Abstract
Depression is common in end-stage renal disease and is associated with poor quality of life and higher mortality; however, little is known about depressive affect in earlier stages of chronic kidney disease. To measure this in a risk group burdened with hypertension and kidney disease, we conducted a cross-sectional analysis of individuals at enrollment in the African American Study of Kidney Disease and Hypertension Cohort Study. Depressive affect was assessed by the Beck Depression Inventory II and quality of life by the Medical Outcomes Study-Short Form and the Satisfaction with Life Scale. Beck Depression scores over 14 were deemed consistent with an increased depressive affect and linear regression analysis was used to identify factors associated with these scores. Among 628 subjects, 166 had scores over 14 but only 34 were prescribed antidepressants. The mean Beck Depression score of 11.0 varied with the estimated glomerular filtration rate (eGFR) from 10.7 (eGFR 50–60) to 16.0 (eGFR stage 5); however, there was no significant independent association between these. Unemployment, low income, and lower quality and satisfaction with life scale scores were independently and significantly associated with a higher Beck Depression score. Thus, our study shows that an increased depressive affect is highly prevalent in African Americans with chronic kidney disease, is infrequently treated with antidepressants, and is associated with poorer quality of life. Sociodemographic factors have especially strong associations with this increased depressive affect. Because this study was conducted in an African-American cohort, its findings may not be generalized to other ethnic groups.
Keywords: AASK (African American Study of Kidney Disease and Hypertension), chronic kidney disease, clinical epidemiology, depression, quality of life
Depression is a common condition among individuals with end-stage renal disease (ESRD) and increases the risk of serious adverse health outcomes, including mortality, more frequent hospitalization, and poorer quality of life.1–11 Between 15 and 30% of ESRD patients either report symptoms of depression or are clinically diagnosed with this condition.1–4,12–18 Although the effect of antidepressant treatment in ESRD is largely unknown, uncontrolled studies have found possible efficacy.19,20 However, only a small proportion of ESRD patients with depression are prescribed antidepressant medications.7,12,16,17
Although numerous studies of depression have been conducted in people with ESRD, its burden among individuals with earlier stages of chronic kidney disease (CKD) and its relationship with kidney function is less well described.21–25 The reported prevalence of increased depressive affect among patients with CKD before attainment of ESRD has varied substantially, ranging from approximately 15% to over 50%.21,22,25 Moreover, conflicting conclusions have been reached regarding a potential relationship between the severity of CKD and depression.21–25 These heterogeneous findings may be a result of shortcomings in study design such as small sample size and sampling of a single geographic region.
Hypertension is the second leading cause of ESRD in the United States and is especially burdensome to African Americans.26 The African American Study of Kidney Disease and Hypertension (AASK) cohort study was designed to identify important risk factors for decline in kidney function and to improve the understanding of CKD in African Americans with hypertensive kidney disease.27,28 In this analysis, we characterized the prevalence of increased depressive affect and examined its cross-sectional association with sociodemographic factors, comorbid health conditions, level of kidney function, and quality of life in the AASK cohort.
RESULTS
Study participants and baseline BDI-II scores
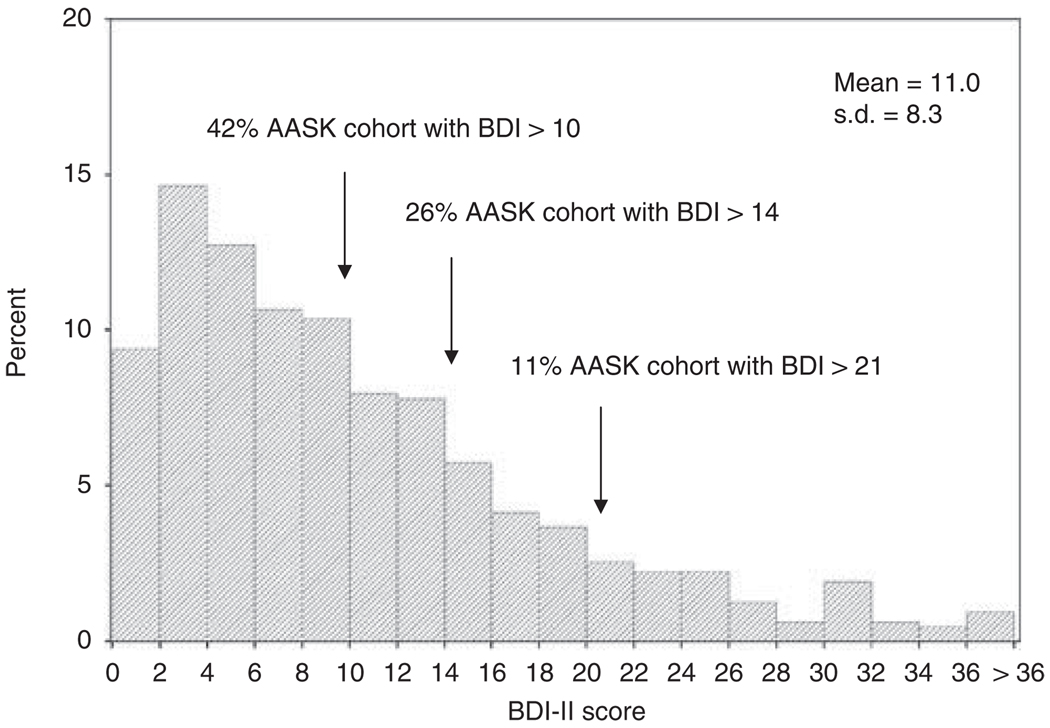
Among the 628 AASK subjects enrolled in the cohort study, the mean (± s.d.) baseline Beck Depression Inventory II (BDI-II) score was 11.0 ± 8.3 (Figure 1). The distribution of the AASK cohort subjects by strata of baseline BDI-II scores was as follows: 58% (0–10), 16%(11–14), 15%(15–21), and 11% (>21).
Figure 1.
Distribution of Beck Depression Inventory II (BDI) scores in the African American Study of Kidney Disease and Hypertension (AASK) cohort at baseline.
Demographic characteristics and baseline BDI-II scores
The mean age of the cohort was approximately 60 years and 38% were females (Table 1). In all, 40% of subjects had not completed high school, 42% had an annual income of <$15,000, and 15% were unemployed. The mean estimated glomerular filtration rate (eGFR) was 43.1 ± 16.1 ml/min per 1.73 m2. Several sociodemographic characteristics differed among the subjects when the range of BDI-II scores was considered (Table 1). A significant inverse relationship was observed between BDI-II scores and unemployment and annual household incomes of <$15,000 (P<0.05). Participants with BDI-II scores of >21 were three times as likely to be unemployed (32 vs 10%), and nearly twice as likely to have an annual household income of <$15,000 (63 vs 34%) when compared with those with BDI-II scores of 0–10.
Table 1.
