Biosciences, Inc., Chelmsford, MA). The mobile phase contained 0.09 M sodium phosphate, 0.05 M sodium citrate, 1.7 mM octanesulfonic acid, 0.05 mM EDTA and 10% acetonitrile (v/v) and adjusted to pH=3.0. All reagents used for the mobile phase were analytical grade. Mobile phase was filtered through a 0.22 µm nylon filter and pumped through the system at a flow rate of 0.7 ml/min. Monoamines were separated on an ESA column (C18, 150mm × 3.2 mm, 3 µm). Guard cell was set at +350 mV, the working electrode potentials E1 and E2 were −150 and +200 mV, respectively. Injection volume was 8 µL and limit of detection (LOD) for DA was 0.019 pg/ µL (or 0.1nM). LOD for DOPAC was not examined since the accumbens microdialysate concentration for this metabolite is usually in the hundred nM range. Retention time for DOPAC, DA were 2.8 and 3.3, respectively. The monoamine levels were quantified by external standard curve calibration using peak area for quantification.
Oral Glucose Tolerance Tests (OGTT)
OGTT tests were performed at 8, 20 and 52 weeks in a different set of rats (n=3–5) with similar body weights to rats used in microdialysis studies. The rationale for not performing OGTTs in the actual subjects was to reduce potential effects from food deprivation and procedural stress on the DA system. The test was administered following a 16hr fast, when an oral glucose load (2g/kg) was delivered to each rat orally via latex gavage. Blood glucose was measured before and at 30, 60, 90, and 120 min post-glucose loading using a standard glucometer LifeScan, One-Touch Basic). Animals were classified as diabetic if the peak level of plasma glucose was ≥ 300 mg/dL (16.7 mmol/l) and a peak glucose level at 120 min > 200 mg/dL (11.1 mmol/l) (42).
Histology
After completing the microdialysis tests, rats were perfused transcardially, brains were removed and 50 µm cresyl violet stained sections were verified under microscope for probe location using Paxinos and Watson atlas (54).
Data Analysis
All data are expressed as mean ± S.D. Statistical analyses were computed with Statistica software for PC (Version 6.1.; StatSoft inc., Tusla, OK).
Body weight and OGTT tests were calculated for each age group and compared between strains at each time point using Student’s t tests.
Neurochemical data from both hemispheres were collapsed for statistical analyses, since there was no statistical difference between samples obtained from the right and the left NAC in either strain at any time point.
All no-net-flux microdialysis data were expressed in nM. The net change of DA (DAin−DAout), where DAin and DAout are the DA concentration in the input and output perfusates, respectively, was calculated for each animal and then averaged for each perfusion group. The average net change in DA was plotted against DAin. No-net-flux data were analyzed using the linear regression, where the slope of the regression line resulted the extraction fraction, whereas the regression line’s intersection with X-axis represented a zero net flux point. Mean slope values and no-net flux points were analyzed by ANOVA with Fisher’s post-hoc analysis.
In potassium-stimulated release experiment, in addition to absolute DA values, data were also standardized and presented as percent from baseline (calculated from three values preceding stimulation). The difference between the effects of potassium infusion was examined using ANOVA with subsequent post-hoc analysis (Fisher test). All differences were considered significant at p<0.05.
Results
Body weight
As expected, body weight significantly increased with aging in both strain (F2,27=10.7, p<0.001). However, post hoc tests revealed that OLETF were heavier than LETO at all ages (8 weeks: 290±8g vs. 264±12g, p<0.001; 18 weeks: 552±23g vs. 473±19g, p<0.001; 56 weeks: 695±58g vs. 539±25g; p< 0.001).
Oral glucose tolerance tests
Fasting blood glucose and responses to intragastric glucose load were tested at 8, 20 and 52 weeks in a separate set of naïve rats from the same shipment which corresponds approximately with the acute microdialyis age groups. There was no statistical difference between the mean body weight of the actual experimental groups and the groups used in the OGTT tests. At 8 weeks, despite no strain difference in fasting blood glucose levels, OLETF had slightly reduced glucose tolerance relative to LETO rats (at 30 min: 12.41±0.73 mmol/l vs. 9.45±0.90 mmol/l, t(2)=3.82, p<0.05, n=3) and an 122% increase in AUC. By 20 weeks, prediabetes progressed dramatically in OLETF as indicated by a two fold rise in blood glucose levels at 30 min compared to LETO (14.02±1.37 mmol/l vs. 7.99±0.11 mmol/l, t(4)=4.26, p<0.01, n=5). Blood glucose levels remained significantly elevated through the 90-min sample (60 min: t(4)=6.65, p<0.01; 90 min: t(4)=4.78, p<0.01). This response profile resulted in a 338% higher AUC in the OLETF group. Although one rat of the five tested was already diabetic at this point, the 20-week cohort overall did not meet the criteria for overt diabetes. In contrast, at 52 week all OLETF rats developed diabetes with elevated fasting glucose levels (6.81±0.83 mmol/l, t(4)=2.29, p<0.05, n=5), a marked increase in their peak responses (19.9±1.03 mmol/l, t(4)=9.03, p<0.001), and significantly elevated glucose levels at 120 min (14.39±0.97 mmol/l, t(4)=7.09, p<0.01).
Histology
All microdialysis probes were placed within the caudomedial aspect of the NAC (A: 1.0–1.4 mm, L: 0.8–1.4 mm with respect to Bregma and the midline, respectively). Because of the size of the probe membrane and the slightly varying ventral and medial position (possible due to different brain size across age groups), we cannot be certain as to whether the samples originated exclusively from the medial shell or also from the adjacent core areas. Nonetheless, there were no statistically significant differences across samples regarding the probe position or placement between hemispheres.
