Abstract
The concept of reward is central to psychology, but remains a cipher for neuroscience. Considerable evidence implicates dopamine in the process of reward and much of the data derives from the nucleus accumbens. Gustatory stimuli are widely used for animal studies of reward, but the connections between the taste and reward systems are unknown. In a series of experiments, our laboratory has addressed this issue using functional neurochemistry and neuroanatomy. First, using microdialysis probes, we demonstrated that sapid sucrose releases dopamine in the nucleus accumbens. The effect is dependent on oral stimulation and concentration. We subsequently determined that this response was independent of the thalamocortical gustatory system, but substantially blunted by damage to the parabrachial limbic taste projection. Further experiments using c-fos histochemistry confirmed that the limbic pathway was the prime carrier for the gustatory afferent activity that drives accumbens dopamine release.
Keywords: Dopamine, parabrachial nucleus, sucrose, taste
The Problem of Reward
Reward is a psychological construct still in search of neural mechanisms. The problems associated with this pursuit arise because the construct itself has several definitions. Natural rewards are produced by sensory stimuli but they are not synonymous with them. Physiological conditions and experience influence, even reverse, the reward value of stimuli. Non natural rewards are produced by drugs and electrical stimulation acting directly on the brain. This provides strong evidence that reward is a central phenomenon. Despite this, the most widely accepted definition of reward is psychological, i.e. a stimulus that increases the probability of a closely antecedent behavior occurring again. This definition is preferred because it is operational but it involves learning and thus becomes entwined with a related concept, incentive motivation [1]. A second definition is introspective, i.e. any stimulus that ‘feels good.’ This is not actually a definition, but a category and, of course, the items in that category vary for each of us. In the neural search, however, this approach has utility because some stimuli are broadly agreed by humans to be in that category and their reward value does not require experience, but they nevertheless do support operant learning. This confluence of definitions permits the inference that animals experience reward from these stimuli much the same way as do humans. This inference underlies research on the neural bases of reward and, perhaps because it is usually left unstated, results in some confusion.
Reward mechanisms in the brain began with the discovery of electrical self-stimulation by Olds and Milner [2] and, for 20 years, they were assigned to the lateral hypothalamus along with biological motivation [3]. The hegemony of the hypothalamus crumbled under evidence from three disparate sources -- the capacity of the isolated brainstem to organize ingestive behavior [4,5], the importance of the vagus in hypothalamic feeding syndromes [6], and the discovery of the mesolimbic dopamine system [7]. Since that low point in the 1970s, motivation has been reinstated slowly in the hypothalamic-preoptic continuum. During this period, neural analysis of female sexual behavior in rodents maintained the credibility of this traditional viewpoint by outlining the neural pathways from the hypothalamic ventromedial nucleus that regulated the spinal mechanisms of the consummatory behavior, lordosis [8]. Subsequently, the number of peptides and peptide hormones involved in energy balance regulation burgeoned and their interactions are centered in the hypothalamus [9].
The hypothalamic mechanisms of reward, however, never recovered their prominence. The dopamine theory of reward shifted attention to the mesencephalic-striatal systems, primarily to its mesolimbic component and the nucleus accumbens [10,11]. Although a great deal is now known about these systems, their roles in reward and motivation remain in flux. At least four theoretical stances vie for position, each supported on substantial empirical bases [11-17]. Much of these data arise from paradigms using complex stimuli, often food, and learning. In some instances, simpler stimuli, such as sucrose, are employed and non-learned responses observed. Regardless, the data consist of some measure of neural activity within the nigrostriatal system or its mesolimbic parallel [18-20]. Theoretically, these systems translate motivation into motor responses, i.e. behavior, but their functions have yet to be clearly determined. Coupled with this, the sensory signals they utilize are even less well understood. Given this synaptic remoteness from the sensory receptors or the motor effectors, it is not surprising that the data are open to interpretation.
