Abstract
The Val158Met polymorphism of the catechol-O-methyltransferase (COMT) gene may be related to individual differences in cognition, likely via modulation of prefrontal dopamine catabolism. However, the available studies have yielded mixed results, possibly in part because they do not consistently account for other genes that affect cognition. We hypothesized that COMT Met allele homozygosity, which is associated with higher levels of prefrontal dopamine, would predict better executive function as measured using standard neuropsychological testing, and that other candidate genes might interact with COMT to modulate this effect. Participants were 95 healthy, right-handed adults who underwent genotyping and cognitive testing. COMT genotype predicted executive ability as measured by the Trail-Making Test, even after covarying for demographics and APOE, BDNF and ANKK1 genotype. There was a COMT-ANKK1 interaction in which individuals having both the COMT Val allele and the ANKK1 T allele showed the poorest performance. This study suggests the heterogeneity in COMT effects reported in the literature may be due in part to gene-gene interactions that influence central dopaminergic systems.
Keywords: cognition, neuropsychological tests, executive control, catechol-O-methyltransferase, polymorphism, epistasis
INTRODUCTION
The catechol-O-methyltransferase (COMT) enzyme plays an important role in regulating prefrontal dopamine levels. The common Val158Met polymorphism of the COMT gene predicts activity of this enzyme. Homozygosity for the COMT Met allele is associated with a several-fold decrease in enzymatic activity and dopamine catabolism relative to Val homozygosity, which is thought to result ultimately in increased availability of dopamine in prefrontal cortex (Dickinson & Elevag, 2009; Egan et al., 2001; Savitz, Solms, & Ramesar, 2006b).
Given the role of the Val158Met polymorphism in prefrontal dopamine catabolism, a number of studies have examined whether this polymorphism is related to variations in executive functioning in humans. The available studies have produced mixed results, possibly due at least partly to factors such as the nature of the cognitive phenotypes and executive sub-processes being studied, the nature and size of the participant populations, general intellectual ability, and ancestry (Barnett, Scoriels, & Munafo, 2008; Bilder, Volavka, Lachman, & Grace, 2004; Dickinson & Elevag, 2009; Goldberg et al., 2003; Savitz et al., 2006b; Tunbridge, Harrison, & Weinberger, 2006).
Studies examining effects of COMT have not consistently accounted for the potential effects of other genes that affect cognition, which may also contribute to the variability in the findings reported in the literature. Because a complex trait such as cognition likely has polygenic determinants, it is probable that other genetic variations interact with COMT to influence cognitive phenotypes (Dickinson & Elevag, 2009; Reuter et al., 2005; Stelzel, Basten, Montag, Reuter, & Fiebach, 2009; Tan et al., 2007; Xu et al., 2007).
In the present study, we examined effects of COMT Val158Met genotype on executive function in healthy adults, and tested for potential interacting effects of three common, well-studied genotype variations thought to influence cognition: Apolipoprotein E (APOE), brain-derived neurotrophic factor (BDNF) and ankyrin repeat and kinase domain containing 1 (ANKK1). There are three common APOE variations, ε2, ε3, and ε4, of which APOE ε4 is associated with increased risk of Alzheimer’s disease and with alterations in brain structure, function and cognition in healthy individuals and other populations ((Bookheimer & Burggren, 2009; Dardiotis et al., 2010; Parasuraman, Greenwood, & Sunderland, 2002; Reitz & Mayeux, 2009) but see (Savitz, Solms, & Ramesar, 2006a)). BDNF is a neurotrophin associated with neuronal resilience and survival, and with activity-dependent plasticity which is important in learning and memory. BDNF expression is widespread in the brain, particularly prefrontal cortex and hippocampus. The common BDNF Val66Met (rs6265) polymorphism has been associated with poorer memory and executive function, lower hippocampal and prefrontal gray matter volumes, and abnormal hippocampal activity in humans (Cowansage, LeDoux, & Monfils, 2010; Egan et al., 2003; Hariri et al., 2003; Savitz et al., 2006b). The polymorphism rs1800497 was originally thought to be part of the dopamine receptor D2 (DRD2) gene, but was later found to be located in the adjacent ANKK1 gene. Although the mechanism is unclear, the T (known historically as A1) allele of the 1800497 polymorphism has been associated with lower D2 receptor density in striatum and other brain regions, poorer cognitive outcome following traumatic brain injury, and various cognitive, psychiatric and behavioral effects in other populations (Dardiotis et al., 2010; McAllister et al., 2008; McAllister et al., 2005; Neville, Johnstone, & Walton, 2004; Ponce et al., 2009; Reuter et al., 2005; Reuter, Schmitz, Corr, & Hennig, 2006; Savitz et al., 2006b). We hypothesized that presence of the COMT Val allele would be associated with poorer cognition, as measured by the Trail-Making Test, a standardized neuropsychological measure of executive abilities. We further hypothesized there would be interacting effects of APOE, BDNF and ANKK1 genotype; specifically, that the presence of the APOE ε4, BDNF Met allele, or ANKK1 T allele, would be associated with a further reduction in cognitive performance. We also assessed other factors that could affect the relationship between COMT and cognition, including age, gender and baseline intellect.
