Abstract
This systematic review summarizes existing evidence regarding the efficacy, safety, and abuse/misuse potential of opioids as treatment for chronic non-cancer pain (CP) in older adults. Multiple databases were searched to identify relevant studies published in English (1/1/80-7/1/09) with a mean study population age of 60 years or above. Forty-three articles were identified and retained for review. The weighted mean subject age was 64.1 years (mean age range: 60-73). Studies enrolled patients with osteoarthritis (70%), neuropathic pain (13%), or other pain-producing disorders (17%). The mean duration of treatment studies (n=40) was 4 weeks (range = 1.5–156 weeks), and only 5 (12%) lasted longer than 12 weeks. In meta-analyses, effect sizes were −0.557 (p<0.001) for pain reduction, −0.432 (p<0.001) for physical disability reduction, and 0.859 (p=0.309) for improved sleep. The effect size for the SF-36 physical component score was 0.191 (p = 0.171) and −0.220 (p =0.036) for the mental component score. Adults ages 65 and above (vs. less than 65) were equally likely to benefit from treatment. Common adverse events included constipation (median frequency of occurrence = 30%), nausea (28%), dizziness (22%), and prompted opioid discontinuation in 25% of cases. Abuse/misuse behaviors were negatively associated with advancing age. Among older adults with CP and no significant comorbidity, short-term use of opioids is associated with reductions in pain intensity, improved physical functioning, but decreased mental health functioning. The long-term safety, efficacy, and abuse potential of this treatment practice in diverse populations of older persons remain to be determined.
Keywords: opioid, pain, older adults
INTRODUCTION
Prior systematic reviews have reported on short-term outcomes associated with opioid medications as a treatment for chronic non-cancer pain.1-5 Opioids reduced pain scores significantly among patients with osteoarthritis,1-3 as well as those with neuropathic pain,1,4 but not chronic back pain.5 Opioids were also associated with improved functional outcomes in two reviews.2,3 These benefits may be limited, however, by the occurrence of adverse events, which prompted opioid discontinuation in up to 31% of cases.1-3
Of note, none of the reviews focused on older populations or reported age-stratified results. An examination of the evidence regarding opioid safety and efficacy in older populations is needed for several reasons. Chronic non-cancer pain is a highly prevalent, costly, and often disabling disorder in later life.6-8 Prevalence studies indicate that 40% to 50% of older adults report the presence of a chronic pain disorder.7-9 The deleterious consequences of inadequately treated pain are far-reaching and include impaired quality of life, sleep, immune function, as well as activities of daily living (ADL) impairment.10-15 In addition, non-steroidal anti-inflammatory agents, the most commonly prescribed class of analgesic medications, have potentially serious gastrointestinal and cardiovascular side effects and can exacerbate comorbid conditions that are prevalent in later life.16,17 Some authors have suggested that opioids are underutilized as a treatment for chronic pain in older populations,18 possibly due to provider concerns about the uncertainty of the value and safety of opioids as a treatment for this disorder,19 as well as their concerns about patient addiction and untoward side effects.20 Accordingly, we conducted a comprehensive review of the literature to identify studies that examined opioid medications as a treatment for chronic non-cancer pain in older persons and reported efficacy, safety, or abuse/misuse outcome data.
METHODS
Data Sources and Searches
We searched the Ovid/Medline, PubMed, MD Consult, CINAHL, and Cochrane Controlled Trail databases (1/1/80-7/1/09) to identify pertinent articles for review. MeSH terms included opioid analgesics, pain, elderly, aged, treatment outcome, and adverse effects. Other keywords included chronic pain, non-malignant pain, efficacy, abuse, and misuse. Citation abstracts were independently reviewed by two investigators to determine their suitability for inclusion in the review. Clinical experts and clinicaltrials.gov were also queried.
Study Selection
Studies were eligible if they: 1) were published in English; 2) evaluated one or more opioid medications (administered orally or transdermally); and 3) reported results (i.e., efficacy, safety, or abuse/misuse data) on older adults as evidenced by a minimum mean study population age of ≥60 years or reported age-stratified results for older age subgroups. Because tramadol is used to treat chronic pain in older persons,21,22 it was included along with the conventional opioids. Due to the small number of studies that examined opioid abuse/misuse outcomes, articles examining this outcome were also retained if the mean age of the sample was < 60 years but included some subjects ages ≥ 60 years and examined age as a predictor of opioid abuse/misuse.
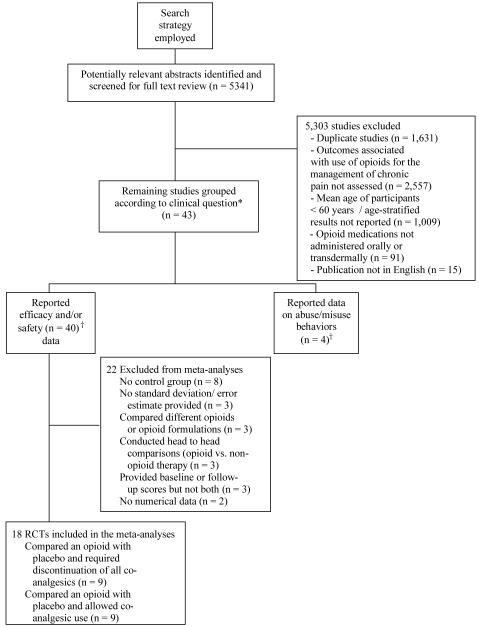
A QUORUM (Quality of Reporting of Meta-analyses) flow diagram (see Figure) shows an overview of the study selection process. The reference lists from all 38 articles meeting the study criteria were reviewed. Five additional studies were included after reviewing the reference lists, yielding a final sample of 43 articles.
Figure.
Flow diagram of included and excluded studies
Data Abstraction
Two investigators independently abstracted study outcomes. Information regarding eligibility criteria used for subject selection, study design, study duration, participants’ demographic and clinical characteristics, source of study funding, condition studied, as well as type and dosage of opioid studied was abstracted. We focused on three pre-specified outcomes: 1) efficacy; 2) safety/tolerability; and 3) occurrence of abuse/misuse behaviors. As most studies employed multiple pain measures, we selected average pain intensity and pain relief scores when present; otherwise pain severity was extracted if present. We extracted Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores that were the most commonly used physical function measures. Quality of life was most often measured by the SF-36 instrument.
Quality Assessment
Retained studies were evaluated for methodological quality.23,24 Previously employed threshold scores5 were used to assign a quality score of ‘excellent’. For clinical trials (n=31), a quality score of ≥ 10 (score range, 0-13), was considered excellent,5 whereas for observational studies (n=12), a score of ≥ 12 (score range, 0-15)25 was considered excellent.5
Data Synthesis and Analysis
Data Synthesis for Univariate Analyses
For the primary efficacy outcomes (pain, physical function, physical quality of life, mental quality of life and sleep), we calculated an average change score by subtracting baseline from follow-up score and then dividing the result by the respective baseline score for the active treatment and placebo control groups. We abstracted data on the most commonly reported adverse events only, i.e., prevalence rate ≥15% in either the treatment or control arm. Dropout rates due to adverse events or lack of efficacy were recorded when present.
Data Synthesis for Meta Analyses
Pain intensity was most often measured on a 0-10 scale, but sometimes on a 0-100 visual analog scale. Physical function was measured by WOMAC in all nine studies reporting this outcome, and quality of life was measured by the SF-36 instrument in the four studies with this outcome available to be included in the meta-analyses. Sleep quality was measured in a variety of ways. We used the overall measure of sleep where it was reported alone or, if not, other measures of sleep functioning.
Most studies reported mean outcomes with standard deviations or standard errors for placebo and active treatment either at baseline and follow up or at one of the 2 time points plus differences scores over time with standard errors for the difference. From these data, we derived for each study a 2×2 set of variables (treatment by time); the 2 difference scores (follow up minus baseline) for placebo and active; and standard deviations for these 6 variables. For the dose-ranging studies (n=3), we examined the medication protocols and averaged the outcomes across the active treatment conditions (with pooled standard deviations) to provide a 2-level treatment variable comparable with the majority of studies that had only a single active treatment.
Statistical Models
We carried out meta-analyses for pain and physical function outcomes using 2 types of models. The first was a statistical mixed model with treatment group (placebo versus active) and time of assessment (a repeated measure, baseline versus follow up) as fixed classification factors; the interaction of these 2 factors; and studies included in the model as levels of a random classification factor. The dependent variables were the outcome means (e.g., pain reduction) for the treatment and time groups divided by their standard deviations. Treatment effects across studies are tested by the interaction of treatment and time in this model.
The second model eliminated the repeated-measures factor for time of assessment and used the standardized mean difference over time (the mean difference divided by the standard deviation of the difference) as the dependent variable. The effect of opioids on the outcome is examined by the treatment main effect in this model. This model allowed inclusion of 2 additional studies in the analysis.
An examination of the results for pain and physical function outcomes revealed consistency between the 2 models, and only results from the second model using standardized difference scores are reported below.