Taste as Reward
The research reviewed here attempts to reduce this remoteness by tracing a well characterized sensory system, taste, to the neurons of the nucleus accumbens whose activity appears to track reward value. The first task was to determine if a normally rewarding gustatory signal, sucrose, released DA in the accumbens. Mark and his colleagues [21] demonstrated that saccharin intake increased dopamine flux in the NAc. More importantly, when they rendered the saccharin aversive by associating it with a toxin, licking the stimulus not only failed to elicit dopamine release from the NAc, but actually reduced it. The most obvious interpretation of these data is that accumbens dopamine levels track the reward value of the stimulus, but other possibilities exist. Both the volume ingested and the ingestive behavior change when the stimulus switches from rewarding to aversive.
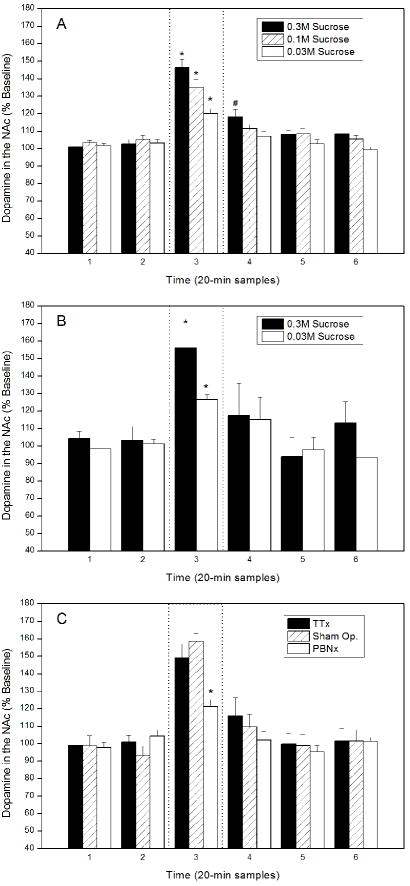
In a similar experiment, we trained rats to ingest water and sucrose and, once they were well practiced, measured dopamine flux in the NAc using microdialysis while they sampled both stimuli. Although food and fluid deprived overnight, the rats licking water did not stimulate NAc dopamine release. In these same animals, however, licking 0.3 M sucrose increased accumbens dopamine up to 300% ([22]). In a subsequent study, we used only water deprivation (14 h overnight), different concentrations of sucrose, fixed fluid volumes, and sham ingestion. These experiments determined that NAc dopamine overflow was a positive function of sucrose concentration, but not of the volume consumed or gastrointestinal feedback (Fig. 1A & B; [23]). With these data, we controlled for the novelty of the stimulus, the amount of behavior elicited, and its nutritional value. This sidelined the most obvious confounding variables and left the relative reward value of the sucrose to account for its effect on NAc dopamine [16,17].
Figure 1.
Dopamine release from the nucleus accumbens shell before, during, and after licking 0.3 M sucrose expressed as a percentage of prestimulus baseline. Dopamine was collected by microdialysis in 20-min samples and measured with high performance liquid chromatography. The dotted lines indicate the sample taken during sucrose licking. Statistically significance differences from baseline (p < 0.05) are indicated by asterisks. A. Concentration-response functions using 0.03 M, 0.1 M, and 0.3 M sucrose during sham feeding. Only one solution was tested per day, but most rats were tested over at least 2 days and thus licked more than one concentration. B. Sham feeding a fixed volume of either 0.03 M or 0.3 M sucrose. The volume was 75% of the average that the rats ingested of the 0.03 M stimulus during a 20 min sham feeding session. C. The effect of central gustatory lesions on dopamine release in nucleus accumbens while ingesting 0.3 M sucrose. Abbreviations: PBNx – Bilateral lesions of the parabrachial nuclei; Sham. Op. - Combined data from 2 groups of full surgical controls; TTAx – Bilateral lesions centered on the thalamic taste relay. The data are published elsewhere in a different form [23, 44].
Although the reward value of sucrose is correlated with dopamine release, it is not clear where in the process the hedonic tone of the afferent message is determined. For taste, it might begin on the periphery, at least for reward values dictated by motivation. Sodium depletion sufficient to induce a salt appetite changes the response characteristics of chorda tympani axons [24-26]. Despite these alterations and further coding changes in the in the nucleus of the solitary tract [27-29], when sodium depleted, chronic decerebrate rats do not alter their behavioral responses to NaCl [30]. This implies that the forebrain is needed to determine the reward value of the afferent activity elicited by the consummatory stimulus. The case for forebrain participation is even stronger for learned changes in reward value, such as conditioned taste aversions [4,5, 31].