Baseline demographic characteristics by BDI-II scores
| Overall (N=628) | BDI 0–10 (N=363) | BDI 11–14 (N=99) | BDI 15–21 (N=95) | BDI >21 (N=71) | ||
|---|---|---|---|---|---|---|
| Variable | % (n) or mean ± (s.d.) |
% (n) or mean ± (s.d.) |
% (n) or mean ± (s.d.) |
% (n) or mean ± (s.d.) |
% (n) or mean ± (s.d.) |
P-value |
| Age | 60.1 ± 10.2 | 60.5 ± 10.4 | 60.6 ± 9.35 | 58.9 ± 11.0 | 59.1 ± 8.71 | 0.43 |
| Female | 38% (238) | 35% (128) | 44% (44) | 40% (38) | 39% (28) | 0.37 |
| Education (from trial) | 0.15 | |||||
| Not a HS graduate | 40% (250) | 37% (135) | 42% (42) | 39% (37) | 51% (36) | |
| HS graduate or beyond | 60% (377) | 63% (228) | 58% (57) | 61% (58) | 49% (34) | |
| Marital status | 0.05 | |||||
| Married/married-like relationship | 36% (228) | 40% (146) | 32% (32) | 28% (27) | 32% (23) | |
| Not married/married-like | ||||||
| Relationship+not living alone | 29% (183) | 28% (102) | 24% (24) | 30% (29) | 39% (28) | |
| Not married/married-like | ||||||
| Relationship+living alone | 34% (216) | 31% (114) | 43% (43) | 41% (39) | 28% (20) | |
| Income | <0.001 | |||||
| <$15,000 | 42% (261) | 34% (123) | 44% (44) | 52% (49) | 63% (45) | |
| ≥$15,000 | 37% (232) | 41% (149) | 38% (38) | 33% (31) | 20% (14) | |
| Declined to answer | 21% (135) | 25% (91) | 17% (17) | 16% (15) | 17% (12) | |
| Health insurance | 0.054 | |||||
| Private/HMO/other | 30% (191) | 32% (115) | 37% (37) | 23% (22) | 24% (17) | |
| Medicaid only | 11% (71) | 10% (35) | 12% (12) | 14% (13) | 15% (11) | |
| Medicare | 29% (184) | 28% (103) | 25% (25) | 27% (26) | 42% (30) | |
| None/uninsured | 29% (181) | 30% (109) | 25% (25) | 36% (34) | 18% (13) | |
| Employment status | <0.0001 | |||||
| Employed | 37% (232) | 42% (153) | 41% (41) | 25% (24) | 20% (14) | |
| Unemployed | 15% (94) | 10% (37) | 12% (12) | 23% (22) | 32% (23) | |
| Retired | 36% (223) | 37% (134) | 37% (37) | 33% (32) | 28% (20) | |
| Other | 12% (78) | 10% (38) | 9% (9) | 18% (17) | 20% (14) | |
| Patient currently exercises | 61% (381) | 63% (230) | 58% (59) | 60% (57) | 51% (36) | 0.22 |
| Smoking status | 0.74 | |||||
| Never smoked | 40% (252) | 42% (151) | 39% (39) | 42% (40) | 31% (22) | |
| Currently smoking | 17% (104) | 16% (58) | 15% (15) | 18% (17) | 20% (14) | |
| Past smoker | 43% (271) | 42% (153) | 45% (45) | 40% (38) | 49% (35) | |
| Alcohol use | 15% (94) | 15% (55) | 16% (16) | 18% (17) | 8% (6) | 0.37 |
| Recreational i.v. drug use | 0.6% (4) | 0.3% (1) | 1% (1) | 2% (2) | 0% (0.0) | 0.19 |
Abbreviations: BDI-II, Beck Depression Inventory II; HMO, health maintenance organization; HS, high school; i.v., intravenous.
Clinical characteristics and baseline BDI-II scores
No consistent or significant trends were observed between strata of BDI-II scores and prevalence of most selected comorbid health conditions (Table 2). However, patients with BDI-II scores of >21 were between two and four times more likely to have a history of stroke or psychiatric problem, respectively, compared with those with BDI-II scores of 0–10 (P<0.05). eGFR and proteinuria did not vary significantly across the range of BDI-II scores (P>0.05). Medical Outcomes Study-Short Form-36-Item (MOS-SF-36) Mental Health Component (MHC) scores, MOS-SF-36 Physical Health Component (PHC) scores, and Satisfaction with Life Scale (SWLS) scores decreased significantly with increasing BDI-II scores (P<0.0001).
Table 2.
Baseline clinical characteristics by BDI-II scores
| Overall (N=628) | BDI 0–10 (N=363) | BDI 11–14 (N=99) | BDI 15–21 (N=95) | BDI >21 (N=71) | P-value | |
|---|---|---|---|---|---|---|
| Weight (kg) | 92.1 ± 22.4 | 92.0 ± 21.3 | 93.0 ± 22.1 | 91.8 ± 23.0 | 91.9 ± 27.5 | 0.98 |
| Body mass index (kg/m2) | 31.5 ± 7.12 | 31.3 ± 6.79 | 31.8 ± 7.39 | 31.8 ± 7.25 | 31.3 ± 8.34 | 0.89 |
| Systolic BP (mm Hg) | 135 ± 21.8 | 134 ± 20.5 | 137 ± 22.7 | 139 ± 25.2 | 133 ± 21.7 | 0.20 |
| Diastolic BP (mm Hg) | 80.7 ± 12.1 | 80.0 ± 11.6 | 82.0 ± 13.5 | 81.4 ± 12.0 | 81.4 ± 12.9 | 0.41 |
| Serum creatinine (mg/dl) | 2.32 ± 1.32 | 2.28 ± 1.15 | 2.14 ± 1.04 | 2.65 ± 2.02 | 2.31 ± 1.24 | 0.04 |
| Estimated GFR (ml/min per 1.73 m2) | 43.1 ± 16.1 | 43.2 ± 15.8 | 44.0 ± 14.8 | 41.1 ± 17.6 | 43.5 ± 17.0 | 0.62 |
| Urine protein/creatinine | 0.38 ± 0.85 | 0.34 ± 0.72 | 0.47 ± 1.08 | 0.52 ± 1.06 | 0.31 ± 0.74 | 0.19 |
| Urine protein/creatinine >0.22 | 30% (188) | 28% (103) | 32% (31) | 39% (36) | 27% (18) | 0.24 |
| Serum albumin (g/dl) | 4.00 ± 0.33 | 4.02 ± 0.32 | 3.95 ± 0.34 | 3.96 ± 0.33 | 4.01 ± 0.30 | 0.17 |
| Hemoglobin (g/dl) | 12.7 ± 1.82 | 12.8 ± 1.86 | 12.8 ± 1.88 | 12.5 ± 1.80 | 12.6 ± 1.55 | 0.53 |
| History of cardiovascular disease | 29% (185) | 25% (91) | 39% (39) | 23% (22) | 46% (33) | 0.0002 |
| History of stroke | 18% (115) | 16% (58) | 14% (14) | 21% (20) | 32% (23) | 0.006 |
| History of peripheral vascular disease | 6% (35) | 4% (15) | 9% (9) | 3% (3) | 11% (8) | 0.027 |
| History of cancer | 6% (36) | 6% (23) | 4% (4) | 6% (6) | 4% (3) | 0.077 |
| History of asthma or COPD | 7% (47) | 6% (21) | 7% (7) | 8% (8) | 15% (11) | 0.042 |
| Psychiatric problem | 4% (23) | 1% (5) | 4% (4) | 7% (7) | 10% (7) | 0.0008 |
| MOS-SF-36 MHC score | 52.4 ± 9.90 | 56.6 ± 6.60 | 49.8 ± 9.52 | 47.7 ± 10.8 | 40.7 ± 10.1 | <0.0001 |
| MOS-SF-36 PHC score | 41.1 ±11.3 | 43.5 ± 10.7 | 39.3 ± 11.1 | 39.3 ± 11.7 | 34.2 ± 10.3 | <0.0001 |
| SWLS score | 4.44 ± 1.52 | 4.94 ± 1.35 | 4.14 ± 1.37 | 3.65 ± 1.46 | 3.32 ± 1.47 | <0.0001 |
Abbreviations: BDI-II, Beck Depression Inventory II; BP, blood pressure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MHC, Mental Health Component; MOS-SF-36, Medical Outcomes Study-Short Form-36-Item; PHC, Physical Health Component; SWLS, Satisfaction with Life Scale.