Too few studies reported analysis for physical and mental quality of life and quality of sleep to include studies in the model as levels of a random factor (or to regard studies as fixed and include them in that way). For these outcomes, we carried out an analysis of the standardized difference scores in a model that included the fixed factor for treatments.
We also examined models in which additional independent variables were included. Pain type (osteoarthritis versus neuropathic), opioid potency (low/medium versus high), duration of action (short- versus long-acting agents), and whether the study allowed for co-analgesic use (yes versus no) were each included as additional fixed classification factors in the above models for pain and physical function (separate models for each additional variable). We focused on the interaction of these variables with treatment and time (or simply with treatment in the second basic model), as well as on the treatment contrasts at each level of the added variable. Methodologic quality score (entered as a quantitative variable) was examined as a covariate in the 2 basic models. To assess for age effects on treatment outcomes, mean participant age (range = 60-73) was entered as a covariate in both models. The homogeneity of the regressions of outcomes such as pain on age and on methodologic quality score were tested for treatment and time—i.e., the interaction of age and study quality with treatment and time.26
Most of the studies in the meta-analyses were not restricted to patients ages 65 or older and did not report results stratified by age. To augment the analysis of mean age described above, we also carried out an analysis limited to the studies that reported data specific to older age groups (i.e., those 65 years of age and older), comparing outcomes for older and younger age groups.
Two types of diagnostic analyses pertaining to the meta-analysis itself were carried out. Heterogeneity of treatment differences across studies, which may result from differences in study protocols, variable definitions, implementation, or overall quality, was tested by a standard statistic, Cochran’s Q.27 The hypothesis of study homogeneity was rejected, indicating that mixed models (studies random) were more appropriate than fixed effect models. Mixed models are also preferable by general principles and for greater generalizability of results.
We also examined the question of publication bias—whether there was a tendency in the sample of studies for there to be a lack of publication of certain types of studies—by a funnel plot with 1/(standard error), a measure of sample size, plotted against effect size.28
RESULTS
Study Characteristics
Of the 43 studies, 40 (93%) provided efficacy data.29-68 One of the 40 treatment studies58 also provided information on rates of abuse/misuse, while three provided data only on rates of abuse/misuse.69-71 The mean duration of the treatment studies was 4 weeks (range = 1.5–156 weeks), with only 5 (12%) lasting longer than 12 weeks. Thirty-one of the 40 treatment studies employed a randomized control design: 19 compared opioid treatment with placebo, 5 examined the effects of opioid treatment as ‘add on’ therapy, 4 compared different types of opioids with one another or with different opioid dosing schedules, and 3 compared opioid therapy to another active treatment. Of the remaining 9 studies, 8 were open-label observational studies with no control group and one was a retrospective cohort study. Of the 3 studies that provided data on abuse/misuse outcomes, one was a secondary analysis, another employed a retrospective cohort design, and the third was a prospective cohort investigation. Most efficacy studies (78%) were sponsored by pharmaceutical companies.
Study Quality
Of the 31 randomized trials, 24 (77%) were assigned an excellent methodologic quality score5,23 with a median quality score of 10 (range, 9-13). Of the remaining 12 articles (all observational studies), 7 (58%) were deemed to have excellent methodologic quality with a median quality score24,25 of 13 (range, 9-15).
Study Participant Characteristics
The 40 treatment studies provided information on 8,690 patients. The weighted mean age for this group of studies was 64.1 years (range, 60 to 73). In the 21 studies that reported race/ethnicity data, all but one51 assessed outcomes in largely non-Hispanic white populations.
Table 1 shows that most treatment studies enrolled patients with osteoarthritis-related pain (n=28). Twenty-nine studies (72%) excluded subjects with a current or past history of substance abuse, while 25 (63%) excluded subjects with significant concomitant disease(s), but these were not described. The 4 studies that examined abuse/misuse outcomes included 16,098 patients. [One study analyzed administrative data on 15,160 patients.69] The weighted mean age for this subgroup of studies was 60.3 years (range 52 to 62.4).
Table 1.
Studies reporting on opioid efficacy and safety among older adults with chronic non-malignant pain
| Ref. # | Condition Site | Study / Subject Characteristics | % of Treatment or Control Group Reporting/Experiencing |
% Discontinuing Treatment Due To |
||||
|---|---|---|---|---|---|---|---|---|
| Opioid / Reference Group |
Size / Race Ethnicity* |
Mean Age ± SD or (range)† |
Length | Efficacy Outcomeठ|
Adverse Event Outcomes§ |
Adverse Events§ |
Lack of Efficacy§ |
|
| Osteoarthritis | ||||||||
| [29] | Spine, knee or other | N = 133 NRM |
62 (32-90) | 2 weeks (placebo- controlled trial) |
Pain intensity ↓ 28% / 31% / 13%1¶ | Nausea 27% / 41% / 11% Dizziness 30% / 20% / 9% Somnolence 25% / 27% / 4% Constipation 23% / 32% / 7% Pruritus 18% / 16% / 2% |
27% / 32% / 4% | 27% / 11% / 49% |
| Controlled Release Oxycodone (10 mg bid, 20 mg bid) / Placebo |
||||||||
| Controlled Release Oxycodone (dose escalated to maximum of 40 mg qd) |
N = 106 | 62 (32-90) | 24 weeks (extension trial) |
Pain intensity ↓ 27% | Constipation 52% Somnolence 30% Nausea 24% Pruritus 20% Nervousness 15% |
37% | 8% | |
| [30] | Hip or knee | N = 230 100% W |
67.1 ± 7.1 (Tramadol) |
2 weeks | Pain intensity ↓ 41% / 27%** | Nausea 23% / 7% Vomiting 17% / 1% |
22% / 2% | NR |
| Controlled Release Tramadol (200 mg qd) / Placebo |
66.4 ± 9.2 (Placebo) |
|||||||
| [31] | Hip or knee | N = 103 NR |
61.6 ± 11.2 | 4 weeks | Pain intensity ↓ 55% / 16%†† WOMAC scores‡‡ Stiffness ↓ 48% / 17%** Physical function ↑ 49% / 17%‡‡ |
Constipation 49% / 11% Somnolence 39% / 10% Dizziness 33% / 8% |
29% / 8% | 1% / 10% |
| Controlled Release Codeine (50 mg bid; dose escalated up to 200 mg bid) / Placebo |
||||||||
| [32] | Hip or knee | N = 295 84% W 14% AA 2% O |
62.6 ± 9.5 (24-hr prep in AM) |
4 weeks (placebo- controlled trial) |
Pain intensity ↓ 26% / 22% / 22% / 14% WOMAC scores: Stiffness ↓ 17% / 17% / 14% / 11% Physical function ↑ 18% / 19% / 14% / 8% |
Constipation 49% / 40% / 29% / 4% Nausea 21% / 32% / 26% / 10% Somnolence 16% / 12% / 12% / 0% Vomiting 6% / 16% / 8% / 1% |
23% / 25% / 24% / 7% |
12% / 16% / 11% / 19% |