Extracellular DA concentration
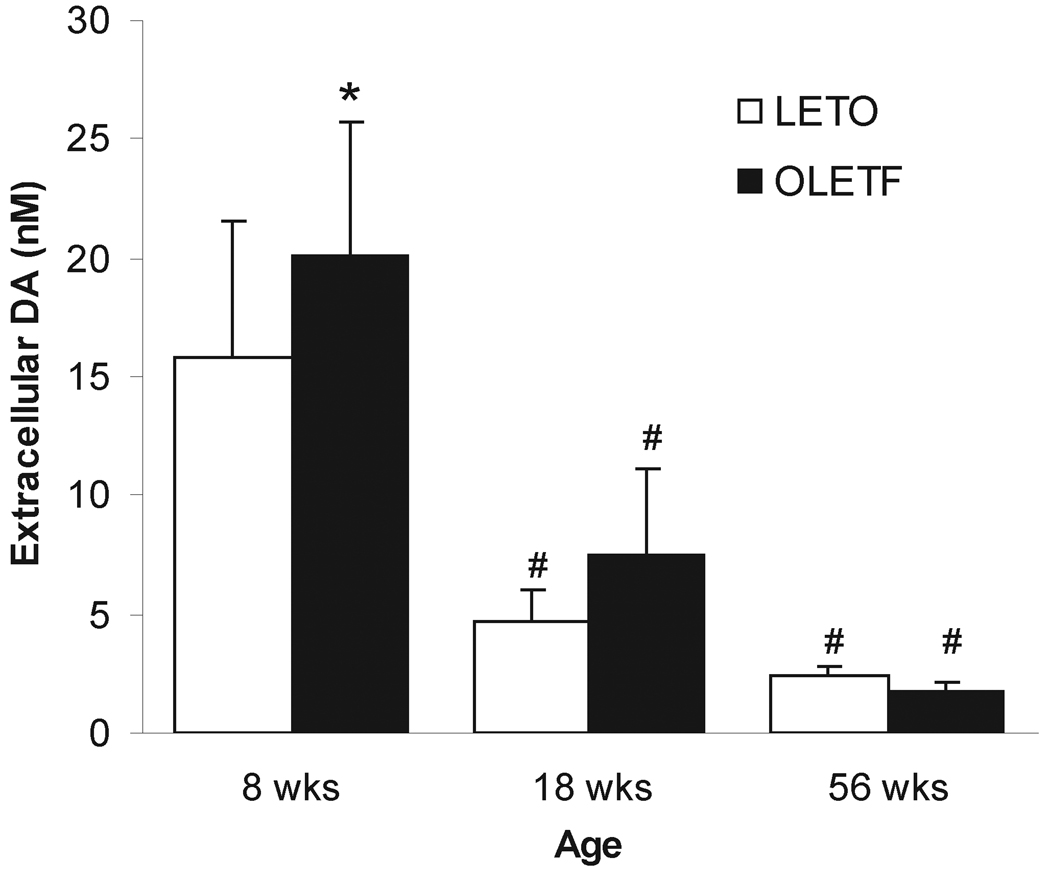
Two-way ANOVA revealed an overall effect of age (F2,45=92.680, p<0.001) and strain (F1,45=9.899, p=0.003) on basal extracellular concentrations of DA in NAC. The analysis of no-net-flux microdialysis data showed that DA levels (mean ± S.D.) were 15.85±5.63 nM and 20.10±5.61 nM in 8 weeks old rats; 4.75±1.25 nM and 7.37±3.71 nM in 18 weeks old rats, and 2.399±0.421 and 1.777±0.405 nM in 56 weeks old LETO and OLETF rats, respectively (Fig.1). Post-hoc analysis demonstrated a significant difference between LETO and OLETF rats at 8 weeks (p<0.001), whereas at 18 weeks the difference barely missed the level of significance (p=0.052). No statistical difference was noted at 56 weeks (p= 0.748; Fig. 1).
Fig. 1.
Extracellular concentration of DA (nM, mean ± S.D.) in the NAC of LETO (open bars) and OLETF (dashed bars) rats calculated from a point of intersection of regression lines and X-axis. *, significant difference between strains, p<0.05; #, significant difference between age groups within a strain in comparison to the 8-week values, p<0.05.