METHODS
Participants
Ninety-five healthy adults, recruited via advertisements in local newspapers and workplaces, participated as healthy controls in a larger program of clinical research in our laboratory and had data available for this study. Inclusion criteria included age 18–85 years, at least 10 years of education or general equivalency degree, and fluency in English. Participants were of Northern European descent. Exclusion criteria included history of neurological disorder such as epilepsy, stroke and dementia; Axis I psychiatric disorder; or medical condition that can be associated with cognitive deficits, such as uncontrolled hypertension or insulin-dependent diabetes. Individuals with a history of traumatic brain injury causing a loss of consciousness exceeding five minutes were also excluded. Written informed consent was obtained according to procedures approved by the Dartmouth College Committee for the Protection of Human Subjects.
Genotyping
Genetic polymorphisms were analyzed from patient DNA isolated from peripheral blood samples using the Gentra Puregene Blood Kit (Qiagen; Valenica, CA). Allele status for APOE, BDNF (rs6265), COMT (rs4680) and ANKK1 (rs1800497) were determined by using TaqMan genotyping assays designed for use on the Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA) using allele specific primer and probes. Two single nucleotide polymorphisms (SNPs; 334 T/C and 472 C/T) were examined to determine APOE genotype as previously described (Koch et al., 2002). The APOE allotype is determined by the combination of SNPs at either one or both of these positions (334 and 472). BDNF (rs6265), COMT (rs4680) and ANKK1 (rs1800497) were genotyped using pre-designed allele-specific primer and probes from Applied Biosystems (Foster City, CA). Applied Biosystems TaqMan® SNP Genotyping Assays contain a pair of polymerase chain reaction oligonucleotide primers flanking the region of interest and one wild-type oligonucleotide probe fluorescently labeled with VIC and another probe for the mutation labeled with FAM. All primers and probes were used at final concentrations of 900 nM and 200 nM, respectively in 1X TaqMan® Universal PCR Master Mix (Applied Biosystems; Foster City, CA) with 5–20 ng of genomic DNA on a 7500 Fast Real-Time PCR System using allelic discrimination analysis with the 7500 Software v2.0 (Applied Biosystems).
Cognitive Testing
Participants completed the Trail Making Test (TMT, Reitan & Wolfson, 1993) or the Delis-Kaplan Executive Function System Trail Making subtest (DKEFS, Delis & Kaplan, 2001) as part of a larger battery of neuropsychological tests. These were the only executive measures available for the entire sample and were therefore selected for this study. Both TMT Part A and DKEFS Trial 2 require sequencing, psychomotor speed, and visuoperceptual ability while TMT Part B and DKEFS Trial 4 make additional demands on cognitive flexibility and working memory (Sanchez-Cubillo et al., 2009). For the purpose of this article, we use the terms “Trails A” and “Trails B” to refer to the two conditions for simplicity. Scores were z-transformed based on the appropriate published normative data to help account for age-related variance and so we could analyze the data for the two forms both separately and together (using test form as a covariate). The Barona formula, which uses demographic data such as age, education and area of residence to estimate baseline IQ, was employed as a measure of estimated baseline intellect (Barona, 1984).