| Controlled Release Morphine preparations (Once daily MS04, 30 mg given in AM, once daily MS04 given in PM; controlled release MS04 15 mg given twice daily / Placebo |
63.1 ± 11.1 (24-hr prep in PM) |
|||||||
| 61.9 ± 10.4 (Twice daily prep) |
||||||||
| 61.9 ± 10.7 (Placebo) |
||||||||
| 24 hour prep in AM / 24 hour prep in PM (both doses escalated if needed) |
N = 181 | 26 weeks (open- label extension trial) |
Significant treatment effects observed for pain and physical function outcomes during weeks 4 to 26§§ |
Constipation 37% / 32% Nausea 12% / 20% Diarrhea 15% / 10% Somnolence 15% / 10% |
36% / 30% | 11% / 7% | ||
| [33] | Hip, knee, spine or other | N = 109 93% W 6% AA 1% H |
63 (38-89) | 13 weeks | Pain intensity ↓ 26% / 9%¶ Pain relief ↑ 21 % / ↓ 8%** Overall WOMAC score ↓ 28% / 1%††, ∥∥ |
Constipation 48% / 10% Nausea 41% / 14% Somnolence 32% / 10% Dizziness 32% / 6% Pruritus 21% / 0% Headache 20% / 20% |
36% / 4% | 16% / 67% |
| Controlled Release Oxycodone (10 mg bid, dose escalated up to a maximum of 60 mg bid / Placebo |
||||||||
| [34] | Hip or knee | N = 159 NR |
67 (47-88) | 4 weeks | Pain relief ↑ 64% WOMAC scores: Stiffness ↓ 23%†† Physical function ↑ 23%†† SF-36 quality of life (physical) ↑ 15%†† SF-36 quality of life (mental) ↑ 6%¶ |
Nausea 32% Vomiting 26% Somnolence 16% |
22% | 1% |
| Fentanyl (25 μg/h, dose escalated if needed) / No reference group |
||||||||
| [35] | Hip | N = 161 NR |
66 (27-82) (Codeine + Paracetamol) |
4 weeks | Decreased pain intensity reported by 45% / 40% of patients |
Nausea 41% / 8% Dizziness 31% / 1% Vomiting 23% / 4% Constipation 21% / 9% Somnolence 17% / 7% |
48% / 14% | NR |
| Codeine (60 mg tid) with paracetamol (1 g tid) / Paracetamol (1 g tid) |
67 (42-82) (Paracetamol) |
|||||||
| [36] | Site not specified | N = 107 93% W 7% O |
62.6 ± 12.2 (CR Oxycodone) |
2 weeks | Pain intensity ↓ 25% / 7%†† | NR | 36% / 4% | 16% / 67% |
| Controlled Release (CR) Oxycodone (10 mg bid, dose escalated up to maximum daily dose of 120 mg) / Placebo |
64 ± 10.7 (Placebo) |
|||||||
| [37] | Hip or knee | N = 81 93%W 7%O |
60 ± 11 | 8 weeks | Pain intensity ↓ 28%†† WOMAC scores: Stiffness ↓ 27%†† Physical function ↑ 24%†† |
Nausea 43% Dizziness 26% Headache 23% Constipation 21% |
42% | NR |
| Fentanyl (25 μg/h, dose escalated if needed) / No reference group |
||||||||
| [38] | Hip or knee | N = 399 NR |
66 ± 0.7 (Fentanyl) |
6 weeks | Pain intensity ↓ 32% / 24%¶ Significant treatment effects reported for overall WOMAC score + pain & physical function subscales§§ |
Nausea 44% / 19% Vomiting 28% / 3% Somnolence 22% / 4% |
27% / 10% | 7% / 32% |
| Fentanyl (25 μg/h, dose escalated if needed) / Placebo |
66 ± 0.7 (Placebo) |
|||||||
| [39] | Hip or knee | N = 140 86% W 9% AA 5% O |
63.6 (38-91) | 6 weeks | Pain intensity ↓ 24% / 16% Pain relief ↑ 64% / 53% WOMAC scores: Stiffness ↓ 33% / 32% Physical function ↑ 31% / 28% |
Nausea 35% / 30% Constipation 30% / 25% Somnolence 25% / 18% Vomiting 17% / 12% Dizziness 14% / 22% |
35% / 33% | 1% / 4% |
| Controlled Release Hydromorphone (8 mg qd, dose escalated as needed) /Controlled Release Oxycodone (10 mg bid, dose escalated if needed) / No reference group |
||||||||
| [40] | Hip or knee | N = 370 90% W 8% AA 2% O |
62¶¶ | xs2 weeks | Pain intensity ↓ 21% / 28% / 29% / 17%** |
Nausea 23% / 41% / 55% / 9% Constipation 18% / 27% / 22% / 4% Dizziness 16% / 22% / 31% / 6% Headache 11% / 15% / 19% / 10% Vomiting 10% / 27% / 35% / 2% Somnolence 10% / 23% / 21% / 3% Pruritus 5% / 20% / 24% / 1% |
25% / 55% / 52% / 10% |
7% / 5% / 4% / 16% |
| Controlled Release Oxymorphone (10 mg bid, 40 mg bid, 50 mg bid) / Placebo |
||||||||
| [41] | Hip or knee | N = 491 86% W 11% AA 2% H 1% O |
63.4 ± 1 (Oxymorphone 20 mg) |
4 weeks | Pain intensity ↓ 25%¶ / 27%¶ / 22% / 17% |
Nausea 61% / 60% / 43% / 11% Constipation 40% / 32% / 36% / 11% Somnolence 30% / 31% / 27% / 5% Dizziness 29% / 31% / 26% / 4% Vomiting 23% / 34% / 10% / 2% Pruritus 19% / 20% / 8% / 2% Dry mouth 12% / 12% / 15% / 1% Headache 6% / 11% / 18% / 11% |
38% / 47% / 25% / 5% |
4% / 7% / 10% / 27% |
| Controlled Release Oxymorphone (20 mg bid, 40 mg bid) / Controlled Release Oxycodone (20 mg bid) / Placebo |
61.4 ± 1 (Oxymorphone 40 mg) |
|||||||
| 62.7 ± 1 (Oxycodone 20 mg) |
||||||||
| 61.7 ± 1 (Placebo) |
||||||||
| [42] | Hip or knee | N = 97 NR |
62.2 ± 7.3 | 6 weeks | Pain intensity ↓ 28% / 31% WOMAC scores: Stiffness ↓ 27% / 32% Physical function ↑ 29% / 29% |
Nausea 24% / 11% Dizziness 24% / 18% Constipation 21% / 15% Somnolence 18% / 8% |
16% / 15% | NR |
| Controlled Release Tramadol titrated up to 200, 300, or 400 mg Qd / Diclofenac SR 75 mg |
||||||||
| [43] | Knee | N = 129 91% W 7% AA 2% O |
62.5 (35-75) | 12 weeks | Pain intensity ↓ 23% / 13%¶ Pain relief ↑ 43% / ↓ 57%** Overall WOMAC score ↓ 17%¶, ∥∥, *** Significant treatment effects reported for WOMAC pain, stiffness and physical function subscales‡‡ |
Nausea 18% / 3% | 22% / 15% | 41% / 65% |
| Tramadol (50 mg every 2 days, dose escalated up to 200 mg qd) / Placebo |
||||||||
| [44] | Knee | N = 240 82% W 15% AA 3% O |
61¶¶ | 13 weeks | Total daily dose of naproxen needed for pain relief decreased by 78% in tramadol arm |
Nausea 27 % / NR Dizziness 21% / NR Constipation 17% / NR Somnolence 15% / NR |
22% / 13% | NR |
| Tramadol (200 mg qd) plus naproxen (1 g qd) / Placebo plus naproxen (1 g qd) |
||||||||
| [45] | Knee | N = 246 82% W 12% AA 3% H 3% O |
61¶¶ (Tramadol) |
12 weeks | Pain intensity ↓ 49% / 27%†† WOMAC scores: Stiffness ↓ 43% / 18%†† Physical function ↑ 44% / 21%†† |
Dizziness 33% / 12% Nausea 24% / 8% Constipation 26% / 6% Headache 15% |
27% / 7% | 15% / 37% |
| Controlled Release Tramadol (100 mg qd, dose escalated up to 400 mg qd / Placebo |
||||||||
| [46] | Hip or knee | N = 135 99% W 1% O |
64.4 (Buprenorphine patch) |
12 weeks | Pain intensity ↓ 49% / 27% | Nausea 30% / 25% Constipation 18% / 8% Dizziness 16% / 5% Fatigue 13% / 18% |
15% / 29% | NR |
| Transdermal Buprenorphine patches (5 ug/hr escalated to a maximum dose of 20 ug/hr /Controlled Release Tramadol 75, 100, 150 or 200 mg/day escalated to maximum daily dose of 400mg/day |
64.2 (Tramadol) |
|||||||
| [47] | Hip or knee | N = 318 85% W 11% AA 2% H 1% A 1% O |
69¶¶ | 12 weeks (post-hoc analysis with subjects ≥ 65 years of age) |
Pain intensity ↓ 23% / 26%¶ / 33%** / 20% / 16% WOMAC scores: Stiffness ↓ 30% / 38%¶ / 39%¶ / 29% / 21% Phys. function ↑ 28% / 34% / 38%¶ / 25% /21% |
Constipation 13% / 20% / 38% / 42% / 10% Dizziness 17% / 17% / 27% / 30% / 4% Nausea 13% / 28% / 29% / 25% / 8% Somnolence 11% / 15% / 11% / 24% / 2% Headache 16% / 15% / 11% / 22% / 4% Pruritus 9% / 9% / 9%/ 15% / 2% |
23% / 26% / 42% / 39% / 6% |
16% / 14% / 9% / 13% / 29% |
| Controlled Release Tramadol (100 mg qd, 200 mg qd, 300 mg qd, 400 mg qd) / Placebo |
||||||||
| [48] | Knee | N = 646 85% W 8% H 5% AA 2% O |
63 (40-80) | 12 weeks | Pain intensity ↓ 42% / 32%†† | Nausea 15% / 6% | 10% / 5% | 8% / 10% |
| Tramadol (200 mg or 300 mg qd) / Placebo |
||||||||
| [49] | Knee | N = 431 NR |
60.3 ± 9.3 (Tramadol bid) |