Extraction fraction of DA
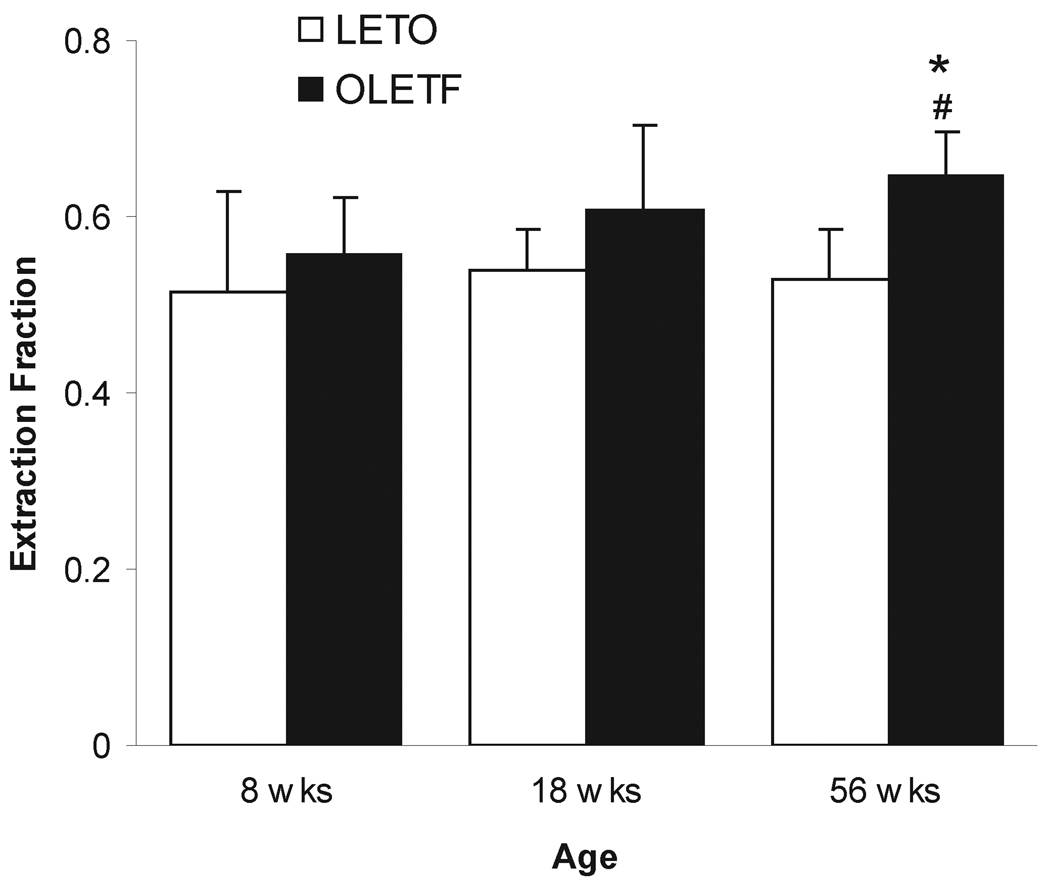
The extracellular fraction was affected by both age (F2,46=11.852, p<0.001) and strain (F1,46=7.928, p=0.007), with no significant interaction between age and strain (F2,46=0.855, p=0.432). The actual extracellular fraction values were 0.514±0.115 and 0.557±0.066 at 8 weeks, 0.538 ±0.048 and 0.608 ±0.096 at 18 weeks, 0.527±0.057 and 0.648±0.049 at 56 weeks, in LETO and OLETF, respectively (Fig. 2). Post-hoc analysis between strains revealed a significantly increased extraction fraction in the 56 weeks old OLETF compared to LETO (p<0.03) and also relative to 8 weeks old OLETF (p<0.05). There was no difference between strains at 8 weeks (p=0.521) and 18 weeks (p=0.058), nor there was any difference in extraction fraction across all ages in LETO (Fig. 2).
Fig. 2.
No-net-flux microdialysis of DA (mean ± S.D.) in the NAC of LETO (open bars) and OLETF (dashed bars) rats. The slope index of regression line represents the extracellular fraction. *, significant difference between strains, p<0.05; #, significant difference between 56 week age group of OLETF and the 8 as well as 18 week OLETF cohorts, p<0.05.
Potassium-induced DA release
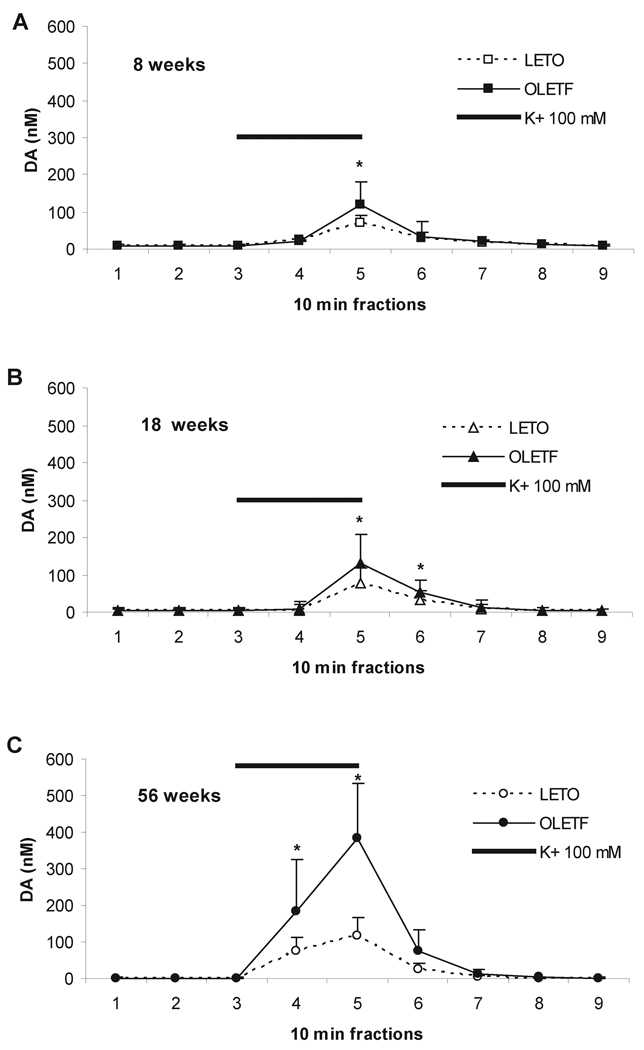
The reverse dialysis of aCSF containing 100 mM K+ (i.e. infused trough the implanted probes) over a period of 20 min resulted in an increase in extracellular DA outflow. Data from these tests are summarized in Figure 3.
Figure 3.
Effect of infusion of high-potassium aCSF (100 mM) on the absolute content of DA (mean ± S.D.) in microdialysates from the NAC at 8 weeks (A), 18 weeks (B), and 56 weeks of age (C) in OLETF (solid lines and squares) and LETO (dashed lines and open squares). Bar indicates duration (30 min) of the chemical stimulation. Asterisks denote a significant difference between strains (p<0.05).