RESULTS
Genotype distributions
The distribution of COMT genotypes in the participant sample was Met/Met (n = 26), Met/Val (n = 46) and Val/Val (n = 23). There were no significant differences among these groups in age, sex, education or estimated baseline IQ (all p > .05; Table 1). Thirty participants were APOE ε4 carriers; 33 were BDNF Met positive; and 34 were ANKK1 T positive. There were no significant relations between these genotypes and age, sex or education (all p > .05). All genotypes were consistent with proportions expected under Hardy-Weinberg Equilibrium (p>0.05). That is, the genotype frequencies (p2, 2pq, q2) were predicted from the allele frequencies (p, q) using the Hardy-Weinberg equation of p2 + 2pq +q2 = 1. Deviation from expected Hardy-Weinberg proportions can sometime indicate genotyping error or other population phenomena such as natural selection. In addition, each of the loci evaluated was in linkage equilibrium. That is, there was no significant correlation between the alleles at each of the loci suggesting that genetic information at each of the loci is statistically independent. The two versions of the TMT (Reitan and DKEFS) were evenly distributed across all four sets of genotype groups (all p > .05).
Table 1.
Group Characteristics.
| Met/Met (n=26) |
Val/Met (n=46) |
Val/Val (n=23) |
p | |
|---|---|---|---|---|
| Age (yrs.) | 54.5 (18.3) | 55.5 (18.6) | 46.9 (19.9) | NS |
| Education (yrs.) | 16.3 (1.9) | 16.2 (2.4) | 15.3 (2.9) | NS |
| Gender (M, F) | 6, 20 | 13, 33 | 9, 14 | NS |
| Barona Index | 115.4 (4.9) | 115.0 (5.6) | 112.5 (6.4) | NS |
| Trails A (z) | 0.97 (0.81) | 0.88 (0.83) | 0.66 (0.68) | NS |
| Trails B (z) | 1.33 (0.75) | 0.72 (0.63) | 0.87 (0.82) | 0.003 |
Simple correlations
Simple correlational analyses were performed to provide an initial assessment of relationships of genotype, demographic variables and test form to the Trails A and B results. There was no significant skewness in the dependent variables. In addition to COMT genotype, variables included age, sex, education, Barona score, TMT test form, and APOE, BDNF and ANKK1 genotype. COMT genotype, coded 1 for Val carriers and 0 for Met homozygotes in order to perform a point-biserial