The Central Gustatory System
In order for the forebrain to participate in assigning hedonic value to afferent activity, the sensory systems involved must interact with the neural mechanisms that elaborate reward and aversion. This interaction might occur in forebrain reward areas, such as the mesolimbic dopamine system, or in the sensory nuclei themselves. For taste, there is evidence for both, but only the former is addressed here in any detail. The first central synapse for the gustatory system is in the rostral half of the nucleus of the solitary tract (NST), an area that also receives primary afferent intraoral trigeminal axons [32]. In rodents, second order taste neurons ascend from the NST largely ipsilaterally to the pontine parabrachial nuclei (PBN; 33-35]. From the PBN, third order taste neurons take two routes to the forebrain [36]. One projection terminates bilaterally in a medial extension of the thalamic trigeminal relay. Thalamic gustatory neurons then ascend to the cortical taste area, a thin strip of agranular or dysgranular cortex straddling the middle cerebral artery just dorsal to the rhinal fissure [37,38]. This thalamocortical system is supported by unambiguous anatomical evidence and electrophysiological confirmation of its gustatory function. Two other reported connections in this system -- one directly from PBN to taste cortex, the other from the gustatory thalamus to the amygdala -- are less consistently supported by the anatomical evidence and have no electrophysiological confirmation whatsoever (see Lundy and Norgren [39], for a review).
The second projection from the parabrachial nuclei is mostly ipsilateral, more extensive, but probably less gustatory. Its major targets are the central gray, hypothalamus, amygdala, and bed nucleus of the stria terminalis [36]. Of these, incontrovertible evidence that PBN axons convey gustatory afferent activity is available for the amygdala, lateral hypothalamus, and the bed nucleus of the stria terminalis [36, 40-42]. In these experiments, PBN neurons that responded to sapid stimuli were antidromically invaded from stimulating electrodes in these nuclei. Parabrachial neurons project less robustly to other areas in the limbic forebrain, such as the ventral tegmental area, preoptic area, and substantia innominata, but their sensory functions have not been tested. The gustatory function of the PBN ventral projections is an issue because the majority of these axons probably carry visceral afferent information rather than taste (see Saper [43], for a review).
The Logic of Taste Reward
In the present context, the bifurcated central taste projection provides two potential routes by which gustatory afferent activity could interact with central reward systems. Our research has focused on which of these routes supports the release of dopamine from nucleus accumbens when rats are licking sucrose. We chose this index because the evidence reviewed above indicates that NAc dopamine tracts the reward value of substances that taste sweet to humans. To test the route of afferent activity from the tongue to NAc, we conducted two sets of experiments that used different dependent variables -- NAc dopamine flux and immunohistochemical staining of the protein product of the immediate early gene c-fos. The logic for both is similar. If the gustatory afferent activity that influences accumbens dopamine requires cortical processing, then bilateral lesions of the thalamic taste relay should block the response. If the dopamine release during sucrose licking depends on the direct limbic projections, then thalamic lesions should have little effect, but parabrachial gustatory lesions should interfere with the response.
We first repeated the NAc dialysis experiments summarized earlier but did so in separate groups of rats that had ibotenic acid lesions of either the thalamic taste relay or the parabrachial nuclei. Because these lesions can influence ingestive behavior differently, we also had separate groups of control rats that had surgery identical to the experimental rats but had intracerebral infusions of saline rather than ibotenic acid. During sucrose licking, the rats with sham lesions in either gustatory relay produced a spike of accumbens dopamine equivalent to that in unoperated animals. Given the same sapid stimulus, rats with lesions of the thalamic taste area also released similar levels of accumbens dopamine. In rats with bilateral parabrachial lesions, however, licking 0.3 M sucrose elicited a significant, but much blunted NAc dopamine response. It measured only one third that of the PBN controls (Fig. 1C; [44]). Given that accumbens dopamine tracts the reward value of sapid sucrose, it appears that much if not all of the gustatory afferent activity needed for that response is carried by the parabrachial limbic projection.