12 weeks | Pain intensity ↓ 31% / 30% WOMAC scores: Stiffness ↓ 49% / 49% Physical function ↑ 52% / 50% |
Dizziness 37% / 26% Nausea 34% / 33% Constipation 30% / 34% Somnolence 21% / 30% Headache 18% / 13% |
10% / 9% | 1% / 1% |
| Controlled Release Tramadol (100–400 mg bid) / Controlled Release Tramadol (100-400 mg qd) |
60.8 ± 9 .3 (Tramadol qd) |
|||||||
| [50] | Hip or knee | N = 307 86% W 13% AA 1% A |
61 (40-75) | 13 weeks | Pain intensity ↓ 40% / 31%¶ WOMAC scores: Stiffness ↓ 27% / 22% Physical function ↑ 30% / 24%¶ SF-36 quality of life (physical) ↑ 20% / 17% SF-36 quality of life (mental) ↑ 2% / 2% |
All adverse effects <15% | 13% / 4% | 8% / 17% |
| Tramadol / Acetaminophen (37.5 mg / 325 mg) qid (dose escalated if needed) plus COX2 inhibitor / Placebo plus COX2 inhibitor |
||||||||
| [51] | Knee | N = 250 100% A |
60.2 ± 7.8 | 2 weeks | Pain intensity ↓ 26% / 27%†† WOMAC scores: Stiffness ↓ 24% / 31% Physical function ↑ 29% / 25% |
Nausea 12% / 25% Vomiting 4% / 17% Dizziness 10% / 22% |
11% / 26% | 0% / 0% |
| Tramadol / Acetaminophen titration group (37.5 mg / 325 mg qd to tid)/ Tramadol / Acetaminophen non- titration group (37.5 mg / 325 mg tid) |
||||||||
| [52] | Flare of OA pain / Hip or knee |
N = 308 88% W 12% AA |
60.1 ± 9.9 | 1.5 weeks | Pain intensity ↓ 42% / 29%†† WOMAC scores: Stiffness ↓ 36% / 30% Physical function ↑ 37% / 30%¶ |
Nausea 17% / 4% | Nausea 17% / 4% | 1% / 0% |
| Tramadol / Acetaminophen (37.5 mg / 325 mg qid) / Placebo |
||||||||
| [53] | Flare of OA pain / Hip or knee |
N = 113 89% W 11% AA |
70.3 ± 3.4 | 1.5 weeks | Pain intensity ↓ 40% / 32%¶ WOMAC scores: Stiffness ↓ 34% / 25% Physical function ↑ 37% / 26%¶ |
Nausea 19% / 5% | 16% / 9% | 0% / 0% |
| Tramadol / Acetaminophen (37.5 mg / 325 mg) plus NSAID qid / Placebo plus NSAID qid |
||||||||
| [54] | Breakthrough musculoskeletal pain / Hip, knee or spine |
N = 42 NR |
65.9¶¶ (Tramadol) |
2 weeks | % reporting moderate / severe pain at rest 15% / 43%¶ |
Constipation 45% / 0% Nausea 35% / 14% Drowsiness 25% / 14% Vertigo 20% / 5% Dizziness 15% / 0% |
5% / 24% | 14% / 38% |
| Tramadol (250 mg qd) / Placebo |
67¶¶ (Placebo) |
|||||||
| [55] | Spine, hand or knee | N = 114 NR |
64.7¶¶ (Propoxy- phene) |
24 weeks | Pain intensity ↓ 56% / 59% | Nausea 25% / 24% Epigastric distress 20% / 7% Dizziness 15% / 13% |
34% / 24% | 2% / 4% |
| Propoxyphene (65 mg qid) / Suprofen (200 mg qid) |
59.2¶¶ (Suprofen) |
|||||||
| [56] | Back pain and chronic pain, site not specified |
N = 148 89%W 11%O |
73.5 ± 5.5 | 4 weeks | Pain intensity ↓ 33%†† | Constipation 20% | 19% | 2% |
| Polymer coated controlled release morphine; initial dose varied with each patient / Dose escalated if needed / No reference group |
SF-36 quality of life (mental) ↑ 15%** SF-36 quality of life (physical) ↑ 19%‡‡ |
|||||||
| Rheumatoid Arthritis | ||||||||
| [57] | Site not specified Fentanyl (25 μg/h, dose escalated if needed) / No reference group |
N = 226 NR |
66 ± 12 (Fentanyl) |
4 weeks | Pain intensity ↓ 50%‡‡ Functional ability (ADL) ↑ 37%¶ Functional ability (Social) ↑ 38%¶ |
All adverse effects <15% | 10% | NR |
| [58] | Site not specified Codeine and / or Oxycodone (doses varied) |
N = 342 NR |
62.7 ± 12.8 (short-term use) |
3 year (retro- spective cohort study) |
Pain severity ↓ 56% / 57%††† | Constipation 12% / 16% | NR | NR |
| 62.4 ± 11 (long-term use) |
||||||||
| Back Pain | ||||||||
| [59] | Vertebral fracture related pain |
N = 64 NR |
71 ± 9 | 4 weeks | Pain intensity at rest ↓ 55%†† Pain intensity on movement ↓ 47%†† Quality of life ↑ 38%†† |
Nausea / Vomiting 28% Dizziness 19% |
20% | NR |
| Fentanyl (25 μg/h, dose escalated if needed) / No reference group |
||||||||
| [60] | Chronic low back pain due to diverse causes |
N = 348 NR |
312 < 65 36 ≥65 |
12 weeks | Oxymorphone responders randomized to either continued oxymorphone or placebo in this randomized withdrawal trial Pain intensity levels remained stable in treatment arm but increased 59% in subjects receiving placebo†† |
All adverse effects < 15% | 9% / 9% | 11% / 43% |
| Controlled Release Oxymorphone (5mg q12 for opioid naïve patients, higher doses for opioid experienced patients, dose escalated if needed / Placebo |
||||||||
| Neuropathic Pain | ||||||||
| [61] | Diabetic Neuropathy | N = 45 NR |
63 ± 9.4 | 4 weeks | Pain intensity ↓ 68% / 28%†† Total pain and disability ↓ 47 % / 19%** |
Nausea 36% / 18% Constipation 29% / 9% Somnolence 20% / 24% Dizziness 16% / 7% |
32% / 17% | 5% / 30% |
| Controlled Release Oxycodone (10 mg bid, escalated to maximum dose of 40 mg bid) / Active Placebo (benztropine 0.25 mg bid; escalated to maximum dose of 1 mg bid) |
||||||||
| [62] | Diabetic Neuropathy | N=338 99%W |
60.1 | 12 weeks | Pain intensity ↓ 33% / 18%** | Constipation 27% /6% Nausea 26% / 11% Somnolence 22% / 5% Fatigue 18% / 8% Dizziness 15% / 4% |
16% / 5% | 3% / 12% |
| Controlled Release Oxycodone (5mg bid, dose escalated if needed plus gabapentin) / gababentin + placeb |
||||||||
| [63] | Post-Herpetic Neuralgia | N=50 NR |
70 ± 11 | 4 weeks | % with at least moderate pain relief 58% / 18%†† |
All adverse effects <15% | 10% / 6% | 0% / 2% |
| Controlled Release Oxycodone (10 mg bid) / Placebo |
||||||||
| [64] | Post-Herpetic Neuralgia | N = 76 88 % W 11 % AA 1% O |
71 ± 12 | 8 weeks | Pain intensity ↓ 32% / 19% / 3%†† Pain relief ↑ 38% / 32% / 11%†† |
Nausea 39% / 6% / 7% Constipation 30% / 11% / 11% Drowsiness 30% / 11% / 14% Dizziness 18% / 17% / 7% Loss of appetite 17% / 2%/ 2% |
9% / 3% / NR | 0% / 0% / NR |
| Morphine (mean dose = 91 mg qd) or Methadone (15 mg qd) / Tricyclic anti-depressant (nortriptylline, mean dose = 89 mg qd or desipramine, mean dose = 63 mg qd) / Placebo |
||||||||
| [65] | Post-Herpetic Neuralgia | N = 127 NR |
65.7 ± 11.9 (tramadol) |
4 weeks | Pain intensity ↓ 58% / 44%¶ Quality of life ↑ 46% / 46% |
All adverse effects <15% | 9% / NR | NR |
| Tramadol (100 mg, escalated to maximum dose of 400 mg for age < 75 and 300 mg for age ≥ 75) / Placebo |
67.9 ± 11.7 (placebo) |
|||||||
| Neuropathic or Non-Neuropathic Pain | ||||||||
| [66] | Neuropathic or non- neuropathic pain |
N = 236 NR |
66.2 (30-91) | 24 weeks | Pain intensity ↓ 47%¶ Quality of life ↑ 35%** |
Dizziness 25% Somnolence 23% Nausea 22% Vomiting 15% Constipation 15% |
29% | NR |
| Fentanyl (25 μg/h, dose escalated if needed) / No reference group |
||||||||
| [67] | Neuropathic or osteoarticular pain |
N = 150 NR |
60 (26-85) Neuropathic group |
6 weeks | Pain intensity ↓ 17% neuro Pain intensity ↓ 35% osteo |
NR | 53% neuro 26% osteo |
NR |
| Oxycodone + Acetaminophen / No reference group |
68 (26-84) Osteoarticular group |
|||||||
| [68] | Arthritic (71%) or neuropathic (29%) pain |
N = 100 NR |
61 ± 12 | 4 weeks | Pain intensity ↓ 72%** for arthritis pain patients Pain intensity ↓ 66%** for neuropathic pain patients |
NR | 0% | NR |
| Controlled Release Tramadol, initial dose 100-200 mg qd, dose escalated up to 400 mg qd if needed / No reference group |
||||||||
Race/ethnicity codes: W = Non-Hispanic White, AA = African-American, H = Hispanic, A = Asian, O = Other.