correlation, was related to Trails B (r = −0.33, p < 0.001) but not Trails A (r = −0.09, p = 0.18) in the combined sample of 95. This pattern remained the same, with a significant relationship between COMT genotype and Trails B performance but not Trails A, when analyzing the Reitan and DKEFS versions of the TMT separately (Reitan Trails B n=38, r = −0.41, p < 0.01; DKEFS Trails B n=57, r = − 0.28, p < 0.02). Across the entire sample, APOE, BDNF and ANKK1 genotypes did not show significant simple correlations with performance on either Trails A or Trails B (all p > 0.05). Of the remaining variables, there were negative correlations for age with performance on both Trails A and Trails B (both p < 0.01), and for test form and Barona score with Trails B performance (both p < .05). These variables were used as covariates in subsequent analyses.
COMT effects
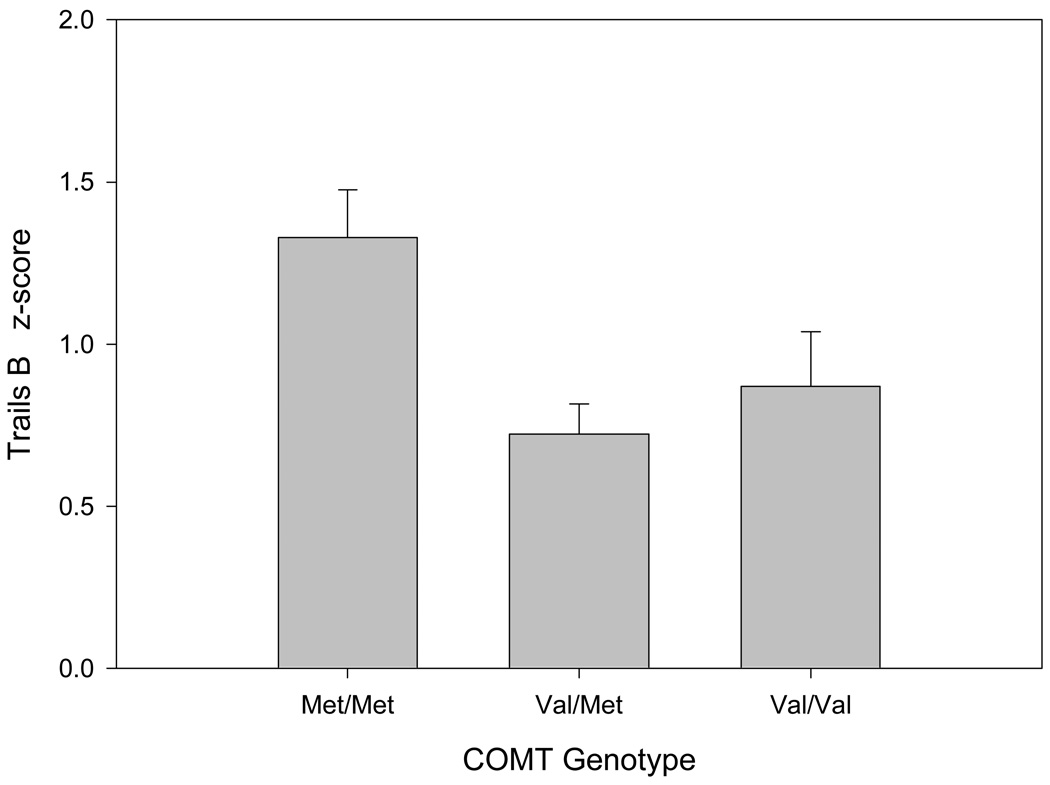
Analyses of covariance were conducted separately for Trails A and B data. Z-transformed data from the two test forms (Reitan, DKEFS) were combined and test form was used as a covariate in all analyses. There was an overall effect of COMT genotype, coded for Met homozygotes, heterozygotes, and Val homozygotes, on Trails B [F(2,91) = 6.24, p = 0.003, partial eta2 = 0.12, Fig. 1] but not Trails A [F(2,91) = 0.98, p = 0.38, partial eta2 = 0.02]. Simple pairwise comparisons revealed that COMT Met homozygotes performed better than the Val/Met (p = 0.001) and Val/Val groups (p = 0.03), who did not differ from each other (p > 0.05). The effect of COMT genotype on Trails B remained significant after additionally covarying for age, sex, education, and estimated baseline intellect (F(2,87) = 6.62, p = 0.002) and APOE, BDNF and ANKK1 genotype (F(2,88) = 6.89, p = 0.002). COMT genotype was unrelated to Trails A in all analyses (all p > .05).
Figure 1.
Relationship between COMT Val158Met genotype and Trail-Making Test B performance (means, standard error). Met homozygotes performed better than Val/Met and Val/Val individuals, who did not differ from each other.
Genetic interaction effects
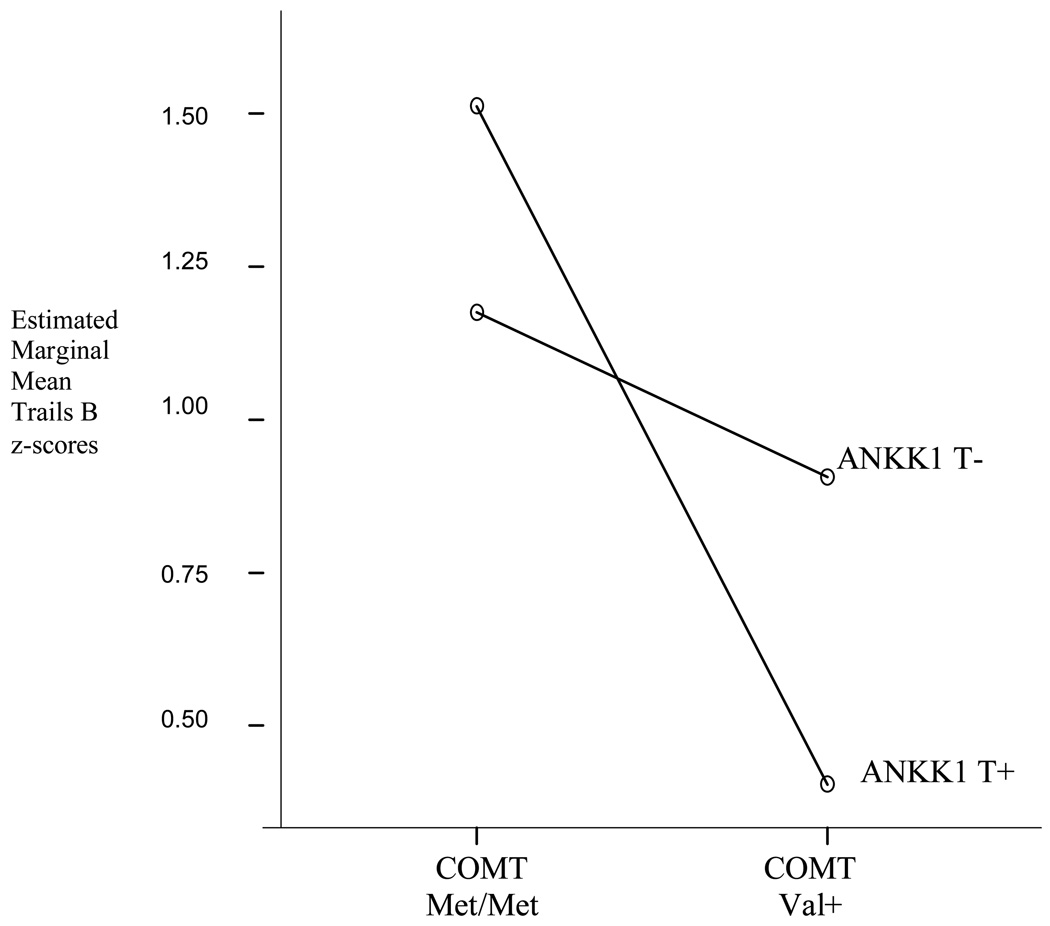