Demographic and clinical characteristics by increased depressive affect and prescription of antidepressants
The mean BDI-II scores varied as follows: 6.9±3.8 for participants without depressive affect (BDI-II of ≤14), 21.7±6.4 for those with increased depressive affect (BDI-II of >14) but without antidepressant medication, and 18.2±11.4 for those prescribed antidepressants (Table 3). There were a few significant differences in characteristics between these groups. Compared with participants without depressive affect, those prescribed antidepressants were significantly more likely to have a history of current smoking, unemployment, chronic obstructive pulmonary disease, psychiatric illness and to be of younger age (P<0.05). Compared with participants with increased depressive affect, those prescribed antidepressants differed significantly only in a greater prevalence of a history of smoking and psychiatric illness (P<0.05). MOS-SF-36 MHC scores and MOS-SF-36 PHC scores were progressively lower from those without depressive affect to those with increased depressive affect and to those prescribed antidepressants (P<0.0001). SWLS scores were similar among participants with increased depressive affect and prescribed antidepressants but significantly lower compared with those without depressive affect (P<.0001). eGFR did not vary significantly across these groups (P>0.05). A sensitivity analysis was conducting using a BDI-II score of ≥11 as a cutoff for increased depressive affect and the comparison results for the three groups were not significantly changed.
Table 3.
Significant baseline demographic and clinical characteristic differences by depressive affect and prescription of antidepressant medications
| Variable | Depressive affect absent (N=447) |
Depressive affect present (N=147) |
Prescribed antidepressant (N=34) |
Absent depressive affect vs present depressive affect (P-value) |
Absent depressive affect vs antidepressant (P-value) |
Present depressive affect vs antidepressant (P-value) |
|---|---|---|---|---|---|---|
| BDI-II score | 6.9 ± 3.8 | 21.7 ± 6.4 | 18.2 ± 11.4 | <0.001 | <0.001 | 0.078 |
| Age | 60.6 ± 10.2 | 59.6 ± 10.2 | 56.2 ± 9.4 | 0.322 | 0.044 | 0.148 |
| Income | 0.001 | 0.248 | 0.609 | |||
| ≥$15,000 | 40% (179) | 28% (41) | 35% (12) | |||
| <$15,000 | 36% (162) | 55% (81) | 53% (18) | |||
| Declined to answer | 24% (106) | 17 % (25) | 12% (4) | |||
| Employment status | <0.0001 | <0.0014 | 0.238 | |||
| Employed | 42% (187) | 25% (36) | 27% (9) | |||
| Unemployed | 11% (47) | 23% (34) | 38% (13) | |||
| Retired | 37% (165) | 35% (51) | 21% (7) | |||
| Other | 11% (47) | 18% (26) | 15% (5) | |||
| Smoking status | 0.811 | 0.0013 | 0.0030 | |||
| Never smoked | 42% (185) | 39% (57) | 29% (10) | |||
| Currently smoking | 15% (67) | 15% (22) | 44% (15) | |||
| Past smoker | 44% (194) | 46% (68) | 27% (9) | |||
| History of psychiatric problem | 1% (6) | 5% (7) | 29% (10) | 0.022 | <0.001 | <0.001 |
| History of asthma or COPD | 6% (25) | 10% (14) | 24% (8) | 0.123 | 0.003 | 0.076 |
| MOS-SF-36 MHC score | 55.4 ± 7.46 | 46.0 ± 10.7 | 39.9 ± 12.2 | <0.001 | <0.001 | <0.001 |
| MOS-SF-36 PHC score | 42.7 ± 10.8 | 37.9 ± 11.0 | 34.9 ± 13.4 | <0.001 | <0.001 | 0.155 |
| SWLS score | 4.77 ± 1.39 | 3.57 ± 1.46 | 3.76 ± 1.72 | <0.001 | <0.001 | 0.490 |
| Estimated GFR (ml/min per 1.73 m2) |
43.4 ± 15.6 | 42.7 ± 17.0 | 39.8 ± 18.1 | 0.692 | 0.630 | 0.692 |
Abbreviations: BDI-II, Beck Depression Inventory II; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MHC, Mental Health Component; MOS-SF-36, Medical Outcomes Study-Short Form-36-Item; PHC, Physical Health Component; SWLS, Satisfaction with Life Scale.
Kidney function, baseline BDI-II scores, and prescription of antidepressants
Mean BDI-II scores and distribution of BDI-II score strata were similar among subjects with eGFR values of >15 ml/ min per 1.73 m2 (Table 4), but they were higher in subjects with an eGFR of ≤15 ml/min per 1.73 m2. Compared with those with an eGFR of >15 ml/min per 1.73 m2, the mean BDI-II score (10.7 vs 16.0) and proportion of BDI-II scores of >14 (22 vs 52%) were greater in subjects with an eGFR of ≤15 ml/min per 1.73 m2. However, there was no overall statistically significant association between BDI-II strata and levels of eGFR (P>0.05).
Table 4.
BDI-II scores by baseline eGFRa
| Variable | Overall (N=622) |
eGFR ≤15 (N=21) |
eGFR 16–30 (N=115) |
eGFR 30–40 (N=113) |
eGFR 40–50 (N=132) |
eGFR 50–60 (N=136) |
eGFR >60 (N=105) |
|---|---|---|---|---|---|---|---|
| BDI score (mean ± s.d.) |
11.0 ± 8.27 | 16.0 ± 12.4 | 10.4 ± 7.32 | 11.0 ± 8.45 | 11.1 ± 8.82 | 10.7 ± 7.93 | 10.8 ± 7.49 |
| BDI groups (% (n)) | |||||||
| 0–10 | 58% (362) | 38% (8) | 63% (73) | 55% (62) | 56% (74) | 64% (87) | 55% (58) |
| 11–14 | 16% (97) | 10% (2) | 12% (14) | 18% (20) | 19% (25) | 14% (19) | 16% (17) |
| 15–21 | 15% (94) | 33% (7) | 17% (19) | 14% (16) | 13% (17) | 14% (19) | 15% (16) |
| >21 | 11% (69) | 19% (4) | 8% (9) | 13% (15) | 12% (16) | 8% (11) | 13% (14) |
Abbreviations: BDI-II, Beck Depression Inventory II; eGFR, estimated glomerular filtration rate.