Two-way ANOVA revealed an overall significant time (i.e. sample) × strain interaction on the extracellular absolute DA levels in 18 weeks (F8,169=2.189, p=0.031) and 56 weeks old (F8,107=12.037, p<0.001), but not in 8 weeks old rats (F(8,153=1.928, p=0.059). The magnitude of release as well as the time dynamics of the effect varied with age (Fig. 3). Post-hoc analysis on the main effects showed that NAC DA release in response to potassium in 18 weeks old OLETF was higher than in LETO at 20 min (p<0.001), but it lasted longer, with the strain difference being significant at 30 min after the onset of stimulation (p<0.02; Fig. 3B). In 56 weeks old rats, the effect of stimulation on DA release reached higher levels in OLETF than LETO at 10 and 20 min (p<0.02, p<0.001, respectively; Fig. 3C). Subsequent one way ANOVAs, however, demonstrated that in OLETF rats stimulated DA release was higher than in LETO across all ages, including 8 weeks old rats. Specifically, at 8 weeks, there was a significant strain difference of DA concentrations in the 5th fraction (F1,17=11.794, p=0.003); at 18 weeks, in the 5th and 6th fractions (F1,15=5.517, p=0.033, and F1, 18=5.043, p=0.038, respectively); at 56 weeks, in the 4th and the 5th fractions (F1,11=4.875, p=0.049, and F1,12=22.495, p<0.001, respectively).
When percent change from baseline were analyzed, there was a significant time × age interaction in LETO (F16,209=18.433, p<0.001) and OLETF rats (F16,207=55.434, p<0.001). At 56 weeks, the magnitude of stimulated DA release was significantly higher in OLETF than in LETO (p<0.05). Because the time-course of the effect varied between strains and across ages (see below), we performed an area under curve (AUC) analysis to compare total DA released in response to stimulation (i.e. above the baseline). Two-way ANOVA revealed an effect of age (F2,49=154.05, p<0.001) and strain (F3,49=38.135, p< 0.001) on AUC. Post-hoc analysis showed a significant difference between 56 weeks old LETO and OLFET rats (p<0.001). There were also a significant within strain difference between 56 weeks and the two younger groups (for all p<0.001).
Although in both strains, the levels of extracellular DA were different with respect to age during and after potassium stimulation, the overall dynamics were different across strains (Fig. 4). In both strains, 10 min after the onset of potassium perfusion, there was a statistical difference in DA levels between 56 weeks and 8 weeks and between 56 and 18 weeks old animals (p<0.001). No difference between 18 weeks and 8 weeks old was noted. At 20 min, DA release in all age groups were significantly different in LETO (p<0.01) with a gradual escalation in response magnitude (Fig. 4A). In contrast, OLETF rats had higher 20-min DA levels at 56 weeks, but the 8 and 18 weeks old cohorts did not differ in their responsiveness to DA stimulation (p<0.001; Fig. 4B). This suggests that the increase in the vesicular DA capacity occurs at an accelerated rate in OLETF than in LETO rats.
Figure 4.
Relative changes (above the basal levels) in net DA output evoked by potassium-stimulation (100 mM) based on area under curve (AUC) analysis (mean ± S.D.). *, significant difference between strains, p<0.05; #, significant difference between age groups within a strain in comparison to oldest rats, p< 0.05.
Effect of potassium stimulation on DOPAC
The reverse dialysis of 100 mM K+ trough the implanted probes decreased the extracellular levels of DOPAC within 20 min of the onset of delivery. The magnitude of relative decrease in DOPAC extracellular level was identical in all experimental groups showing an about 50 % drop relative to basal values with a recovery to 100% in about 30 min following stimulation. Accordingly, two-way ANOVA showed significant time×age effects on DOPAC extracellular level in NAC under a depolarization elicited by potassium infusion in both LETO (F16,213=6.9599, p<0.001) and OLETF (F16,213=2.3059, p=0.004) rats. There was no statistical difference between obese and lean strains at any age: 8 weeks: (F8,151=1.904, p=0.064); 18 weeks: (F8,174=1.232, p=0.283); and 56 wks: (F8,105=1.078, p=0.384).
Discussion
In the present study we sought to investigate basal and stimulated DA release in the NAC as a function of obesity and metabolic complications leading to type-2 diabetes in a rat model of hyperphagia-induced obesity, the OLETF rat. Our findings showed that basal and potassium-stimulated DA release as well as DA clearance in the NAC of the obese OLETF rats were different from the lean controls. However, the direction and the magnitude of these effects are age dependent corresponding with the development of obesity and insulin resistance.
Altered regulation of basal and stimulated DA levels in OLETF
In 8 weeks old animals, the extracellular DA concentrations were significantly higher in OLETF than in LETO rats. Then, both strains showed a continous reduction of NAC DA throughout all time points tested with a profound drop in basal DA levels by 56 weeks. At 18 weeks of age, however, OLETF rats still demonstrated a trend (P=0.052) of higher unstimulated extracellular DA compared to LETO. In OLETF, but not in LETO rats this declining trend in extracellular DA concentration coincided with an increase in extraction fraction of DA, an index of uptake. Nonetheless, this measure was significantly higher in 56 weeks OLETF rats compared to age-matched lean cohorts.
Findings from the potassium stimulated-release tests showed an increased sensitivity as a function of age in both strains. This observation reinforces previous reports using different methods (27, 65). Further analyses, however, demonstrated that in OLETF rats both the amplitude and the duration of potassium-evoked DA release was augmented compared to LETO rats at all ages. This difference was apparent even when the stimulated release was normalized with respect to differential ambient DA levels across strains and ages, and indicates an increased vesicular capacity in obese OLETF.