To assess hypothesized interactions between COMT and each of the three other candidate genotypes, separate two-way analyses of covariance were performed with COMT genotype as one independent variable and APOE, BDNF or ANKK1 genotype as the other. Given our directional a priori hypotheses about the potential effects of each genotype, we did not employ corrections for multiple comparisons for these analyses. Genotypes were coded dichotomously to maximize cell sizes. Covariates were age, sex, education, Barona score, and test form. In all three analyses, the same pattern emerged showing a main effect of COMT genotype on Trails B and no significant effect on Trails A. There were no significant interaction effects for COMT with APOE or BDNF genotype (p > .05). There was a significant interaction of COMT and ANKK1 for Trails B (F(1, 86) = 6.60, p = 0.01, partial eta2 = 0.07); individuals having both the T allele of ANKK1 and the Val allele of COMT showed the lowest performance (Table 2; Fig. 2). To provide a basic test for possible demographic interactions, we also divided the sample into approximately equal numbers of subjects in older (≥ 60 years, n=49) and younger (< 60 years, n=46) groups. There were no significant interactions of COMT genotype with either sex or age group (p > 0.05).
Table 2.
Genotype Interactions: Means and standard deviations for Trail Making Test z-scores.
| COMT Met Homozygotes | COMT Val Carriers | ||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | ||
| APOE E− | APOE E+ | APOE E− | APOE E+ | ||
| n | 17 | 9 | 48 | 21 | |
| Trails A | 0.88 (0.82) | 1.15 (0.82) | 0.81 (0.89) | 0.80 (0.48) | NS |
| Trails B | 1.17 (0.76) | 1.62 (0.68) | 0.80 (0.67) | 0.70 (0.76) | p < .001 a |
| BDNF Met− | BDNF Met+ | BDNF Met− | BDNF Met+ | ||
| n | 18 | 8 | 44 | 25 | |
| Trails A | 0.81 (0.80) | 1.33 (0.76) | 0.84 (0.77) | 0.75 (0.82) | NS |
| Trails B | 1.22 (0.83) | 1.56 (0.48) | 0.75 (0.72) | 0.81 (0.66) | p < .001 a |
| ANKK1 T− | ANKK1 T+ | ANKK1 T− | ANKK1 T+ | ||
| n | 12 | 14 | 49 | 20 | |
| Trails A | 0.80 (0.89) | 1.13 (0.73) | 0.82 (0.78) | 0.77 (0.81) | NS |
| Trails B | 1.21 (0.81) | 1.43 (0.71) | 0.85 (0.65) | 0.57 (0.77) | p = .01 b |
Main Effect: COMT Met Homozygotes > COMT Val Carriers
Interaction Effect: Group 2 = Group 1 > Group 3 > Group 4
Figure 2.