Six patients with missing baseline eGFR were excluded.
The overall proportion of study subjects who were prescribed antidepressant medications was 5.4% and ranged from 3% in subjects with BDI-II scores between 0 and 10 to 17% in subjects with BDI-II scores of >21. Higher BDI-II score was significantly associated with a higher likelihood of prescription of antidepressants (P<0.001). No significant relationship was observed between prescription of anti-depressants and levels of eGFR (P>0.05).
Independent association of demographic factors with baseline BDI-II scores
In linear regression analysis, three sociodemographic factors were significantly and independently associated with BDI-II scores after adjusting for eGFR (Table 5). Study participants who currently exercised had lower BDI-II scores (parameter = −1.74, s.e. = 0.66, P = 0.009) compared with subjects who did not exercise. Annual household income of <$15,000 (referent: household income of ≥$15,000) and unemployment (referent: employment) were strongly associated with higher BDI-II scores (parameter = +2.30, s.e. = 0.85, P = 0.007 and parameter = +4.94, s.e. = 1.09, P<0.0001, respectively). Sensitivity analyses were performed excluding patients with baseline antidepressant medication use and the resulting findings were not significantly changed.
Table 5.
Association of baseline sociodemographic factors and eGFR with BDI-II scorea
| Variable | Parameter estimate | Standard error | P-value |
|---|---|---|---|
| Age (per 10 years) | −0.66 | 0.42 | 0.12 |
| Female | −0.21 | 0.70 | 0.76 |
| Less than HS degree | 1.05 | 0.73 | 0.15 |
| Married/married-like relationship vs not married/married-like relationship+living alone | −0.99 | 0.83 | 0.23 |
| Not married/married-like relationship+not living alone vs not married/married-like relationship+living alone |
−0.45 | 0.82 | 0.59 |
| <$15,000 vs ≥$15,000 | 2.30 | 0.85 | 0.007 |
| Declined to answer vs ≥$15,000 | −0.83 | 0.89 | 0.35 |
| Private/HMO/other vs Medicare | 0.75 | 1.04 | 0.47 |
| Medicaid only vs Medicare | −0.28 | 1.16 | 0.81 |
| None/uninsured vs Medicare | 0.09 | 0.90 | 0.92 |
| Retired vs employed | 1.79 | 0.93 | 0.05 |
| Unemployed vs employed | 4.94 | 1.09 | <0.0001 |
| Other vs employed | 3.80 | 1.15 | 0.001 |
| Patient currently exercises | −1.74 | 0.66 | 0.009 |
| Current smokers vs never smoked | 0.67 | 0.98 | 0.49 |
| Past smokers vs never smoked | 1.00 | 0.72 | 0.17 |
| Alcohol use | 0.08 | 0.90 | 0.93 |
| Recreational i.v. drug use | 2.36 | 4.03 | 0.56 |
| Estimated GFR (per 10ml/min per 1.73 m2) | 0.05 | 0.20 | 0.82 |
Abbreviations: BDI-II, Beck Depression Inventory II; eGFR, estimated glomerular filtration rate; HMO, health maintenance organization; HS, high school; i.v., intravenous.
Regression coefficients shown are from multiple regression jointly relating BDI score to each of the listed predictor variables. The regression coefficients indicate the mean difference in the BDI score associated with the indicated characteristic for dichotomous predictor variables, or a one-unit increase in quantitative predictor variables.
Independent association of clinical factors with baseline BDI-II scores
eGFR did not have a significant independent association with BDI-II scores upon linear regression analysis incorporating other clinical factors (P = 0.75; Table 6). History of stroke was independently associated with higher BDI-II scores (parameter = +1.97, s.e. = 0.68, P = 0.004). Lower MOS-SF-36 MHC scores (parameter = −0.39, s.e. = 0.03, P ≤ 0.0001), PHC scores (parameter = −0.14, s.e. = 0.03, P<0.0001), and SWLS scores (parameter = −0.94, s.e. = 0.20, P<0.0001) were all associated with higher BDI-II scores. If MOS-SF-36 MHC, PHC, and SWLS scores are excluded from this regression, history of psychiatric problem (parameter = +8.06, s.e. = 1.73, P<0.0001) and cardiovascular disease (parameter = +1.70, s.e. = 0.72, P = 0.02) also become significantly associated with higher BDI-II scores. Sensitivity analyses were performed excluding patients with baseline antidepressant medication use and the resulting findings were not significantly changed.
Table 6.
Association of baseline comorbidities, functional health status, and eGFR with BDI-II scorea
| Variable | Parameter estimate |
Standard error |
P-value |
|---|---|---|---|
| Age (per 10 years) | 0.33 | 0.27 | 0.22 |
| Female | −0.35 | 0.54 | 0.52 |
| History of cancer | −1.63 | 1.16 | 0.1598 |
| History of stroke | 1.97 | 0.68 | 0.004 |
| History of peripheral vascular disease |
0.35 | 1.17 | 0.77 |
| History of psychiatric problem | 1.78 | 1.43 | 0.21 |
| History of asthma or COPD | 0.44 | 1.01 | 0.66 |
| History of cardiovascular disease | 0.54 | 0.58 | 0.36 |
| MOS-SF-36 MHC score | −0.39 | 0.03 | <0.0001 |
| MOS-SF-36 PHC score | −0.14 | 0.03 | <0.0001 |
| SWLS score | −0.94 | 0.20 | <0.0001 |
| Estimated GFR (per 10 ml/min per 1.73 m2) |
−0.05 | 0.16 | 0.75 |
Abbreviations: BDI-II, Beck Depression Inventory II; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MHC, Mental Health Component; MOS-SF-36, Medical Outcomes Study-Short Form-36-Item; PHC, Physical Health Component; SWLS, Satisfaction with Life Scale.
Regression coefficients shown are from multiple regression jointly relating BDI score to each of the listed predictor variables. The regression coefficients indicate the mean difference in the BDI score associated with the indicated characteristic for dichotomous predictor variables, or a one-unit increase in quantitative predictor variables.