An increase in depolarization-evoked DA release may be mediated by increased permeability of K+ channels and/or voltage-sensitive Ca2+ channels (18, 71). There is direct evidence that CCK redistributes intracellular Ca2+ or changes calcium influx through neuronal membranes (63, 78). It has also been demonstrated that modulation of methamphetamine-induced DA release by CCK is highly Ca2+ dependent (74). Thus, decreased expression of CCK-1 receptor may cause changes in Ca2+-depended components of neurotransmitter release. Independent of CCK, prediabetes in OLETF may also affect calcium-related DA homeostasis. Specifically, it has been shown that activity of calmodulin protein kinase II is altered under experimental hyperglycaemic condition (59) with potential effect on altered catecholamine release (58). In addition to hyperglycemia, diabetic keto-acidosis may promote monoamines sequestrations (22) that, in turn, cooperate on replenishing the vesicular pool of DA.
To our knowledge, only one study investigated DA release in the OLETF rat. Feifel et al. (23) reported that in OLETF rats the amphetamine- and cocaine-induced DA release is altered. These effects, however, were region specific, and showed an opposite direction, i.e., an increased stimulation in the core and a reduced response in the shell region of NAcc. As noted, the size of microdialysis probes used and the nature of placement for this type of studies do not allow for differentiation between the core and shell, thus a direct comparison between our results and Feifel et al. is impractical. Furthermore, Feifel et al. used the conventional microdialysis method that does not account for potential differences in the absolute basal levels. Thus, studies using multiple distinct probe locations would be useful since a heterogeneity in CCK receptor distribution along the medio-lateral and rostro-caudal axis of the NAC and multiplicity in affinity sites have been suggested with plausible role in CCK/DA interaction (3, 17, 36, 41, 70).
Increased vesicular capacity could be attributed to an altered function of the vesicular monoamine transporter (VMAT2) that is primarily responsible for vesicular packing. In fact, our observation that the extracellular levels of the DA metabolite DOPAC showed a consistent reduction following high-potassium stimulation suggests a shift from metabolism to storage. The finding that change in DOPAC levels did not differ between strains at any age, however, somewhat mitigates this proposition. Caution must be exercised though, since other critical variables such as precursor availability, synthesis and DAT functions, may change differentially between strains over time, that in turn influence vesicular storage size (56).
Of particular interest, is the accumulating evidence showing that insulin can regulate DAT. Specifically, insulin modifies phosphoinositol 3-kinase mediated signaling leading to increased DAT expression on cell surface and [3H]-DA uptake (26). Relevant to our discussion, it has been reported that DAT expression in obese Zucker rat’s VTA/ substantia nigra (SN) complex is higher than in lean controls (24). Furthermore, this effect seems not to be secondary to the deficits in leptin signaling, or insulin resistance, since short-term food deprivation in normal rats results in the opposite effect, i.e. a decrease in mRNA expression and activity of the DAT (53). Thus, with progression of insulin resistence, the accompanying increase in circulating insulin levels may result in increased DAT availability in the OLETF rats. Increased DA removal from the extracellular compartment due to upregulated DAT in rats with advanced prediabetic and diabetic status can result in lower basal DA levels. We demonstrated this by directly showing reduced basal DA, and by estimating increased uptake based on the extraction index. Due to its extrasynaptic localization and to the response dynamics, the DAT in the striatum has been proposed to regulate tonic rather than phasic DA release. This together with an increased cytosolic pool (i.e. inferred from increased reuptake) supplying DA to the releasable pool, may explain sustained or even increasing capacity of the terminals to release more DA in response to depolarization in the diabetic OLETF rats. Thus, it is not impossible that an augmented phasic DA signaling in response to stimulation with palatable meals may contribute to overeating in this strain.
Effect of ageing on DA and development of diabetes in OLETF rats
In this study, aging played a critical role in the manifestation of strain differences observed in DA regulation between the OLETF and LETO rats. In general, it has been known for some time that aging results in reduced number of DA neurons as well as impaired DA functions in various brain areas of both human and rats (47, 60). It has also been demonstrated that extensive reduction in the number of DA neurons in the SN is also causing diminished DA levels in the striatum and the basal ganglia, and in turn motor deficits characteristic to Parkinson’s disease (37). Despite this information, to our knowledge this is the first study demonstrating ageing’s diminishing effect on the actual DA concentration in the NAC of rats in vivo using quantitative microdialysis. Nonetheless, the current study cannot differentiate between strain effect of age in the absence of increased adiposity and metabolic abnormalities.
Potential relationship between CCK-1 receptors and altered DA regulation
There is evidence showing a direct influence of CCK-ergic transmission on DA neuronal functions (3, 17). CCK is co-localized in a subpopulation of DA neurons of the VTA and SN projecting to the limbic and basal ganglia structures including the NAC (36). It regulates the firing rate of the DA neurons and also the DA release in terminal regions (3). CCK and CCK-ergic drugs variously affect DA neurotransmission in the brain due to heterogeneity in CCK receptor population and difference in CCK receptor distribution (61). Disruption of CCK-1 mediated neurotransmission by the CCK-1 receptor antagonist devazepide has been reported to elicit an increase in DA content of the NAC (44). Of particular interest, unlike the CCK-2 receptors that are abundant in the forebrain, CCK-1 receptors are expressed only in select brain structures, particularly those involved in reward and motivation (34). In this context, it is plausible that the lack of functional CCK-1 receptors in the OLETF rats may also result in behavioral alterations that are related to CCK/DA interaction (3, 61). For example, Bednar et al. (2) demonstrated the potency of DA D1 or D2 receptor antagonists to augment the inhibitory effect of CCK on intraorally infused sucrose. Recently, we showed that OLETF rats were more sensitive to peripheral administration of D1 or D2 receptor antagonists in reducing sucrose intake (20). Thus, it seems that in the OLETF rats an altered DA regulation may indeed contribute to the changes in feeding behaviors observed in this strain (i.e. increased sweet preference and overeating in general).