The second set of experiments used the same logic but, for technical reasons, required a more elaborate design [45]. Although the dopamine release occurs during sham licking of sucrose, the visceral axons in the PBN limbic projections raised the possibility that vagal afferent activity contributed to the accumbens response. To control for this, two sets of PBN lesions were included -- one medial, centered on taste neurons, the other lateral, aimed at the visceral afferent relay. In addition, unlike accumbens dopamine, which is modulated on a second by second time scale [46], c-fos requires stimulation over many minutes to reach detectable levels. Therefore, the rats needed to be trained to sham drink sucrose and water for an hour. This required implanting chronic gastric cannulas, overnight food and water deprivation, and training for more than a week to achieve stable sham intake. To control for experience, each rat had 0.6 M sucrose one day and water the next. Thirty minutes after the last sham drinking session, the rats were sacrificed and their brains processed for immunohistological localization of the c-fos protein.
The brains of control rats that sham licked sucrose contained substantially more c-fos label in a distinct nuclear pattern than did those of control rats ingesting water [45]. As expected, the central gustatory relays each contained significantly more label when rats licked sucrose. More surprisingly, so did some limbic areas that receive direct projections from the parabrachial nuclei, including the central nucleus of the amygdala and the bed nucleus of the stria terminalis (Fig. 2A & B). Perhaps most telling, compared with water, sham sucrose licking increased the number of c-fos labeled cells in the shell of the nucleus accumbens, but not in its core. After bilateral thalamic lesions, the differential label disappeared in the gustatory cortex but, in the other areas, licking sucrose still produced a significant increase in c-fos. Lesions in the lateral, non-gustatory PBN had no significant effect on forebrain c-fos label provoked by sham ingestion of water or sucrose (Fig. 2D). As might be expected from the anatomy of the central taste system, similar lesions centered more medially in the gustatory PBN eliminated differential c-fos label in the thalamic taste area and the gustatory cortex. The same lesions also blocked the c-fos response to sham sucrose intake in the amygdala, bed nucleus, and the accumbens shell (Fig. 2C).
Figure 2.
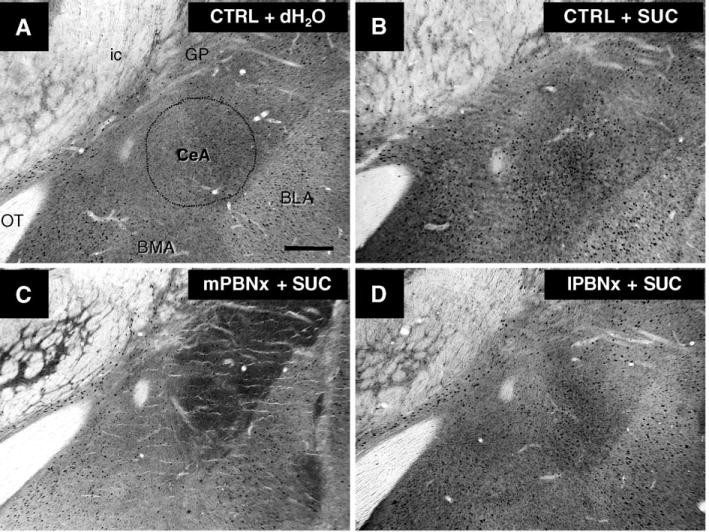
Digital photomicrographs of c-fos immunohistochemical staining in the coronal brain sections through the central nucleus of amygdala (CeA) from rats elicited by 1-h sham drinking of distilled water (dH2O) or 0.6 M sucrose (SUC). The c-fos positive neurons are shown in a control (CTRL) animal given dH2O (A), sucrose (B). The mPBNx (C) vs. lPBNx (D) rats were given with sucrose. Calibration bar = 50 μm.
Discussion
Taken with the dialysis data, these results add weight to the inference that the sensory activity produced by sapid sucrose reaches the nucleus accumbens via the parabrachial limbic projections rather than the thalamocortical route. The fact that all the rats were sham feeding confirms that the differential activation elicited by sucrose was due to oral sensory stimulation. The group with lateral, visceral afferent PBN lesions, which had little if any effect on c-fos labeling, adds emphasis to this claim. Aside from distinguishing between the two gustatory projections to the forebrain, however, neither the dialysis data nor the c-fos experiments determine the synaptic organization of the gustatory influence on accumbens dopamine.