COMT – ANKK1 interaction. Individuals having both the Val allele of the COMT gene and the T allele of the ANKK1 gene showed the lowest performance. Estimated marginal means from the covariance analysis are presented in this figure. Raw mean values are shown in Table 2.
DISCUSSION
COMT Met homozygotes performed better than Val carriers on Trails B, a neuropsychological measure of executive function. This effect was independent of age, gender, education, estimated baseline intellect, and APOE and BDNF genotype. An interaction of COMT and ANKK1 was observed; individuals having both the T allele of ANKK1 and the Val allele of COMT showed the lowest cognitive performance. This finding suggests that neuropsychological testing is a useful endophenotype for research on the genetics of individual differences in cognition in a healthy adult sample.
The finding of a COMT-ANKK1 interaction suggests that genetic variations related to central dopaminergic systems may act together to influence individual differences in executive abilities. Other gene interactions and effects on other neurotransmitter systems are also possible. Specifically, COMT is also a major catabolic enzyme for norepinephrine and epinephrine, and there are noradrenergic projections to the entire cerebral cortex and other brain regions (Briand, Gritton, Howe, Young, & Sarter, 2007; Guldberg & Marsden, 1975). More generally, the finding of a COMT-ANKK1 interaction serves to highlight the issue of considering gene-gene interactions in cognitive genetics research. Prior research on the effects of COMT Val158Met genotype on cognition has yielded mixed results [for summary (Barnett et al., 2008)] possibly due at least partly to genetic interactions, among other factors (Dickinson & Elevag, 2009). In addition to interactions with other genes, there is within gene variability to consider. COMT haplotypes and polymorphisms other than Val158Met may be relevant, and are of considerable interest for further hypothesis-driven selection of genotype predictors of cognition (Barnett, Scoriels, & Munafo, 2009; Funke et al., 2005; Meyer-Lindenberg et al., 2006; Nackley et al., 2006).
It has been suggested that the relationship between COMT and various cognitive abilities may in fact be due to effects of COMT on general intellectual ability. However, covarying for estimated baseline intellect did not diminish the relationship between genotype and cognitive performance in our sample. This suggests some specificity of the relationship, though concurrent intelligence testing would be necessary to fully evaluate that possibility. It should also be noted that mean estimated baseline intellect was in the high average range in our sample, which could alter the extent to which variations in intellect could play a role in moderating the relationship between genotype and executive function. An additional point with regards to the cognitive specificity of the finding is that Trails B draws on a range of abilities, including visuoperceptual ability, psychomotor speed, sequencing, set-shifting and working memory (Sanchez-Cubillo et al., 2009) and our data alone are insufficient to distinguish which aspect or aspects drove the genotype-phenotype relationships in this case. While we refer to the phenotype examined in this study as “executive” ability, this is a broadly defined version of that category of neuropsychological function.
There are several theories regarding effects of COMT genotype on cognition, including the “inverted U” hypothesis and the tonic-phasic hypothesis (Bilder et al., 2004; Tunbridge et al., 2006). The “inverted U” hypothesis suggests that when dopamine levels are either too high or too low, cognition is adversely affected. Where on this continuum healthy adults with different Val158Met genotypes may fall is uncertain. The tonic-phasic hypothesis posits that the Met allele is associated with higher cortical D1 transmission, higher tonic subcortical dopamine levels, and lower phasic subcortical dopamine, resulting in greater stability of cognitive processing at the cost of decreased flexibility (Bilder et al., 2004). The Trail-Making Test, like most if not all neuropsychological tests, makes multiple cognitive demands (Sanchez-Cubillo et al., 2009) and studies using tests constructed specifically to distinguish cognitive stability and flexibility are important for directly testing the tonic-phasic hypothesis (Colzato, Waszak, Nieuwenhuis, Posthuma, & Hommel, 2010; Dickinson & Elevag, 2009).