In this study, we have expanded the investigation of the relationship between CCK-1 receptors and DA in the NAC to include corollaries of dietary obesity and late-onset diabetes. Specifically, based on our observation that in OLETF rats, both changes in the basal and phasic DA release is accentuated by age one may assume that CCK-1 receptors may play a permissive role in maintaining DA homeostasis against challenges by environmental factors. In this context, one may explain the observed alterations in the DA system of the OLETF rats as a result of developmental adaptation to their deficit in functional CCK-1 receptors. An alternative explanation could be that DA regulatory deficits in OLETF rats are rather secondary to their hyperphagic phenotype downstream from the mutant genotype. In the first case, however, DA alterations would be expected to facilitate eating, whereas in the latter case, would result in curbing appetite. Although the exact role of DA in behavioral reward in general, and its relationship with eating in particular, even in non-obese, is far from being settled (4), accumulating evidence have linked increased NAC DA levels with increased eating. For example, stimulation that results in DA release in the NAC also stimulates food consumption (29, 33) and intake of sucrose solution (30). Further evidence for a positive correlation between tonic level of DA and food consumption had been shown in DAT knock-down mice. Particularly, these mice express high basal DA levels and similar to the OLETF rats not only have an increased meal-size and sucrose consumption but they are also heavier than wild-type mice (55). Although the DAT knock-out mice demonstrate a retarded growth that makes food intake and body weight less comparable with the wild-type, these mice also express an increased avidity for sucrose (15). These and other evidences together with our present findings suggest that the altered DA regulation, particularly the increased basal DA levels, seen in the OLETF rats occurring very early in their lives, even before marked increase in body weight or deficits in glucose control develop, are rather contributory to increased eating than consequence of it. Indeed, it has been shown recently that OLETF rats are hyperphagic as early as the first postnatal day (75) pointing to a critical prenatal interaction between CCK and DA systems in the developing brain. Nonetheless, the gradual development in obesity and metabolic impairments associated with occurrence of diabetes as demonstrated in the present report may also challenge DA homeostasis. Whether these effects are direct or indirect with respect to the DA systems as well as to the behavior remains to be elucidated
Acknowledgements
The authors wish to thank Otsuka Pharmaceutical Co. (Tokushima, Japan) for the generous donation of the OLETF and LETO animals used to perform this study. This research was supported by National Institute of Diabetes & Digestive & Kidney Diseases Grant DK065709.
List of abbreviations
- CCK
cholecystokinin
- DA
dopamine
- DAT
dopamine transporter
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- LETO
Long Evans Tokushima Otsuka
- NAC
nucleus accumbens
- OLETF
Otsuka Long Evans Tokushima Fatty
- SN
substantia nigra
- VTA
ventral tegmental area
References
- 1.Baptista T. Body weight gain induced by antipsychotic drugs: mechanisms and management. Acta Psychiatr Scand. 1999;100:3–16. doi: 10.1111/j.1600-0447.1999.tb10908.x. [DOI] [PubMed] [Google Scholar]
- 2.Bednar I, Carrer H, Qureshi GA, Sodersten P. Dopamine D1 or D2 antagonists enhance inhibition of consummatory ingestive behavior by CCK-8. Am J Physiol. 1995;269:R896–R903. doi: 10.1152/ajpregu.1995.269.4.R896. [DOI] [PubMed] [Google Scholar]
- 3.Beinfeld MC. What we know and what we need to know about the role of endogenous CCK in psychostimulant sensitization. Life Sci. 2003;73:643–654. doi: 10.1016/s0024-3205(03)00384-9. [DOI] [PubMed] [Google Scholar]
- 4.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 5.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 6.Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- 7.Bungay PM, Morrison PF, Dedrick RL. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46:105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- 8.Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chefer VI, Shippenberg TS. Changes in basal and cocaine-evoked extracellular dopamine uptake and release in the rat nucleus accumbens during early abstinence from cocaine: quantitative determination under transient conditions. Neuroscience. 2002;112:907–919. doi: 10.1016/s0306-4522(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 10.Chefer VI, Zapata A, Shippenberg TS, Bungay PM. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab. 2005;7:307–317. doi: 10.1111/j.1463-1326.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen KC. Evidence on extracellular dopamine level in rat striatum: implications for the validity of quantitative microdialysis. J Neurochem. 2005;92:46–58. doi: 10.1111/j.1471-4159.2004.02848.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen KC. Preferentially impaired neurotransmitter release sites not their discreteness compromise the validity of microdialysis zero-net-flux method. J Neurochem. 2005;92:29–45. doi: 10.1111/j.1471-4159.2004.02847.x. [DOI] [PubMed] [Google Scholar]
- 14.Conway B, Rene A. Obesity as a disease: no lightweight matter. Obes Rev. 2004;5:145–151. doi: 10.1111/j.1467-789X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 15.Costa RM, Gutierrez R, de Araujo IE, Coelho MR, Kloth AD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Dopamine levels modulate the updating of tastant values. Genes Brain Behav. 2006 doi: 10.1111/j.1601-183X.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- 16.Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides. 2001;22:1339–1348. doi: 10.1016/s0196-9781(01)00461-2. [DOI] [PubMed] [Google Scholar]