Mean age provided for entire sample or for treatment/comparison arm groups when no overall mean age provided.
Results are reported as proportionate change scores, (follow-up–baseline scores)/baseline score, unless otherwise specified.
Results in this column correspond to the treatments or placebo listed (in the order in which they appear) in column 1.
NR = Not reported.
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
A lower total WOMAC score indicates a better outcome.
Proportionate changes could not be calculated as the study did not report baseline scores for these outcomes.
Changes in the overall WOMAC score are reported as the study did not report baseline scores for the WOMAC subscales.
Neither standard deviation nor range were reported for age.
Tested for differences in treatment vs. reference group scores at follow-up.
Subjects rated pain severity before and after a single dose of pain medication.
Study Drug Characteristics
Twenty-six studies examined outcomes associated with low- to medium-potency opioids (e.g., tramadol, codeine), whereas 14 reported data on high-potency opioids (e.g., fentanyl, morphine), and most (72%) used extended-release formulations. Over half (59%) of the efficacy studies allowed for dose adjustments. The average oral morphine equivalent opioid dose was 63 mg/day (range = 24-165).
Efficacy Outcomes
Results of Studies Comparing Opioid Treatment with Placebo or with Other Treatments as an Add-On Therapy
Eighteen treatment studies provided sufficient data to permit meta-analysis; all 18 employed a randomized, placebo-controlled trial design. Most of the studies (78%) had an excellent methodologic quality score (14/18 = 78%). Meta-analyses (Table 2) revealed significant reductions in both pain intensity and physical disability, along with non-significant improvements in sleep and physical quality of life. A small, but significant reduction in mental health functioning was found among patients receiving opioids versus those who received a placebo.
Table 2.
Meta-Analysis of Primary Outcomes
| Outcome | Number of studies* |
No. subjects receiving opioid treatment |
No. subjects receiving placebo or non- opioid therapy |
Distributional assumption for studies |
Placebo mean change† |
Active mean change‡ |
Effect size (active – placebo) § |
Probability for test of effect size∥ |
|---|---|---|---|---|---|---|---|---|
| Pain | 18 | 3,005 | 1,865 | random | −.6845 | −1.2461 | −.5571 | <.0001 |
| Physical Function |
9 | 1,822 | 935 | random | −.5660 | −.9977 | −.4317 | .0015 |
| Quality of Life (Physical) |
4 | 972 | 512 | fixed | .4099 | .6010 | .1911 | .1713 |
| Quality of Life (Mental) |
4 | 972 | 512 | fixed | .1844 | −.0361 | −.2205 | .0305 |
| Sleep | 6 | 1,019 | 435 | fixed | .7723 | 1.6309 | .8586 | .3092 |
Each outcome was analyzed in a separate model. The dependent variables were standardized difference scores (Time 2 – Time 1). The model for pain and physical function included a fixed classification factor for Treatment (placebo versus active) and studies as levels of a random factor. For the other outcomes, because of the limited number of studies reporting these outcomes, studies were regarded as fixed but not included in the model.
Studies included only randomized, placebo controlled trials reporting sufficient data to allow for an estimation of effect size.
Least squares means from the model for placebo.
Least squares means from the model for active treatment.
Difference of the 2 least squares means (active – placebo).
Test of the treatment effect.
Table 3 summarizes the association of other independent variables on pain and physical function outcomes. The effect size for pain intensity reduction among patients with neuropathic pain was more than double the one found for patients with osteoarthritis. Drug potency, duration of action, and allowing co-analgesic use during the trial had no effect on these outcomes. In other sensitivity analyses, there was no association between pain and physical function outcomes and study methodologic quality score or mean age of study participants.
Table 3.
Meta-Analyses of Pain and Physical Function Outcomes by Pain Type, as well Potency, Formulation of Study Drug, and Co-analgesic Use
| Variable | Out- come |
Number of Studies* |
No. Subjects Receiving Opioid Treatment |
No. Subjects Receiving Placebo or Non-opioid Therapy |
Distributional Assumption for Studies |
Placebo Mean Change† |
Active Mean Change‡ |
Effect Size (Active – Placebo)§ |
Probability For test of Effect Size∥ |
Probability for Test of Interaction of Treatment and Second Variable¶ |
|---|---|---|---|---|---|---|---|---|---|---|
| Pain Type | ||||||||||
| Osteoarthritis | Pain | 14 | 2,571 | 1,428 | random | −.6830 | −1.1403 | −.4573 | <.0001 | .0259 |
| Neuropathic | 4 | 434 | 437 | −.6895 | −1.5961 | −.9065 | <.0001 | |||
| Drug Potency | ||||||||||
| Low/Medium | Pain | 13 | 1,910 | 1,346 | random | −.6848 | −1.2463 | −.5615 | <.0001 | .9374 |
| High | 5 | 1,095 | 519 | −.6837 | −1.2293 | −.5456 | .0053 | |||
| Low/Medium | Phys Fxn |
5 | 771 | 460 | random | −.5831 | −1.0570 | −.4739 | .0080 | .6394 |
| High | 4 | 1,051 | 475 | −.5446 | −.9236 | −.3791 | .0342 | |||
| Drug Formulation | ||||||||||
| Short-Acting | Pain | 4 | 464 | 382 | random | −.9495 | −1.3649 | −x.4154 | .0393 | .3981 |
| Long-Acting | 14 | 2,541 | 1,483 | −.6088 | −1.2064 | −.5976 | <.0001 | |||
| Short-Acting | Phys Fxn |
3 | 517 | 211 | random | −.9589 | −1.2014 | −.2425 | .2144 | .2989 |
| Long-Acting | 6 | 1,305 | 724 | −.4537 | −.9395 | −.4858 | .0021 | |||
| Co-analgesic Use Allowed | ||||||||||
| Yes | Pain | 9 | 1,181 | 1097 | random | −.7277 | −1.2220 | −.4942 | .0052 | .9520 |
| No | 9 | 1,824 | 768 | −.6975 | −1.2050 | −.5075 | .0043 | |||
| Yes | Phys Fxn |
4 | 608 | 513 | random | −.6908 | −1.0859 | −.3951 | .0301 | .7462 |
| No | 5 | 1,214 | 422 | −.4662 | −.9271 | −.4610 | .0095 | |||
Each outcome was analyzed in a separate model for each of the 4 additional independent variables. The dependent variables were standardized difference scores (Time 2 – Time 1). The models included fixed classification factors for Treatment (placebo versus active), one of the additional independent variables, the interaction of the 2 variables, and studies as levels of a random factor. The table presents results for 7 models, 4 for pain and 3 for physical function (there are no studies reporting the outcome for physical function that examine neuropathic pain).
Studies included only randomized, placebo controlled trials reporting sufficient data to allow for an estimation of effect size.
Least squares means from the model for placebo.
Least squares means from the model for active treatment.
Difference of the 2 least squares means (active – placebo). Larger negative differences are results in favor of the active treatment.
Test of the treatment effect for a given level of pain type, potency, short/long acting, or co-analgesic use.
Test of the interaction of treatment and either pain type, potency, short/long acting, or co-analgesic use; that is, the test of whether the treatment effect differs by level of the additional variable.
Results of Studies Conducting Head-to-Head Comparisons
Only three studies compared opioid therapy to another active treatment (either a non-steroidal or a tricyclic antidepressant).42,55,64 One found a nonsignificant difference in level of neuropathic pain reduction (effect size = 0.206) for long-acting morphine use versus tricyclic anti-depressant (nortriptylline or desipramine) therapy.64 Among patients with osteoarthritis, no difference in level of pain reduction was found (effect size = −0.066) between long-acting tramadol use and non-steroidal (diclofenac) therapy.42 Another study of osteoarthritis pain found that a weak opioid (propoxyphene) provided comparable analgesic efficacy to suprofen, but provided inadequate data to calculate an effect size.55
Results of Studies Examining the Effect of Age on Treatment Outcomes
Six studies29,30,47,53,56,60 included 788 subjects ages 65 and older and assessed for age effects. A meta-analysis could not be conducted because of data limitations. All six studies reported that analgesic efficacy was independent of age and documented significant pain reductions in both older (65 years and above) and younger (less than 65 years) study patients. Significant treatment effects in favor of opioid therapy for patients 65 years and above were also reported for other outcomes including physical functioning,47,53,56 sleep,47,56 and quality of life.47
Abuse/Misuse Outcomes
Four studies58,69-71 reported outcomes regarding opioid abuse/misuse. Three studies operationalized drug abuse/misuse as the presence of selected patient behaviors (e.g., seeking opioid prescriptions from multiple physicians, forging prescriptions, or reports of lost or stolen prescriptions, while the fourth required that an ICD code for opioid abuse or dependence be present in the patient’s medical record. One 36-month retrospective cohort study of 644 patients with osteoarthritis (mean age 63) found that 3% of participants demonstrated opioid abuse behaviors.58 In a study of 15,160 veterans receiving opioid medications, fewer than 1% of patients 60 years of age or older over versus 4% of those under 60 (p < 0.001) had a recorded diagnosis of opioid abuse or dependence.69 A prospective cohort study70 of 196 opioid-treated patients with chronic pain found that advancing age was associated with a lower likelihood of abuse/misuse (adjusted OR = 0.95; 95% CI = 0.90-0.99). Finally, a retrospective cohort study of 98 primary care patients with chronic pain,71 found that advancing age was also associated with a decreased likelihood of abuse/misuse behaviors (adjusted OR = 0.94; 95% CI = 0.89-0.99).