DISCUSSION
We observed a prevalence of increased depressive affect of at least 26% among a large cohort of African Americans with hypertensive CKD, based on a BDI-II score of > 14. An additional 2% of subjects without increased depressive affect as assessed by the BDI-II were prescribed antidepressant medications, suggesting that nearly one-third of the AASK cohort study participants have clinically significant depressive affect. The prevalence of increased depressive affect in this cohort of African Americans with CKD is substantially elevated compared with that in the general healthy population. In a study of over 9000 non-medically ill adults across the United States, the NCS-R (National Comorbidity Survey Replication survey, the 12-month period prevalence of a major depressive disorder was found to be 6.6%.29 This contrast is even more striking considering that we used a conservative BDI threshold to define clinically significant depressive affect. If a less conservative BDI cutoff of ≥11 is used, as suggested by a recent study,30 over 4 in 10 participants of the AASK cohort have increased depressive affect. It is important to underscore the applicability of these observations. The AASK cohort was recruited from over 20 clinical centers in cities across the United States and is a large diverse sample of African Americans with hypertensive CKD.28 Findings from the AASK cohort are well recognized to be inherently generalizable to African Americans with CKD, who constitute a vulnerable population subject to health disparities and overrepresented in the CKD and ESRD community.27,28,31–33
To our knowledge, this report is the first large detailed epidemiologic study of depression in people with earlier stages of CKD before the onset of chronic dialysis. Previous small studies have reported a wide range of prevalent depressive symptoms in patients with CKD, which may be attributed to differences in study populations, definitions and stage of CKD, comorbid conditions, and instruments used to assess and define depressive affect.21–25 Although only 17% of subjects with moderate CKD in the Heart and Soul Study had depressive symptoms, the mean BDI score in a small group of outpatients with severe CKD from Iowa was quite elevated at 12.8, suggestive of mild depressive affect.22,34 More similar to the urban African-American cohort of this study, two analyses of outpatients with moderate-to-severe CKD in Washington DC found substantially lower mean BDI scores that ranged from 7.4 to 8.23,24 However, these two previous works were single-center studies without published details regarding socioeconomic data. Therefore, their representativeness is unclear and fully accounting for their disconcordance from these present results is not possible.23,24 A recent study conducted at a Veterans Affairs Medical Center in Texas suggests that 21% of male veterans with CKD stages 2 to 5 have an episode of clinically diagnosed major depression.25
The prevalence of increased depressive affect in the AASK cohort was similar, using respective criteria, to that observed in chronic ESRD patients undergoing maintenance dialysis, in which the prevalence of increased depressive affect or the clinical diagnosis of depression has generally ranged from 15 to 30%.1–4,12–18 Previous studies of predominately African-American chronic hemodialysis patients have found mean BDI scores between 11 and 13.5,8,18 The prevalence of increased depressive affect among the AASK cohort did not vary substantially by level of kidney function except among people with very low kidney function (eGFR of <15 ml/min per m2), in which increased depressive affect was noticeably the highest. Regression analysis supported this observation by finding eGFR not to be independently associated with BDI-II score. Similar to findings reported in this study, although previous studies have reported no overall significant correlation between depression and eGFR, noteworthy increases in depressive symptoms were found with very severe decrements in kidney function.21,23–25 Although inconsistencies in the literature exist because of differences in the timing of depression assessments and unmeasured qualitative factors,5,7,9,34 previous analyses of patients initiating hemodialysis have revealed rates of depression to be much higher than that in their chronic hemodialysis counterparts.6,7,10 Major lifestyle and psychological adjustments, and multiple losses that occur during the transition from severe CKD to ESRD requiring dialysis, may account for these observations.7,16,35,36 Importantly, only 4% of the AASK cohort had an eGFR of <15 ml/min per m2; therefore, our ability to assess and interpret the relationship between depressive affect and this very low level of eGFR is limited. Furthermore, the current understanding of the relationship between loss of kidney function and development of depression is severely limited by the lack of longitudinal studies.
Our findings confirm previous concerns regarding under-treatment of depression in patients with CKD.7 Overall, approximately 5% of AASK study subjects were prescribed antidepressants, including only 12% with increased depressive affect. In previous studies of chronic hemodialysis patients with depression, 15 to 50% of such individuals were prescribed antidepressants.6,7,14,17 Treatment of depression has been noted to be especially poor in African Americans.35,37,38 In a study of urban African Americans on chronic hemodialysis, only 13% of depressed patients were treated by a mental health provider and only 5% were prescribed antidepressant medications.18 Although the AASK study is limited by not possessing data regarding non-pharmacologic depression treatments, our findings raise further concerns regarding the effectiveness of current depression management. Even among the small number of AASK participants prescribed antidepressant medications, average BDI-II scores remained quite elevated. Considering the negative health outcomes associated with depression and the potential efficacy of antidepressants in ESRD, greater scrutiny should be directed toward optimizing care for depression in CKD patients.1,2,4–6,12,16,18,20
We found strong independent relationships between lower income, unemployment, and a higher likelihood of increased depressive affect in the AASK cohort. Adverse socioeconomic factors are well known to have important associations with depression.1,17,29,35 In NCS-R survey, unemployment and poverty were two of the most significant correlates for major depressive disorder.29 However, in contrast to some previous works, this present study, despite its extremely detailed participant data, found relatively few demographic and clinical factors, such as age, gender, or comorbid illness, to be significantly associated with increased depressive affect.1,2,17,19 It is important to underscore that the intricate relationship between depression and chronic illness is not fully understood.6,13,16,35,39
Nonetheless, these findings suggest that increased depressive affect may be an occult condition in this high-risk population; therefore, clinicians must be careful to elicit indirect evidence of depression in this population. The presence of worsening somatic symptoms (for example, fatigue and anorexia) without substantial deterioration in kidney function, verbal cues (for example, loss of interest and indecisiveness), or nonverbal signs (for example, changes in posture or speech pattern) may be markers of depression.40 Furthermore, clinicians should strongly consider routinely asking open-ended questions regarding how African-American patients with CKD are feeling to motivate patients to disclose and clarify symptoms as well as to foster the intimacy of the patient–physician relationship, which may increase the detection of depression.40
Finally, these findings raise the issue of formal screening for depression in African Americans with CKD. Screening for depression in general medical populations remains controversial because of conflicting data regarding its effectiveness in improving the care and outcomes of depressed patients.12,41 Although depression seems to be especially common in African Americans with CKD and negatively affects quality of life, its effect on other medical outcomes is unknown. Further studies are needed to delineate the clinical sequelae of depression and to properly assess the utility of screening in this vulnerable patient population.
There are limitations to this study. First, although the BDI is a reliable and accurate tool for identifying depression in ESRD, it has been less well analyzed in CKD.21,23,24,30 We stressed that this study assessed the extent of increased depressive affect rather than a clinical diagnosis of depression.12,16,36 Moreover, previous concerns about the performance of the BDI were chiefly focused on the misclassification of uremic symptoms as somatic symptoms of depression,4–5,12,14,16,21,35 which is less likely in a population with earlier stage CKD before ESRD. Second, we assessed depressive affect only at baseline in the AASK cohort and used a cross-sectional study design. The directionality of associations between depressive affect and other factors cannot be inferred, and these relationships may evolve over time. Third, information regarding important qualifying variables, such as illness intrusiveness and stressful life events, was not available; therefore, we are limited in making psychological inferences.42 Fourth, some content overlap does exist between the BDI-II and the SF-36, and this measurement redundancy may account for part of the observed association between quality of life and increased depressive affect.43 Last, approximately 9% of the AASK study cohort (63 subjects) did not complete a BDI-II questionnaire at enrollment and were excluded from this analysis. However, it is unlikely that their inclusion would substantially alter our findings because important characteristics among excluded and included subjects were not significantly different.