- 17.Crawley JN. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991;12:232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- 18.Dawson LA, Routledge C. Differential effects of potassium channel blockers on extracellular concentrations of dopamine and 5-HT in the striatum of conscious rats. Br J Pharmacol. 1995;116:3260–3264. doi: 10.1111/j.1476-5381.1995.tb15133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R292–R300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- 20.De Jonghe BC, Hajnal A, Covasa M. Increased sensitivity to D1 and D2 receptor antagonism in sucrose feeding OLETF rats. Society for the Study of Ingestive Behavior: Annual Meeting. 2005:329–392. Appetite. [Google Scholar]
- 21.Drewnowski A. Energy density, palatability, and satiety: implications for weight control. Nutr Rev. 1998;56:347–353. doi: 10.1111/j.1753-4887.1998.tb01677.x. [DOI] [PubMed] [Google Scholar]
- 22.Erecinska M, Pastuszko A, Wilson DF, Nelson D. Ammonia-induced release of neurotransmitters from rat brain synaptosomes: differences between the effects on amines and amino acids. J Neurochem. 1987;49:1258–1265. doi: 10.1111/j.1471-4159.1987.tb10018.x. [DOI] [PubMed] [Google Scholar]
- 23.Feifel D, Shilling PD, Kuczenski R, Segal DS. Altered extracellular dopamine concentration in the brains of cholecystokinin-A receptor deficient rats. Neurosci Lett. 2003;348:147–150. doi: 10.1016/s0304-3940(03)00767-5. [DOI] [PubMed] [Google Scholar]
- 24.Figlewicz DP, Patterson TA, Johnson LB, Zavosh A, Israel PA, Szot P. Dopamine transporter mRNA is increased in the CNS of Zucker fatty (fa/fa) rats. Brain Res Bull. 1998;46:199–202. doi: 10.1016/s0361-9230(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 25.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–335. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- 26.Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol Pharmacol. 2005;68:102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- 27.Gerhardt GA, Maloney RE., Jr Microdialysis studies of basal levels and stimulus-evoked overflow of dopamine and metabolites in the striatum of young and aged Fischer 344 rats. Brain Res. 1999;816:68–77. doi: 10.1016/s0006-8993(98)01095-6. [DOI] [PubMed] [Google Scholar]
- 28.Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, pre-diabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajnal A, Mark GP, Rada PV, Lenard L, Hoebel BG. Norepinephrine microinjections in the hypothalamic paraventricular nucleus increase extracellular dopamine and decrease acetylcholine in the nucleus accumbens: relevance to feeding reinforcement. J Neurochem. 1997;68:667–674. doi: 10.1046/j.1471-4159.1997.68020667.x. [DOI] [PubMed] [Google Scholar]
- 30.Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 31.Hamdi A, Porter J, Prasad C. Decreased striatal D2 dopamine receptors in obese Zucker rats: changes during aging. Brain Res. 1992;589:338–340. doi: 10.1016/0006-8993(92)91296-q. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton ME, Redondo JL, Freeman AS. Overflow of dopamine and cholecystokinin in rat nucleus accumbens in response to acute drug administration. Synapse. 2000;38:238–242. doi: 10.1002/1098-2396(20001201)38:3<238::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- 34.Hill DR, Shaw TM, Graham W, Woodruff GN. Autoradiographical detection of cholecystokinin-A receptors in primate brain using 125I-Bolton Hunter CCK-8 and 3H-MK-329. J Neurosci. 1990;10:1070–1081. doi: 10.1523/JNEUROSCI.10-04-01070.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 36.Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature. 1980;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- 37.Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology. 1998;51:S2–S9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 38.Huang XF, Yu Y, Zavitsanou K, Han M, Storlien L. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res Mol Brain Res. 2005;135:150–161. doi: 10.1016/j.molbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, Lawrence AJ, Deng C. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res. 2006;175:415–419. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Hurd YL, Lindefors N, Brodin E, Brene S, Persson H, Ungerstedt U, Hokfelt T. Amphetamine regulation of mesolimbic dopamine/cholecystokinin neurotransmission. Brain Res. 1992;578:317–326. doi: 10.1016/0006-8993(92)90264-a. [DOI] [PubMed] [Google Scholar]
- 41.Kariya K, Tanaka J, Nomura M. Systemic administration of CCK-8S, but not CCK-4, enhances dopamine turnover in the posterior nucleus accumbens: a microdialysis study in freely moving rats. Brain Res. 1994;657:1–6. doi: 10.1016/0006-8993(94)90946-6. [DOI] [PubMed] [Google Scholar]
- 42.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 43.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Ladurelle N, Durieux C, Roques BP, Dauge V. Different modifications of the dopamine metabolism in the core and shell parts of the nucleus accumbens following CCK-A receptor stimulation in the shell region. Neurosci Lett. 1994;178:5–10. doi: 10.1016/0304-3940(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 45.Levin BE, Triscari J, Sullivan AC. The effect of diet and chronic obesity on brain catecholamine turnover in the rat. Pharmacol Biochem Behav. 1986;24:299–304. doi: 10.1016/0091-3057(86)90354-0. [DOI] [PubMed] [Google Scholar]
- 46.Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 47.Meites J. Role of hypothalamic catecholamines in aging processes. Acta Endocrinol (Copenh) 1991;125 Suppl 1:98–103. [PubMed] [Google Scholar]
- 48.Moran TH. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16:858–865. doi: 10.1016/s0899-9007(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 49.Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48:360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- 50.Olson RJ, Justice JB., Jr Quantitative microdialysis under transient conditions. Anal Chem. 1993;65:1017–1022. doi: 10.1021/ac00056a012. [DOI] [PubMed] [Google Scholar]