The ANKK1 polymorphism we tested, rs1800497, is a non-synonymous coding polymorphism that was originally thought to be in the adjacent DRD2 gene. Though located in ANKK1, this polymorphism is in linkage disequilibrium with many polymorphisms in DRD2 and may be a marker of alterations in one or both genes (McAllister et al., 2008; Ponce et al., 2009) (Entrez Gene, ncbi.nlm.nih.gov, 2010). Rs1800497 has been associated with a wide array of psychiatric disorders and personality characteristics (Ponce et al., 2009) and though the results vary across studies, there is an emerging literature on potential interacting effects of ANKK1 and COMT genotype on cognitive outcomes in humans (e.g., Reuter et al., 2005; Stelzel et al., 2009; Xu et al., 2007). Our group has previously investigated rs1800497 as a predictor of cognitive outcome following mild traumatic brain injury, given its relationship to D2 receptor expression (McAllister et al., 2008; McAllister et al., 2005). In these studies, the T allele was associated with poorer cognitive outcome and a haploblock of polymorphisms in ANKK1 was identified that may act in concert. In future larger scale research, it would be valuable to examine both ANKK1 and COMT haplotypes alone and in combination.
Limitations of the present study include the small sample size; although statistically significant effects were detected in this sample, additional effects might be found in a larger study. Therefore, despite the lack of interaction effects for COMT with APOE and BDNF in this study, larger studies might uncover interaction effects for these genes. Additionally, only a limited number of genotype variations could be tested with this size of sample. We chose to study common polymorphisms for which there was ample research on the basis of which to formulate a priori hypotheses. Very large samples are needed to employ more exploratory or discovery-oriented computational genetics approaches to analyzing genome-wide data, and the risk of false positive findings rises as more genes are examined. A combination of hypothesis-driven and computational approaches may be optimal at this stage of development of neuropsychological genetics research - to advance beyond the single gene approach while avoiding underpowering interaction analyses. Moving to even larger collaborative samples will also permit a higher degree of investigation of genetic interactions.
Another limitation of our study is the large age range of the participants; although we used statistical approaches to control for and investigate age effects, the large age range contributed heterogeneity in our sample and studies with a narrower age range will be necessary to further investigate the potential interaction of COMT and ANKK1 genotypes. Additionally, although we also used statistical approaches to deal with the fact that we had data from two different versions of the Trail-Making Test, overall, more homogeneous, specific, and comprehensive approaches to phenotyping executive function will be advantageous in future research.
In summary, we observed that COMT and ANKK1 genotypes interacted to account for variance in executive function, as measured broadly using the Trail-Making Test, in healthy cognitively intact adults. This supports the value of continued efforts to employ neuropsychological endophenotypes to improve scientific understanding of the role of these and other polymorphisms in individual differences in cognition. Further work integrating structural and functional imaging with neuropsychological measures (Cerasa et al., 2008; Tan et al., 2007; Zinkstok et al., 2006) will help clarify the neural mechanisms by which COMT, ANKK1 and other genetic variations influence individual differences in cognition.
ACKNOWLEDGMENTS
Supported in part by the National Institute of Neurological Disorders and Stroke (K23NS045830, R03NS056228, RO1 NS 40472), National Multiple Sclerosis Society (RG3248, PP1214), Hitchcock Foundation, National Institute on Aging (R01AG019771), National Institute on Disability and Rehabilitation Research (H-133670031, H133G000136) and Ira DeCamp Foundation.
Footnotes
No conflicts exist.