Adverse Events
Among opioid-treated patients, the most commonly reported adverse advents were constipation with a median frequency of occurrence of 30% (range 12–52%); nausea 28% (12–61%); dizziness 22% (10–37%); and somnolence 21% (10–39%). Occurrence rates for these outcomes were lower in the placebo control groups (Table 1). Most adverse events were rated by investigators as either ‘mildly’ or ‘moderately’ severe and all resolved after stopping the medication. Numbers need to harm and corresponding 95% confidence intervals were calculated for the most prevalent adverse events and included: nausea (5.9; 95% CI 4.5-7.3), constipation (6.3; 95% CI, 4.3-8.4) somnolence (8.6; 95% CI, 5.9-11.4) and dizziness (9.1; 95% CI, 6.3-11.9).
Only three studies assessed for possible age effects regarding adverse events.29,47,53 In one study,47 older (≥ 65 years) participants receiving opioid therapy were more likely to report constipation (28% vs. 17%, p < 0.001), fatigue (9% vs. 4%, p = 0.016), and anorexia (6% vs. 3%, p = 0.028), as compared to opioid-treated patients less than 65 years of age. In a second study,53 older patients receiving opioid therapy reported higher rates of somnolence (9% vs. 3%, no p value provided) and vomiting (13% vs. 7%, no p value provided) when compared to patients receiving treatment who were less than 65. In the third study,29 complaints of somnolence among patients 65 years of age and above were greater than for those younger than 65 (p = 0.02), but the study did not provide occurrence rates for either age group.
Discontinuation Rates
One in four opioid treated patients discontinued treatment due to an adverse event, with a median rate of discontinuation of 25% (range = 3–52%). Only 8% (2–24%) of participants receiving a placebo or comparator drug discontinued treatment on account of an adverse event. The median rate of withdrawal due to a lack of drug efficacy was 8% (0–47%) among opioid treated patients and 16% (0–67%) in the control patients.
Examination of Publication Bias
A funnel plot with 1/(standard error), a measure of sample size, plotted against effect size showed no clustering of studies in the lower-right of the funnel that would indicate lack of publication of smaller or nonsignificant studies (data not shown).
DISCUSSION
Among young-old patients (mean age across studies ranged from 60 to 73 years) without significant comorbidities, short-term use of opioids is associated with modest, but favorable effects on both pain and physical functioning. The observed effect sizes are comparable to those reported in other reviews1-4 of opioid analgesic effects in all age groups. Our results further suggest that the effects of treatment on pain may be enhanced among older individuals with neuropathic versus osteoarthritis-related pain. A recent systematic review4 demonstrated significant efficacy associated with opioid use (relative to placebo) for the treatment of neuropathic pain. Opioids are generally considered second-line agents for the treatment of neuropathic pain because of side effects and a paucity of evidence demonstrating long-term efficacy.72 Our result showing greater opioid-related pain reduction for neuropathic (vs. nociceptive) pain conditions should be regarded as preliminary, given the small number of studies (n=4) examining treatment outcomes among patients with neuropathic pain.
Opioid treatment was also associated with moderate (but non-significant) improvements in sleep, while physical quality of life (as measured by the SF-36) was not affected. A small, negative effect on mental health functioning was found. The clinical significance of this finding remains unclear and is in contrast to recent investigations of persons with chronic pain, which found either no effect1,73 or improved mood with treatment among the subgroup of patients that achieved good pain relief.54
In sensitivity analyses, use of high potency opioids was not associated with greater reductions in pain or physical disability relative to use of low-to-medium potency opioids. While long-acting (vs. short-acting) opioid formulations were found to have larger effect sizes for both pain and physical functioning, between group differences were not significant. Establishing the benefits of long versus short-acting opioid agents in older populations is needed, given that guidelines10,74 continue to recommend use of long-acting formulations, whereas clinicians continue to prescribe mostly short-acting agents for chronic pain in their older patients.21,75,76 Few studies reported on abuse/misuse outcomes, which is a particularly salient outcome as many clinicians cite concerns about potential patient addiction as a reason for not prescribing opioid therapy.20,77 Of the four studies retained in our sample, one58 reported a prevalence rate of 3%, while three69-71 found that advancing age was negatively associated with abuse/misuse behaviors. These results contrast with the higher prevalence of aberrant opioid medication taking behaviors (range = 5-24%) reported in one review of nonelderly chronic back pain patients.5 Before concluding that older adults are less likely to abuse/misuse opioids, additional research is needed given the short-term nature of most studies in our review and the fact that a sizeable majority excluded persons with a history of substance abuse, which is a recognized risk factor for opioid abuse.71
An important question in clinicians’ minds is whether opioid therapy is comparable or superior in terms of safety and efficacy when compared with non-opioid analgesic agents. Only three studies42,55,64 in our sample conducted such appraisals. These preliminary results suggest that short-term outcomes of opioid therapy are comparable to those obtained with either non-steroidal or tricyclic antidepressant therapy. What constitutes comparable analgesic therapy given increasing concerns17 about the safety of non-steroidal anti-inflammatory agents is an important and unresolved question. Comparative effectiveness studies are needed and could include evaluations of opioid use versus nonpharmacologic treatments to include complementary therapies.
A recently published guideline calls for minimizing use of non-steroidal analgesic agents in the treatment of chronic non-cancer pain in older adults, because of the significant risk profile associated with the use of these agents.17 The guideline recommends that clinicians consider opioid therapy for older patients who continue to report substantial pain or experience pain-related impairment in function.17 Research published prior to the release of the guideline indicates that as many as one in four older adults with chronic non-cancer pain already receive opioid therapy.21,78 Such a recommendation will likely translate into an increasing number of older adults who receive a course of opioid therapy for chronic non-cancer pain, providing strong support for a careful review of the evidence base regarding the efficacy and safety of this treatment approach among older adults.
Our study confirms an earlier report10 highlighting the paucity of pain treatment research focusing exclusively on older populations. Of the 40 treatment studies retained in this review, only 6 (15%) reported results on participants (n=788) ages 65 and above. All 6 studies (which excluded individuals with significant comorbidity) reported that outcomes were comparable in younger (less than 65 years of age) and older (65+) age groups. With few exceptions, adverse event rates were comparable. In a secondary analysis that included data from 18 studies in our sample, the degree of pain reduction did not vary as a function of participant mean age (range = 60–73 years), suggesting further that older adults may also obtain benefit from opioid therapy. Thus, available evidence suggests that adults ages 65 and above without significant comorbidity are equally likely to benefit from opioid therapy as younger adults with respect to pain reduction. There are currently insufficient data to determine whether and to what extent the positive treatment effects observed in the current study extend to important subgroups of older adults, including those with multiple comorbidities, functional impairment, cognitive deficits, as well as those taking multiple medications.
Additional study limitations associated with the retained articles include the following: First, studies followed patients for brief periods of time. Thus, the long-term effects of opioid use on pain, physical and metabolic function, and other relevant outcomes (e.g., likelihood of developing tolerance) remain to be determined. Second, most studies examined fixed doses of long-acting opioids, while prior studies21,76 suggest that physicians are more likely to prescribe short-acting agents on an as needed basis for patients with chronic non-cancer pain. The positive treatment effects observed in the current study may overestimate the ‘true’ benefits of opioid therapy given that the prescribing patterns of the studies correlate poorly with how these medications are actually prescribed in practice. Finally, 78% of the studies were sponsored by pharmaceutical companies, raising concerns about the possibility of reporting bias.
Limitations of this review include the possibility that our search strategy failed to identify all pertinent articles. However, a broad array of search terms and data bases was employed along with a careful review of the references from all retained articles in an attempt to eliminate this bias. In addition, most of the retained articles generated positive results, raising the question of possible publication bias, although there was no indication in a funnel plot of exclusion of smaller or nonsignificant studies.
In conclusion, the clinical management of older adults with chronic non-cancer pain disorders remains challenging, in part due to complex risk–benefit decisions that clinicians routinely face regarding pharmacologic interventions in this age group. Our findings support recommendations17,79 that short-term opioid trials are reasonable for older adults without comorbidity and either nociceptive or neuropathic pain. Once a decision is made to proceed with an opioid trial, frequent surveillance for ongoing attainment of therapeutic goals and adverse events is mandatory. Given that previous pain treatment studies enrolled few older adults and excluded those with significant comorbidity, it remains unclear whether older adults with multiple comorbidities or functional or cognitive impairment also benefit from such interventions. Future research on the long-term safety and efficacy of this treatment practice—with a particular focus on enrolling diverse groups of older adults with chronic non-cancer pain and ascertaining geriatric relevant outcomes (e.g., fall risk, ADL functioning, cognition, quality of life)—is now needed to improve the management of later-life pain.