- 51.Pan WH, Lee JC. Determination of microdialysis extraction fraction of cocaine by the no net flux method under high, low, and zero steady-state cocaine concentrations in rats. Brain Res. 1995;686:249–253. doi: 10.1016/0006-8993(95)00456-z. [DOI] [PubMed] [Google Scholar]
- 52.Parsons LH, Justice JB., Jr Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J Neurochem. 1992;58:212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- 53.Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology. 1998;68:11–20. doi: 10.1159/000054345. [DOI] [PubMed] [Google Scholar]
- 54.Paxinos G, Watson CA. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic; 1997. [Google Scholar]
- 55.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pothos EN. Regulation of dopamine quantal size in midbrain and hippocampal neurons. Behav Brain Res. 2002;130:203–207. doi: 10.1016/s0166-4328(01)00419-3. [DOI] [PubMed] [Google Scholar]
- 57.Prentice AM, Jebb SA. Fast foods, energy density and obesity: a possible mechanistic link. Obes Rev. 2003;4:187–194. doi: 10.1046/j.1467-789x.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 58.Ramakrishnan R, Nazer MY, Suthanthirarajan N, Namasivayam A. An experimental analysis of the catecholamines in hyperglycemia and acidosis induced rat brain. Int J Immunopathol Pharmacol. 2003;16:233–239. doi: 10.1177/039463200301600308. [DOI] [PubMed] [Google Scholar]
- 59.Ramakrishnan R, Prabhakaran K, Jayakumar AR, Gunasekaran P, Sheeladevi R, Suthanthirarajan N. Involvement of Ca2+/calmodulin-dependent protein kinase II in the modulation of indolamines in diabetic and hyperglycemic rats. J Neurosci Res. 2005;80:518–528. doi: 10.1002/jnr.20499. [DOI] [PubMed] [Google Scholar]
- 60.Roth GS, Joseph JA. Age-related changes in transcriptional and posttranscriptional regulation of the dopaminergic system. Life Sci. 1994;55:2031–2035. doi: 10.1016/0024-3205(94)00383-1. [DOI] [PubMed] [Google Scholar]
- 61.Rotzinger S, Bush DE, Vaccarino FJ. Cholecystokinin modulation of mesolimbic dopamine function: regulation of motivated behaviour. Pharmacol Toxicol. 2002;91:404–413. doi: 10.1034/j.1600-0773.2002.910620.x. [DOI] [PubMed] [Google Scholar]
- 62.Seroogy K, Schalling M, Brene S, Dagerlind A, Chai SY, Hokfelt T, Persson H, Brownstein M, Huan R, Dixon J, et al. Cholecystokinin and tyrosine hydroxylase messenger RNAs in neurons of rat mesencephalon: peptide/monoamine coexistence studies using in situ hybridization combined with immunocytochemistry. Exp Brain Res. 1989;74:149–162. doi: 10.1007/BF00248288. [DOI] [PubMed] [Google Scholar]
- 63.Simasko SM, Wiens J, Karpiel A, Covasa M, Ritter RC. Cholecystokinin increases cytosolic calcium in a subpopulation of cultured vagal afferent neurons. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1303–R1313. doi: 10.1152/ajpregu.00050.2002. [DOI] [PubMed] [Google Scholar]
- 64.Sorensen LB, Moller P, Flint A, Martens M, Raben A. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int J Obes Relat Metab Disord. 2003;27:1152–1166. doi: 10.1038/sj.ijo.0802391. [DOI] [PubMed] [Google Scholar]
- 65.Stanford JA, Currier TD, Purdom MS, Gerhardt GA. Nomifensine reveals age-related changes in K(+)-evoked striatal DA overflow in F344 rats. Neurobiol Aging. 2001;22:495–502. doi: 10.1016/s0197-4580(00)00243-8. [DOI] [PubMed] [Google Scholar]
- 66.Szczypka MS, Mandel RJ, Donahue BA, Snyder RO, Leff SE, Palmiter RD. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron. 1999;22:167–178. doi: 10.1016/s0896-6273(00)80688-1. [DOI] [PubMed] [Google Scholar]
- 67.Szczypka MS, Rainey MA, Palmiter RD. Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nat Genet. 2000;25:102–104. doi: 10.1038/75484. [DOI] [PubMed] [Google Scholar]
- 68.Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/s0378-1119(97)00259-x. [DOI] [PubMed] [Google Scholar]
- 69.Ukkola O, Bouchard C. Role of candidate genes in the responses to long-term overfeeding: review of findings. Obes Rev. 2004;5:3–12. doi: 10.1111/j.1467-789x.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- 70.Vaccarino FJ, Vaccarino AL. Antagonism of cholecystokinin function in the rostral and caudal nucleus accumbens: differential effects on brain stimulation reward. Neurosci Lett. 1989;97:151–156. doi: 10.1016/0304-3940(89)90155-9. [DOI] [PubMed] [Google Scholar]
- 71.Voigt M, Wang RY, Westfall TC. Cholecystokinin octapeptides alter the release of endogenous dopamine from the rat nucleus accumbens in vitro. J Pharmacol Exp Ther. 1986;237:147–153. [PubMed] [Google Scholar]
- 72.Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, Logan J, Gatley SJ, Ding YS, Wong C, Pappas N. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord. 2003;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- 73.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Perng SL, Lin JC, Tsao WL. Cholecystokinin facilitates methamphetamine-induced dopamine overflow in rat striatum and fetal ventral mesencephalic grafts. Exp Neurol. 1994;130:279–287. doi: 10.1006/exnr.1994.1206. [DOI] [PubMed] [Google Scholar]
- 75.Weller A. The ontogeny of postingestive inhibitory stimuli: examining the role of CCK. Dev Psychobiol. 2006;48:368–379. doi: 10.1002/dev.20148. [DOI] [PubMed] [Google Scholar]
- 76.Yeomans MR, Blundell JE, Leshem M. Palatability: response to nutritional need or need-free stimulation of appetite? Br J Nutr. 2004;92 Suppl 1:S3–S14. doi: 10.1079/bjn20041134. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida M, Yokoo H, Mizoguchi K, Kawahara H, Tsuda A, Nishikawa T, Tanaka M. Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: measurement by in vivo microdialysis. Neurosci Lett. 1992;139:73–76. doi: 10.1016/0304-3940(92)90861-z. [DOI] [PubMed] [Google Scholar]
- 78.Zhang W, Segura BJ, Mulholland MW. Cholecystokinin-8 induces intracellular calcium signaling in cultured myenteric neurons from neonatal guinea pigs. Peptides. 2002;23:1793–1801. doi: 10.1016/s0196-9781(02)00136-5. [DOI] [PubMed] [Google Scholar]