Indeed, the data present an embarrassment of riches. The limbic areas most affected by the gustatory PBN lesions, the amygdala and the bed nucleus of the stria terminalis, both have direct and indirect connections to nucleus accumbens [47,48]. In addition, there are areas that receive projections from the PBN in which sucrose licking failed to produce differential c-fos labeling such as the lateral hypothalamus and the ventral tegmental area. The lateral hypothalamus has reciprocal connections with NAc and, of course, the ventral tegmental area contains the neurons whose activity releases dopamine in the accumbens. These possibilities are amenable to experimental sorting but the possibility of a simple solution, say a monosynaptic excitatory projection from the PBN to the ventral tegmental dopamine neurons, seems unlikely. In most neural systems, when a variety of potential possibilities exist, further analysis usually reveals a role for all of them.
Acknowledgments
The authors’ research was supported by grants DC00240, DC05435, and DK065709. Correspondence should be addressed to Dr. Ralph Norgren, Department of Neural and Behavioral Science H181, College of Medicine, The Pennsylvania State University, Hershey, PA 17033-0850, USA. rxn5@psu.edu
Abbreviations
- BLA
basolateral nucleus of amygdala
- BMA
basomedial nucleus of amygdala
- CeA
central nucleus of amygdala
- GP
globus pallidus
- ic
internal capsule
- OT
optic tract
Footnotes
This paper is based on a presentation at a workshop on Peripheral-Central Interactions in the Control of Food Intake and Energy Balance held at the Centro Stefano Franscini in Ascona, Switzerland in August 2005.
References
- 1.Pfaffmann C. Taste: a model of incentive motivation. In: Pfaff DW, editor. The physiological mechanisms of motivation. New York: Springer-Verlag; 1982. pp. 61–97. [Google Scholar]
- 2.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of the rat brain. J Comp Physiol Psychol. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 3.Olds J. Brain stimulation and the motivation of behavior. Prog Brain Res. 1976;45:401–26. doi: 10.1016/S0079-6123(08)61001-8. [DOI] [PubMed] [Google Scholar]
- 4.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978a;143:281–97. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- 5.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978b;201:267–9. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 6.Powley TL. The ventromedial hypothalamic syndrome, satiety and a cephalic phase hypothesis. Psychol Rev. 1977;84:89–126. Review. [PubMed] [Google Scholar]
- 7.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 8.Pfaff DW. Estrogens and Brain Function: Neural Analysis of a Hormone-Controlled Mammalian Reproductive Behavior. Springer-Verlag; New York: 1980. [Google Scholar]
- 9.Woods SC. Signals that influence food intake and body weight. Physiol Behav. 2005;86:709–16. doi: 10.1016/j.physbeh.2005.08.060. Review. [DOI] [PubMed] [Google Scholar]
- 10.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. Review. [DOI] [PubMed] [Google Scholar]
- 11.Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–21. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;8:309–69. doi: 10.1016/s0165-0173(98)00019-8. Review. [DOI] [PubMed] [Google Scholar]
- 13.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–76. doi: 10.1016/j.neubiorev.2003.11.015. Review. [DOI] [PubMed] [Google Scholar]
- 14.Salamone JD, Correa M, Mingote S, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. Review. [DOI] [PubMed] [Google Scholar]
- 15.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 16.Smith GP. Dopamine and food reward. In: Fluharty S, Morrison A, Sprague J, Stellar E, editors. Progress in psychobiology and physiological psychology. New York: Academic Press; 1995. pp. 83–144. [Google Scholar]
- 17.Smith GP. Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite. 2004;43:11–13. doi: 10.1016/j.appet.2004.02.006. Review. [DOI] [PubMed] [Google Scholar]