In conclusion, increased depressive affect afflicts nearly one-third of African Americans with CKD, negatively affects their quality of life, and is rarely treated with pharmacologic therapy. Clinicians caring for African Americans with CKD should remain vigilant for the presence of occult depression in their patients. Current evidence does not warrant screening for depression in African Americans with CKD. However, studies are needed to ascertain the clinical consequences of depression in patients with CKD and to further examine the potential worsening of depressive symptoms in patients near ESRD requiring renal replacement therapy, which will inform the utility of screening. Moreover, clinical trials should be initiated to evaluate the role of antidepressant treatment strategies in this population.
MATERIALS AND METHODS
Study design and sample
We conducted a cross-sectional analysis of depressive affect in subjects at their time of entry (that is, baseline) into the AASK cohort study. The AASK cohort study was a multicenter prospective study of people with hypertensive CKD whose hypertension was managed with a recommended blood pressure goal (<130/ 80 mm Hg) and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. The AASK cohort study enrolled only subjects who had previously participated in the AASK clinical trial. Details of both of these studies have been published previously.27–28 All subjects who were alive at the completion of the clinical trial and had not begun dialysis therapy or received a kidney transplant were eligible to enroll in the cohort study. Out of 764 eligible subjects from the clinical trial who had not begun dialysis therapy or received a kidney transplant, 691 subjects were enrolled in 2002 and followed through 2007, and 628 subjects completed a BDI-II questionnaire at enrollment. Major eligibility criteria for the clinical trial included self-identified African-American race, ages 18 to 70 years, an iothalamate measured GFR between 20 and 65 ml/min per 1.73 m2, and no apparent cause of CKD other than hypertension.28 The study was approved by the institutional review boards of the participating centers. All study participants provided written informed consent.
Variables and data sources
The BDI-II was administered as a self-completed questionnaire to all AASK participants at their baseline visit. The BDI-II is an adaptation of the Beck Depression Inventory, which is a widely used validated instrument to assess depressive affect.13–15,44 Scores for each of the 21 items range from 0 to 3 with a higher score representing a greater problem. The total score range is 0–63, in which a score of <10 is absence of depression, and scores of 10–15, 16–23, and ≥24 are considered mild, moderate, and severe depression, respectively, in the general non-medically ill population.45 Several studies have shown that BDI scores of >14 are accurate at diagnosing depression among patients with ESRD.13–15 The BDI has been used less frequently to evaluate depression in individuals with earlier stages of CKD.21,23,24 Only one study, which focused on a small male veteran population, has evaluated the BDI in patients with CKD, finding that a BDI score of ≥11 was the best cutoff point for a major depression episode.30 Therefore, although we evaluated strata of BDI scores, we adopted the higher more stringent BDI threshold of >14 to define significantly increased depressive affect in AASK participants.
Demographic variables (for example, age, gender, education, marital status, insurance, annual household income, employment status, current exercise, and smoking/alcohol/drug use) were self-reported. Comorbid health conditions (for example, cancer, stroke, cardiovascular disease, peripheral vascular disease, asthma or chronic obstructive pulmonary disease, and psychiatric problem) were self-reported and identified by a review of medical records. Cardiovascular disease included any of the following: coronary artery disease, heart failure or diastolic dysfunction, left ventricular hypertrophy, heart rhythm, or conduction problem. Prescription records of AASK participants were reviewed at baseline and medications designated as antidepressants were independently confirmed by three clinicians. Blood pressure was measured in a standardized manner by trained, certified observers using the Tycos Classic Handheld Aneroid device (Skaneateles Falls, NY, USA). Three blood pressure measurements were obtained in the seated position and one measurement was obtained in the standing position and then averaged for a reported value. For each subject, urine protein/creatinine ratio was measured at baseline as well as eGFR (ml/min per 1.73 m2), which was calculated using an average of serum creatinine values with the AASK study equation obtained within the first 3 months after participant enrollment.46 Quality-of-Life questionnaire instruments administered included the MOS-SF-36 and the SWLS.47,48 As described in detail elsewhere, the MOS-SF-36 consists of 36 items that cover eight domains whose scores are aggregated into a PHC score and a MHC score.49 These component scores are normalized to the US population, with a mean of 50 and an s.d. of 10.50 Higher scores are indicative of better quality of life than lower scores. The SWLS is a five-item scale dealing with ideal life, conditions of life, and satisfaction with present and past life that has a satisfaction rating of 1 to 7 (low to high) for each item.48 The SWLS has reported good reliability, correlation with other subjective well-being scales, and has been used in previous studies, including among patients with CKD.8,9,23,24,48
Statistical analyses
Patient characteristics at baseline were described overall, in four BDI-II strata (0–10, 11–14, 15–21, and >21), and by presence of increased depressive affect (BDI of >14) or antidepressant medications using mean±s.d. for quantitative variables and frequencies and percentages for categorical variables. Bivariate analyses involving chi-square tests and analysis of variance were used as appropriate to assess differences in patient characteristics among the four BDI-II strata. To assess the relationship between BDI-II scores and kidney function, both mean BDI-II scores and BDI-II strata frequency were compared by levels of eGFR using analysis of variance and Mantel–Haenzel tests, respectively. Stepdown Bonferroni adjustment was used when performing pairwise comparisons among subjects with increased depressive affect (BDI of >14) or without depressive affect (BDI of ≤14) or with antidepressant medications.
Multivariable linear regression analysis was used to evaluate the cross-sectional relationship between baseline BDI-II scores (continuous variable) and other baseline patient characteristics. Separate multivariable models were used to examine the relationship between (1) BDI-II scores and sociodemographic factors, and (2) BDI-II scores and comorbid conditions, including kidney function (eGFR), and quality of life. All statistical analyses were conducted with SAS, version 9.1 (Cary, NC, USA).
ACKNOWLEDGMENTS
Part of these results was presented in abstract and poster format at the American Society of Nephrology Annual Meeting in November 2008 (Philadelphia, Pennsylvania, USA). A special acknowledgement is extended to the AASK participants for their time and extraordinary commitment to the AASK trial and now the AASK cohort study. We also acknowledge all members of the AASK Collaborative Research Group, which includes investigators and staff from 21 clinical centers. This study was supported by cooperative agreements from the National Institutes of Diabetes and Digestive and Kidney Diseases (5U01DK045388) and the National Center for Minority Health and Health Disparities at the National Institutes of Health (5M01RR00071). Support was also provided by King Pharmaceuticals, Pfizer, and Astra Zeneca Pharmaceuticals. The following National Institutes of Health institutional grants provided additional support: M01 RR-00080, RR-00071, M01 RR-00827, M01 RR-00032, P20 RR-11145, 2P20 RR-11104, M01 RR-00052, RR-00095, and DK-2818-02. Support was also provided by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (VA HSR&D Career Development Award to MJF).
Appendix
AASK Collaborative Research Group—Investigators and staff from 21 clinical centers
Case Western Reserve University
Principal Investigator—Jackson T. Wright, Jr, Mahboob Rahman.