ACKNOWLEDGMENTS
The Robert Wood Johnson Foundation’s Program of Research Integrating Substance Use in Mainstream Healthcare (PRISM) funded this study. This work was also supported by the John A. Hartford Foundation (Hartford Center of Excellence in Geriatric Medicine Award to Weill Cornell Medical College) and the Cornell-Columbia Translational Research Institute on Pain in Later Life: An Edward R. Roybal Center for Translational Research on (P30 AG22845-02) Award. This research project was supported by grants from the Robert Wood Johnson Foundation, the John A. Hartford Foundation and the National Institute on Aging.
Footnotes
Sponsor’s Role: The John A. Hartford Foundation and NIH had no role in designing, conducting, or reporting the study.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
REFERENCES
- 1.Kalso E, Edwards JE, Moore A, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: A meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2007;15:957–965. doi: 10.1016/j.joca.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Furlan AD, Sandoval JA, Mailis-Gagnon A, et al. Review: Opioids are more effective than placebo but not other analgesics for chronic noncancer pain. CMAJ. 2006;174:1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenberg E, McNichol ED, Carr DM. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin. JAMA. 2005;293:3043–3052. doi: 10.1001/jama.293.24.3043. [DOI] [PubMed] [Google Scholar]
- 5.Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: Opioid treatment for chronic back pain: Prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 6.Elliott AM, Smith BH, Penny KI, et al. The epidemiology of chronic pain in the community. Lancet. 1999;354:1248–1252. doi: 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- 7.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–431. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 8.Blyth FM, March LM, Brnabic AJM, et al. Chronic pain in Australia: A prevalence study. Pain. 2001;89:127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 9.Soldato M, Liperoti R, Landi F, et al. Non-malignant daily pain and risk of disability among older adults in home care in Europe. Pain. 2007;129:304–310. doi: 10.1016/j.pain.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 10.American Geriatrics Society Panel on Persistent Pain in Older Persons The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:S205–224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 11.Bryant LL, Grigsby J, Swenson C, et al. Chronic pain increases the risk of decreasing physical performance in older adults: The San Luis Valley Health and Aging Study. J Gerontol Med Sci. 2007;62:989–996. doi: 10.1093/gerona/62.9.989. [DOI] [PubMed] [Google Scholar]
- 12.Reid MC, Williams CS, Gill TM. Back pain and decline in lower extremity physical function among community-living older persons. J Gerontol Med Sci. 2005;60:793–797. doi: 10.1093/gerona/60.6.793. [DOI] [PubMed] [Google Scholar]
- 13.Scudds RJ, Robertson JM. Empirical evidence of the association between the presence of musculoskeletal pain and physical disability in community-dwelling senior citizens. Pain. 1998;75:229–235. doi: 10.1016/s0304-3959(97)00224-8. [DOI] [PubMed] [Google Scholar]
- 14.Bryant LL, Grigsby J, Swenson C, et al. Chronic pain increases the risk of decreasing physical performance in older adults: The San Luis Valley Health and Aging Study. J Gerontol Med Sci. 2007;62:989–996. doi: 10.1093/gerona/62.9.989. [DOI] [PubMed] [Google Scholar]
- 15.Leveille SG, Fried L, Guralnik JM. Disabling symptoms: What do older women report? J Gen Intern Med. 2002;17:766–773. doi: 10.1046/j.1525-1497.2002.20229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell GM, Schnitzer TJ. Cox-2 inhibitors and other nonsteroidal anti-inflammatory drugs in the treatment of pain in the elderly. Clin Geriatr Med. 2001;17:489–502. doi: 10.1016/s0749-0690(05)70082-3. [DOI] [PubMed] [Google Scholar]
- 17.American Geriatric Society Panel on the Pharmacological Management of Peristent Pain in Older Persons Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 18.Auret K, Schug SA. Underutilization of opioids in elderly patients with chronic pain: Approaches to correcting the problem. Drugs Aging. 2005;22:641–654. doi: 10.2165/00002512-200522080-00002. [DOI] [PubMed] [Google Scholar]
- 19.Upshur CC, Luckmann RS, Savageau JA. Primary care provider concerns about management of chronic pain in community clinic populations. J Gen Intern Med. 2006;21:652–655. doi: 10.1111/j.1525-1497.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799–803. doi: 10.1097/AJP.0b013e3181565cf1. [DOI] [PubMed] [Google Scholar]
- 21.Solomon DH, Avorn J, Wang PH, et al. Prescription opioid use among older adults with arthritis or low back pain. Arthr Rheum. 2006;55:35–41. doi: 10.1002/art.21697. [DOI] [PubMed] [Google Scholar]
- 22.Deshpande A, Furlan A, Mailis-Gagnon A, et al. Opioids for chronic low-back pain. Cochrane Database of Systematic Reviews. 2007;(3):CD004959. doi: 10.1002/14651858.CD004959.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomized studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferro MA, Speechley KN. Depressive symptoms among mothers of children with epilepsy: A review of prevalence, associated factors, and impact on children. Epilepsia. 2009;50:2344–2354. doi: 10.1111/j.1528-1167.2009.02276.x. [DOI] [PubMed] [Google Scholar]
- 26.Henderson CR., Jr Analysis of covariance in the mixed model: higher level, nonhomogeneous, and random regressions. Biometrics. 1982;38:623–640. [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Egger M, Smith G Davey, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roth SH, Fleischmann RM, Burch FX, et al. Around-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: Placebo-controlled trial and long-term evaluation. Arch Intern Med. 2000;160:853–860. doi: 10.1001/archinte.160.6.853. [DOI] [PubMed] [Google Scholar]
- 30.Malonne H, Coffiner M, Sonet B, et al. Efficacy and tolerability of sustained-release tramadol in the treatment of symptomatic osteoarthritis of the hip or knee: a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2004;26:1774–1782. doi: 10.1016/j.clinthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Peloso PM, Bellamy N, Bensen W, et al. Double blind randomized placebo control trial of controlled-release codeine in the treatment of osteoarthritis of the hip or knee. J Rheumatol. 2000;27:764–771. [PubMed] [Google Scholar]
- 32.Caldwell JR, Rapoport RJ, Davis JC, et al. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: Results from a randomized, placebo-controlled, double-blind trial and an open-label extension trial. J Pain Symptom Manage. 2002;23:278–291. doi: 10.1016/s0885-3924(02)00383-4. [DOI] [PubMed] [Google Scholar]
- 33.Markenson JA, Croft J, Zhang PG, et al. Treatment of persistent pain associated with osteoarthritis with controlled-release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain. 2005;21:524–535. doi: 10.1097/01.ajp.0000146215.86038.38. [DOI] [PubMed] [Google Scholar]
- 34.Loet XL, Pavelka K, Richarz U. Transdermal fentanyl for the treatment of pain caused by osteoarthritis of the knee or hip: an open, multicentre study. BMC Musculoskelet Dis. 2005;6:31. doi: 10.1186/1471-2474-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kjaersgaard-Andersen P, Nafei A, Skov O, et al. Codeine plus paracetamol versus paracetamol in longer-term treatment of chronic pain due to osteoarthritis of the hip.A randomized, double-blind, multi-centre study. Pain. 1990;43:309–318. doi: 10.1016/0304-3959(90)90028-C. [DOI] [PubMed] [Google Scholar]
- 36.Zautra AJ, Smith BW. Impact of controlled-release oxycodone on efficacy beliefs and coping Efforts among osteoarthritis patients with moderate to severe pain. Clin J Pain. 2005;21:471–477. doi: 10.1097/01.ajp.0000139910.97211.37. [DOI] [PubMed] [Google Scholar]
- 37.Choquette D, McCarthy TG, Rodrigues JF, et al. Transdermal fentanyl improves pain control and functionality in patients with osteoarthritis: An open-label Canadian trial. Clin Rheumatol. 2008;27:587–595. doi: 10.1007/s10067-007-0751-6. [DOI] [PubMed] [Google Scholar]
- 38.Langford R, McKenna F, Ratcliffe S, et al. Transdermal fentanyl for improvement of pain and functioning in osteoarthritis: A randomized, placebo-controlled trial. Arthritis Rheum. 2006;54:1829–1837. doi: 10.1002/art.21884. [DOI] [PubMed] [Google Scholar]