- 18.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do μ-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310:1337–40. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- 20.Taha SA, Fields HL. Inhibition of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–22. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551:308–10. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- 22.Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 23.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol. 2004;286:R31–7. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 24.Contreras RJ. Changes in gustatory nerve discharges with sodium deficiency: a single unit analysis. Brain Res. 1977;121:373–8. doi: 10.1016/0006-8993(77)90162-7. [DOI] [PubMed] [Google Scholar]
- 25.Setzer AK, Norgren R. The effects of sodium-free diet on gustatory neural responses in the geniculate ganglion. Chemical Senses. 1996;21:668–9. [Google Scholar]
- 26.Setzer AK, Norgren R. Sodium deprivation affects neuronal responses in the geniculate ganglion to an array of gustatory stimuli. Chemical Senses. 1997;22:791. [Google Scholar]
- 27.Cho YK, Smith ME, Norgren R. Low-dose furosemide modulates taste responses in the nucleus of the solitary tract of the rat. Am J Physiol. 2004;287:R706–14. doi: 10.1152/ajpregu.00090.2004. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Norgren R. Sodium-deficient diet reduces gustatory activity in the nucleus of the solitary tract of behaving rats. Am J Physiol. 1995;269:R647–61. doi: 10.1152/ajpregu.1995.269.3.R647. [DOI] [PubMed] [Google Scholar]
- 29.Tamura R, Norgren R. Intracranial renin alters gustatory neural responses in the nucleus of the solitary tract of rats. Am J Physiol. 2003;284:R1108–18. doi: 10.1152/ajpregu.00574.2002. [DOI] [PubMed] [Google Scholar]
- 30.Grill HJ, Schulkin J, Flynn FW. Sodium homeostasis in chronic decerebrate rats. Behav Neurosci. 1986;100:536–43. doi: 10.1037//0735-7044.100.4.536. [DOI] [PubMed] [Google Scholar]
- 31.Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105:944–54. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- 32.Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of the rat. J Neurophysiol. 1995;73:2144–62. doi: 10.1152/jn.1995.73.6.2144. [DOI] [PubMed] [Google Scholar]
- 33.Norgren R, Leonard CM. Taste pathways in rat brain stem. Science. 1971;173:1136–9. doi: 10.1126/science.173.4002.1136. [DOI] [PubMed] [Google Scholar]
- 34.Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol. 1973;150:217–37. doi: 10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- 35.Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res. 1975;91:99–117. doi: 10.1016/0006-8993(75)90469-2. [DOI] [PubMed] [Google Scholar]
- 36.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- 37.Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res. 1975;92:123–9. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- 38.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res. 1986;379:342–52. doi: 10.1016/0006-8993(86)90788-2. [DOI] [PubMed] [Google Scholar]
- 39.Lundy RF, Jr, Norgren R. Gustatory System. In: Paxinos G, Mai J, editors. The rat nervous system. 3. San Diego: Elsevier Academic Press; 2004. pp. 891–921. [Google Scholar]
- 40.Li CS, Cho YK. Program No 281.5.2005 Abstract Viewer/Itinerary Planner. Washington, D.C.: Society for Neuroscience; 2005. Inhibitory projections from the bed nucleus if the stria terminalis modulate activity of taste-responsive neurons in the hamster parabrachial nuclei. Online. [Google Scholar]
- 41.Li CS, Cho YK, Smith DV. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol. 2005;93:1183–96. doi: 10.1152/jn.00828.2004. [DOI] [PubMed] [Google Scholar]
- 42.Norgren R. Gustatory afferents to ventral forebrain. Brain Res. 1974;81:285–95. doi: 10.1016/0006-8993(74)90942-1. [DOI] [PubMed] [Google Scholar]
- 43.Saper C. Central autonomic system. In: Paxinos G, editor. The rat nervous system. 3. New York: Elsevier Academic Press; 2004. pp. 761–96. [Google Scholar]
- 44.Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav. 2005;84:363–9. doi: 10.1016/j.physbeh.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Mungarndee SS, Lundy RF, Jr, Caloiero VG, Norgren R. Forebrain c-fos expression following sham exposure to sucrose after central gustatory lesions: A quantitative study. Society for Neuroscience. Abstract Viewer/Itinerary Planner; Washington, D.C.: 2004. [Google Scholar]
- 46.Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–71. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–41. doi: 10.1111/j.1749-6632.1999.tb09270.x. Review. [DOI] [PubMed] [Google Scholar]
- 48.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. Review. [DOI] [PubMed] [Google Scholar]