Study Coordinator—Renee Dancie, Louise Strauss.
Emory University
Principal Investigator—Janice Lea.
Study Coordinator—Beth Wilkening, Arlene Chapman, and Diane Watkins.
Harbor-UCLA Medical Center
Principal Investigator—Joel D. Kopple.
Study Coordinator—Linda Miladinovich, Jooree Choi, Patricia Oleskie, and Connie Secules.
Harlem Hospital Center
Principal Investigator—Velvie Pogue.
Study Coordinator—Donna Dowie, Jen-Tse Cheng.
Howard University
Principal Investigator—Otelio Randall, Tamrat Retta.
Study Coordinator—Shichen Xu, Muluemebet Ketete, Debra Ordor, Carl Tilghman.
Johns Hopkins University
Steering Committee Chair—Lawrence Appel.
Principal Investigator—Edgar Miller, Brad Astor.
Study Coordinator—Charalett Diggs, Jeanne Charleston, Charles Harris, Thomas Shields.
Charles R Drew University
Principal Investigator—Keith Norris, David Martins.
Study Coordinator—Melba Miller, Holly Howell, Laurice Pitts.
Medical University of South Carolina
Principal Investigator—DeAnna Cheek.
Study Coordinator—Deborah Brooks.
Meharry Medical College
Principal Investigator—Marquetta Faulkner, Olufemi Adeyele.
Study Coordinator—Karen Phillips, Ginger Sanford, Cynthia Weaver.
Morehouse School of Medicine
Principal Investigator—William Cleveland, Kimberly Chapman.
Study Coordinator—Winifred Smith, Sherald Glover.
Mount Sinai School of Medicine and University of Massachusetts
Principal Investigator—Robert Phillips, Michael Lipkowitz, Mohammed Rafey.
Study Coordinator—Avril Gabriel, Eileen Condren, Natasha Coke.
Ohio State University
Principal Investigator—Lee Hebert, Ganesh Shidham.
Study Coordinator—Leena Hiremath, Stephanie Justice.
University of Chicago, Chicago
Principal Investigator—George Bakris, James Lash.
Study Coordinator—Linda Fondren, Louise Bagnuolo, Janet Cohan, Anne Frydrych.
University of Alabama, Birmingham
Principal Investigator—Stephen Rostand, Denyse Thornley-Brown.
Study Coordinator—Beverly Key.
University of California, San Diego
Principal Investigator—Francis B Gabbai, Daniel T O’Connor.
Study Coordinator—Brenda Thomas.
University of Florida
Principal Investigator—C. Craig Tisher, Geraldine Bichier.
Study Coordinator—Cipriano Sarmiento, Amado Diaz, Carol Gordon.
University of Miami
Principal Investigator—Gabriel Contreras, Jacques Bourgoignie Dollie Florence-Green.
Study Coordinator—Jorge Junco, Jacqueline Vassallo.
University of Michigan
Principal Investigator—Kenneth Jamerson, Akinlou Ojo, Tonya Corbin.
Study Coordinator—Denise Cornish-Zirker, Tanya Graham, Wendy Bloembergen.
University of Southern California
Principal Investigator—Shaul Massry, Miroslav Smogorzewski.
Study Coordinator—Annie Richardson, Laurice Pitts.
University of Texas Southwestern Medical Center, Dallas
Principal Investigator—Robert Toto, Gail Peterson, Rames Saxena.
Study Coordinator—Tammy Lightfoot, Sherry-Ann Blackstone, Carlos Loreto.
Vanderbilt University
Principal Investigator—Julie Lewis, Gerald Schulman.
Study Coordinator—Mo Sika, Sandy McLeroy.
National Institute of Diabetes and Digestive and Kidney Diseases
Lawrence Y. Agodoa, Josephine P. Briggs, John W. Kusek.
Data Coordinating Center (Cleveland Clinic Foundation)
Jennifer Gassman, Gerald Beck, Tom Greene, Bo Hu.
Study Coordinator—Karen Brittain, Susan Sherer, Laurie Tuason, Cynthia Kendrick, Sharon Bi, Harvey Litowitz, Xianyou Liu, Xuelei Wang, Kimberly Wiggins, Cheryl A. Tatum.
Central Biochemistry Laboratory
Frederick Van Lente, Joan Waletzky, Cathy O’Laughlin, LaChauna Burton.
External Advisory Committee
William McClellan, Lucile Adams-Campbell, Kathy Faber-Langendoen, Bryce Kiberd, Elisa Lee, Timothy Meyer, David Nathan, John Stokes, Herman Taylor, Peter W. Wilson.
Cardiovascular Research Foundation
Tine deBacker, Alexandra Lansky, Steve Slack.
Footnotes
A list of the AASK Study Group investigators can be found in references 27 and 28 and in the acknowledgements.
DISCLOSURE
All the authors declared no competing interests.
The Data Coordinating Center is part of the Cleveland Clinic Foundation, which is also the site of the Central Biochemical Laboratory and the GFR Laboratory.
The results presented in this paper have not been published previously in whole or part, except in abstract and poster form at the American Society of Nephrology Annual Meeting in Philadelphia, Pennsylvania, on 6 November 2008.