- 39.Hale M, Tudor IC, Khanna S, et al. Efficacy and tolerability of once-daily OROS hydromorphone and twice-daily extended-release oxycodone in patients with chronic, moderate to severe osteoarthritis pain: Results of a 6-week, randomized, open-label, noninferiority analysis. Clin Ther. 2007;29:874–888. doi: 10.1016/j.clinthera.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Kivitz A, Ma C, Ahdieh H, Galer BS. A 2-week, multicenter, randomized, double-blind, placebo-controlled, dose-ranging, phase III trial comparing the efficacy of oxymorphone extended release and placebo in adults with pain associated with osteoarthritis of the hip or knee. Clin Ther. 2006;28:352–364. doi: 10.1016/j.clinthera.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto AK, Babul N, Ahdieh H. Oxymorphone extended-release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: Results of a randomized, double-blind, placebo- and active-controlled phase III trial. Pain Med. 2005;6:357–366. doi: 10.1111/j.1526-4637.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 42.Beaulieu AD, Peloso PM, Haraoui B, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: A randomized controlled trial. Pain Res Manag. 2008;13:103–110. doi: 10.1155/2008/903784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleischmann RM, Caldwell JR, Roth SH, et al. Tramadol for the treatment of joint pain associated with osteoarthritis: A randomized, double-blind, placebo-controlled trial. Curr Ther Res. 2001;62:113–127. [Google Scholar]
- 44.Schnitzer TJ, Kamin M, Olson WH. Tramadol allows reduction of naproxen dose among patients with naproxen-responsive osteoarthritis pain: A randomized, double-blind, placebo-controlled study. Arthritis Rheum. 1999;42:1370–1377. doi: 10.1002/1529-0131(199907)42:7<1370::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 45.Babul N, Noveck R, Chipman H, et al. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: A randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Manage. 2004;28:59–71. doi: 10.1016/j.jpainsymman.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Karlsson M, Berggren AC. Efficacy and safety of low-dose transdermal buprenorphine patches (5,10, and 20 microg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: A 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clin Ther. 2009;31:503–513. doi: 10.1016/j.clinthera.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Vorsanger G, Xiang J, Jordan D, et al. Post hoc analysis of a randomized, double-blind, placebo-controlled efficacy and tolerability study of tramadol extended release for the treatment of osteoarthritis pain in geriatric patients. Clin Ther. 2007;29:2520–2535. doi: 10.1016/j.clinthera.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34:328–338. doi: 10.1016/j.jpainsymman.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Mongin G, Yakusevich V, Kope A, et al. Efficacy and safety of a novel once-daily tablet formulation of tramadol. Clin Drug Invest. 2004;24:545–558. doi: 10.2165/00044011-200424090-00005. [DOI] [PubMed] [Google Scholar]
- 50.Emkey R, Rosenthal N, Wu SC, et al. Efficacy and safety of tramadol/acetaminophen tablets (Ultracet) as add-on therapy for osteoarthritis pain in subjects receiving a COX-2 nonsteroidal antiinflammatory drug: a multicenter, randomized, double-blind, placebo-controlled trial. J Rheumatol. 2004;31:150–156. [PubMed] [Google Scholar]
- 51.Choi CB, Song JS, Kang YM, et al. A 2-week, multicenter, randomized, double-blind, double-dummy, add-on study of the effects of titration on tolerability of tramadol/acetaminophen combination tablet in Korean adults with knee osteoarthritis pain. Clin Ther. 2007;29:1381–1389. doi: 10.1016/j.clinthera.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Silverfield JC, Kamin M, Wu SC, et al. Tramadol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: A multicenter, outpatient, randomized, double-blind, placebo-controlled, parallel-group, add-on study. Clin Ther. 2002;24:282–296. doi: 10.1016/s0149-2918(02)85024-x. [DOI] [PubMed] [Google Scholar]
- 53.Rosenthal NR, Silverfield JC, Wu SC, et al. Tramadol/acetaminophen combination tablets for the treatment of pain associated with osteoarthritis flare in an elderly patient population. J Am Geriatr Soc. 2004;52:374–380. doi: 10.1111/j.1532-5415.2004.52108.x. [DOI] [PubMed] [Google Scholar]
- 54.Roth SH. Efficacy and safety of tramadol HCl in breakthrough musculoskeletal pain attributed to osteoarthritis. J Rheumatol. 1998;25:1358–1363. [PubMed] [Google Scholar]
- 55.Salzman RT, Brobyn RD. Long-term comparison of suprofen and propoxyphene in patients with osteoarthritis. Pharmacology. 1983;27(Suppl 1):55–64. doi: 10.1159/000137900. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki J, Weil AJ, Ross EL, et al. Effectiveness of polymer-coated extended-release morphine sulfate capsules in older patients with persistent moderate-to-severe pain: A subgroup analysis of a large, open-label, community-based trial. Cur Ther Res. 2007;68:137–150. doi: 10.1016/j.curtheres.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berliner MN, Giesecke T, Bornhovd KD. Impact of transdermal fentanyl on quality of life in rheumatoid arthritis. Clin J Pain. 2007;23:530–534. doi: 10.1097/AJP.0b013e318074c9b1. [DOI] [PubMed] [Google Scholar]
- 58.Ytterberg SR, Mahowald ML, Woods SR. Codeine and oxycodone use in patients with chronic rheumatic disease pain. Arthritis Rheum. 1998;41:1603–1612. doi: 10.1002/1529-0131(199809)41:9<1603::AID-ART10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 59.Ringe JD, Faber H, Bock O, et al. Transdermal fentanyl for the treatment of back pain caused by vertebral osteoporosis. Rheumatol Int. 2002;22:199–203. doi: 10.1007/s00296-002-0217-8. [DOI] [PubMed] [Google Scholar]
- 60.Peniston JH, Gould E. Oxymorphone extended release for the treatment of chronic low back pain: A retrospective pooled analysis of enriched-enrollment clinical trial data stratified according to age, sex, and prior opioid use. Clin Ther. 2009;31:347–358. doi: 10.1016/j.clinthera.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Watson CP, Moulin D, Watt-Watson J, et al. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain. 2003;105:71–78. doi: 10.1016/s0304-3959(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 62.Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain. 2008;12:804–813. doi: 10.1016/j.ejpain.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain. A randomized trial in post-herpetic neuralgia. Neurology. 1998;50:1837–1841. doi: 10.1212/wnl.50.6.1837. [DOI] [PubMed] [Google Scholar]
- 64.Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in post-herpetic neuralgia: A randomized, placebo-controlled trial. Neurology. 2002;59:1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- 65.Boureau F, Legallicier P, Kabir-Ahmadi M. Tramadol in post-herpetic neuralgia: A randomized, double-blind, placebo-controlled trial. Pain. 2003;104:323–331. doi: 10.1016/s0304-3959(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 66.Franco ML, Seoane A. Usefulness of transdermal fentanyl in the management of nonmalignant chronic pain: A prospective, observational, multicenter study. Pain Clinic. 2002;14:99–112. [Google Scholar]
- 67.Gatti A, Sabato AF, Carucci A, et al. Adequacy assessment of oxycodone/paracetamol (acetaminophen) in multimodal chronic pain: A prospective observational study. Clin Drug Investig. 2009;29(Suppl 1):31–40. doi: 10.2165/0044011-200929001-00005. [DOI] [PubMed] [Google Scholar]
- 68.Mariconti P, Collini R. Tramadol SR in arthrosic and neuropathic pain. Minerva Anesthesiol. 2008;74:63–68. [PubMed] [Google Scholar]
- 69.Edlund MJ, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Ives TJ, Chelminski PR, Hammett-Stabler CA, et al. Predictors of opioid misuse in patients with chronic pain: A prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reid MC, Engles-Horton LL, Weber MB, et al. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17:173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 73.Dillie KS, Fleming MF, Mundt MP, et al. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008;21:108–117. doi: 10.3122/jabfm.2008.02.070144. [DOI] [PubMed] [Google Scholar]
- 74.Kalso E, Allan L, Dellemijn PL, et al. Recommendations for using opioids in chronic non-cancer pain. Eur J Pain. 2003;7:381–386. doi: 10.1016/S1090-3801(02)00143-X. [DOI] [PubMed] [Google Scholar]
- 75.Won AB, Lapane KL, Vallow S, et al. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J Am Geriatr Soc. 2004;52:867–874. doi: 10.1111/j.1532-5415.2004.52251.x. [DOI] [PubMed] [Google Scholar]
- 76.Reid MC, Papaleontiou M, Olkhovskaya Y, et al. Should we be prescribing opioids for chronic pain in our older patients? J Am Geriatr Soc. 2008;(Suppl):S175. [Google Scholar]
- 77.Nwokeji ED, Rascati KL, Brown CM, et al. Influences of attitudes on family physicians’ willingness to prescribe long-acting opioid analgesics for patients with nonmalignant pain. Clin Therap. 2007;29(Suppl):589–602. doi: 10.1016/j.clinthera.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Barry LC, Gill T, Kerns RD, et al. Effective pain reduction strategies among community living older persons with chronic pain. J Gerontol Med Sci. 2005;60:1569–1575. doi: 10.1093/gerona/60.12.1569. [DOI] [PubMed] [Google Scholar]
- 79.Anonymous The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6–8. [PubMed] [Google Scholar]