REFERENCES
- 1.Lopes AA, Bragg J, Young E, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002;62:199–207. doi: 10.1046/j.1523-1755.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 2.Hedayati SS, Grambow SC, Szczech LA, et al. Physician-diagnosed depression as a correlate of hospitalization in patients receiving long-term hemodialysis. Am J Kidney Dis. 2005;46:642–649. doi: 10.1053/j.ajkd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Drayer RA, Piraino B, Reynolds CF, et al. Characteristics of depression in hemodialysis patients: symptoms, quality of life and mortality risk. Gen Hosp Psychiatry. 2006;28:306–312. doi: 10.1016/j.genhosppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Hedayati SS, Bosworth HB, Briley LP, et al. Death or hospitalization of patients on chronic hemodialysis is associated with physician-based diagnosis of depression. Kidney Int. 2008;74:930–936. doi: 10.1038/ki.2008.311. [DOI] [PubMed] [Google Scholar]
- 5.Kimmel PL, Peterson RA, Weihs KL, et al. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis patients. Kidney Int. 2000;57:2093–2098. doi: 10.1046/j.1523-1755.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 6.Boulware LE, Liu Y, Fink NE, et al. The temporal relation between depression symptoms, cardiovascular disease events and mortality in ESRD: contribution of reverse causality. Clin J Am Soc Nephrol. 2006;1:496–504. doi: 10.2215/CJN.00030505. [DOI] [PubMed] [Google Scholar]
- 7.Watnick S, Kirwin P, Mahnensmith R, et al. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis. 2003;41:105–110. doi: 10.1053/ajkd.2003.50029. [DOI] [PubMed] [Google Scholar]
- 8.Patel SS, Shah VS, Peterson RA, et al. Psychological variables, quality of life, and religious beliefs in ESRD patients treated with hemodialysis. Am J Kidney Dis. 2002;40:1013–1022. doi: 10.1053/ajkd.2002.36336. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel PL, Peterson RA, Weihs KL, et al. Psychologic functioning, quality of life, and behavorial compliance in patients beginning hemodialysis. J Am Soc Nephrol. 1996;7:2152–2159. doi: 10.1681/ASN.V7102152. [DOI] [PubMed] [Google Scholar]
- 10.Walters BA, Hays RD, Spritzer KL, et al. Health-related quality of life, depressive symptoms, anemia, and malnutrition at hemodialysis initiation. Am J Kidney Dis. 2002;40:1185–1194. doi: 10.1053/ajkd.2002.36879. [DOI] [PubMed] [Google Scholar]
- 11.Valderrabano F, Jofre R, Lopez-Gomez JM. Quality of life in end-stage renal disease patients. Am J Kidney Dis. 2001;38:443–464. doi: 10.1053/ajkd.2001.26824. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SD, Norris L, Acquaviva K, et al. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2:1332–1342. doi: 10.2215/CJN.03951106. [DOI] [PubMed] [Google Scholar]
- 13.Watnick S, Wang PL, Demadura T, et al. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis. 2005;46:919–924. doi: 10.1053/j.ajkd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Hedayati SS, Bosworth HB, Kuchibhatla M, et al. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69:1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 15.Craven JL, Rodin GM, Littlefield C. The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med. 1988;18:365–374. doi: 10.2190/m1tx-v1ej-e43l-rklf. [DOI] [PubMed] [Google Scholar]
- 16.Kimmel PL. Depression in patients with chronic renal disease: what we know and what we need to know. J Psychosom Res. 2002;53:951–956. doi: 10.1016/s0022-3999(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 17.Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in DOPPS. Kidney Int. 2004;66:2047–2053. doi: 10.1111/j.1523-1755.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 18.Cukor D, Coplan J, Brown C, et al. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:484–490. doi: 10.2215/CJN.00040107. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein FO, Finkelstein SH. Depression in chronic dialysis patients: assessment and treatment. Nephrol Dial Transplant. 2000;15:1911–1913. doi: 10.1093/ndt/15.12.1911. [DOI] [PubMed] [Google Scholar]
- 20.Wuerth D, Finkelstein S, Finkelstein F. The identification and treatment of depression in patients maintained on dialysis. Semin Dial. 2005;18:142–146. doi: 10.1111/j.1525-139X.2005.18213.x. [DOI] [PubMed] [Google Scholar]
- 21.Hedayati SS, Jiang W, O’Connor CM, et al. The association between depression and chronic kidney disease and mortality among patients hospitalized with congestive heart failure. Am J Kidney Dis. 2004;44:207–215. doi: 10.1053/j.ajkd.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Odden MC, Whooley MA, Shlipak MG. Depression, stress, and quality of life in persons with chronic kidney disease: the Heart and Soul Study. Nephron Clin Pract. 2006;103:c1–7. doi: 10.1159/000090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen SD, Patel SS, Khetpal P, et al. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:919–925. doi: 10.2215/CJN.00820207. [DOI] [PubMed] [Google Scholar]
- 24.Shidler NR, Peterson RA, Kimmel PL. Quality of life and psychosocial relationships in patients with chronic renal insufficiency. Am J Kidney Dis. 1998;32:557–566. doi: 10.1016/s0272-6386(98)70017-4. [DOI] [PubMed] [Google Scholar]
- 25.Hedayati SS, Minhajuddin AT, Toto RD, et al. Prevalence of major depressive episode in CKD. Am J Kidney Dis. 2009;54:424–432. doi: 10.1053/j.ajkd.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Renal Data System. USRDS 2008 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2008 [Google Scholar]
- 27.Appel LJ, Middleton J, Miller ER, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14:S166–S172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 28.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephrol. 2003;14(7 Suppl 2):S154–S165. doi: 10.1097/01.asn.0000070080.21680.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 30.Hedayati SS, Minhajuddin AT, Toto RD, et al. Validation of depression screening scales in patients with CKD. Am J Kidney Dis. 2009;54:433–439. doi: 10.1053/j.ajkd.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19:1261–1270. doi: 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]
- 32.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68:914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 33.Powe NR, Melamed ML. Racial disparities in the optimal delivery of chronic kidney disease care. Med Clin North Am. 2005;89:475–488. doi: 10.1016/j.mcna.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Hoth KF, Christensen AJ, Ehlers SL, et al. A longitudinal examination of social support, agreeableness and depressive symptoms in chronic kidney disease. J Behav Med. 2007;30:69–74. doi: 10.1007/s10865-006-9083-2. [DOI] [PubMed] [Google Scholar]
- 35.Kimmel PL, Patel SS, Peterson RA. Depression in African-American patients with kidney disease. J Natl Med Assoc. 2002;94:925–1035. [PMC free article] [PubMed] [Google Scholar]
- 36.Kimmel PL. Psychosocial factors in adult end-stage renal disease patients treated with hemodialysis: correlates and outcomes. Am J Kidney Dis. 2000;35 Suppl 1:S132–S140. doi: 10.1016/s0272-6386(00)70240-x. [DOI] [PubMed] [Google Scholar]
- 37.Blazer DG, Hybels CF, Simonsick EM, et al. Marked differences in antidepressant use by race in an elderly community sample: 1986–96. Am J Psychiatry. 2000;157:1089–1094. doi: 10.1176/appi.ajp.157.7.1089. [DOI] [PubMed] [Google Scholar]
- 38.Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Med Care. 2003;41:479–489. doi: 10.1097/01.MLR.0000053228.58042.E4. [DOI] [PubMed] [Google Scholar]
- 39.Tossani E, Cassano P, Fava M. Depression and renal disease. Semin Dial. 2005;18:73–81. doi: 10.1111/j.1525-139X.2005.18217.x. [DOI] [PubMed] [Google Scholar]
- 40.Kimmel PL, Weihs K, Peterson RA. Survival in hemodialysis patients: the role of depression. J Am Soc Nephrol. 1993;4:12–27. doi: 10.1681/ASN.V4112. [DOI] [PubMed] [Google Scholar]
- 41.Palmer SC, Coyne JC. Screening for depression in medical care: pitfalls, alternatives, and revised priorities. J Psycho Res. 2003;54:279–287. doi: 10.1016/s0022-3999(02)00640-2. [DOI] [PubMed] [Google Scholar]
- 42.Devins GM. Illness intrusiveness and the psychological impact of lifestyle disruptions in chronic life-threatening disease. Adv Ren Replace Ther. 1994;1:251–263. doi: 10.1016/s1073-4449(12)80007-0. [DOI] [PubMed] [Google Scholar]
- 43.Troidle L, Wuerth D, Finkelstein S, et al. The BDI and the SF36: which tool to use to screen for depression. Adv Perit Dial. 2003;19:159–162. [PubMed] [Google Scholar]
- 44.Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 45.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 46.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38:744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 47.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I: conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 48.Diener E, Emmons RA, Larsen RJ, et al. The Satisfaction with Life scale. J Pers Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 49.Ware JE, Kosinski M, Bayliss MS, et al. Comparison of methods for scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the medical outcomes study. Med Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- 50.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, New England Medical Center; 1994. [Google Scholar]