References
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biological Psychiatry. 2008;64(2):137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafo MR. Reply to Goldman. Biological Psychiatry. 2009;65:e3–e4. [Google Scholar]
- Barona A, Reynolds C, Chastain R. A demographically based index of pre-morbid intelligence for the WAIS-R. Journal of Consulting & Clinical Psychology. 1984;52(5):885–887. [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bookheimer S, Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer's and cognitive aging. Annual Review of Clinical Psychology. 2009;5:343–362. doi: 10.1146/annurev.clinpsy.032408.153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Progress in Neurobiology. 2007;83(2):69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Labate A, Liguori M, Lanza P, Quattrone A. Impact of catechol-O-methyltransferase Val (108/158) Met genotype on hippocampal and prefrontal gray matter volume. Neuroreport. 2008;19(4):405–408. doi: 10.1097/WNR.0b013e3282f5f784. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Waszak F, Nieuwenhuis S, Posthuma D, Hommel B. The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: evidence for a role of dopamine in the control of task-switching. Neuropsychologia. 2010;48(9):2764–2768. doi: 10.1016/j.neuropsychologia.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils M-H. Brain-derived neurotrophic factor: A dynamic gate-keeper of neural plasticity. Current Molecular Pharmacology. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Dardiotis E, Fountas KN, Dardioti M, Xiromerisiou G, Kapsalaki E, Tasiou A, Hadjigeorgiou GM. Genetic association studies in patients with traumatic brain injury. Neurosurgical Focus. 2010;28(1):E9. doi: 10.3171/2009.10.FOCUS09215. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E. Delis-Kaplan Executive Function Battery. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Dickinson D, Elevag B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Weinberger DR. The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Funke B, Malhotra AK, Finn CT, Plocik AM, Lake SL, Lencz T, Kucherlapati R. COMT genetic variation confers risk for psychotic and affective disorders: a case control study. Behavioral & Brain Functions. 2005;1:19. doi: 10.1186/1744-9081-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacological Reviews. 1975;27(2):135–206. [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor Val66Met polymorphism affects human memory-related hippocampal activity and predicts memory performance. The Journal of Neuroscience. 2003;23(17):6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W, Ehrenhaft A, Griesser K, Pfeufer A, Muller J, Schomig A, Kastrati A. TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clinical Chemistry & Laboratory Medicine. 2002;40(11):1123–1131. doi: 10.1515/CCLM.2002.197. [DOI] [PubMed] [Google Scholar]
- McAllister T, Flashman LA, Rhodes CH, Tyler AL, Moore JH, Saykin A, Tsongalis GJ. Single nucleotide polymorphisms in ANKK1 and the dopamine D2 receptor gene affect cognitive outcome shortly after traumatic brain injury: A replication and extension study. Brain Injury. 2008;22(9):705–714. doi: 10.1080/02699050802263019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Rhodes CH, Flashman LA, McDonald BC, Belloni D, Saykin AJ. Effect of the dopamine D2 receptor T allele on response latency after mild traumatic brain injury. American Journal of Psychiatry. 2005;162(9):1749–1751. doi: 10.1176/appi.ajp.162.9.1749. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott J, Ding J, Kolachana B, Buckholtz J, Weinberger D. Impact of complex genetic variation in COMT on human brain function. Molecular Psychiatry. 2006;11:867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314(5807):1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23(6):540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Sunderland T. The apolipoprotein E gene, attention, and brain function. Neuropsychology. 2002;16(2):254–274. doi: 10.1037//0894-4105.16.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce G, Perez-Gonzalez R, Aragues M, Rodriguez-Jimenez R, Jiminez-Arriero M, Hoenicka J. The ANKK1 kinase gene and psychiatric disorders. Neurotoxicology Research. 2009;16:50–59. doi: 10.1007/s12640-009-9046-9. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. 2 ed. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- Reitz C, Mayeux R. Endophenotypes in normal brain morphology and Alzheimer's disease: A review. Neuroscience. 2009;164:174–190. doi: 10.1016/j.neuroscience.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Peters K, Schroeter K, Koebke W, Lenardon D, Bloch B, Hennig J. The influence of the dopaminergic system on cognitive functioning: A molecular genetic approach. Behavioural Brain Research. 2005;164(1):93–99. doi: 10.1016/j.bbr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmitz A, Corr P, Hennig J. Molecular genetics support Gray's personality theory: the interaction of COMT and DRD2 polymorphisms predicts the behavioural approach system. International Journal of Neuropsychopharmacology. 2006;9(2):155–166. doi: 10.1017/S1461145705005419. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society. 2009;15(3):438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. Apolipoprotein E variants and cognition in healthy individuals: a critical opinion. Brain Research Reviews. 2006a;51(1):125–135. doi: 10.1016/j.brainresrev.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes, Brain and Behavior. 2006b;5(4):311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Effects of dopamine-related gene-gene interactions on working memory component processes. European Journal of Neuroscience. 2009;29(5):1056–1063. doi: 10.1111/j.1460-9568.2009.06647.x. [DOI] [PubMed] [Google Scholar]
- Tan H-Y, Chen Q, Sust S, Buckholtz JW, Meyers JD, Egan MF, Callicott JH. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(30):12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60(2):141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Xu H, Kellendonk CB, Simpson EH, Keilp JG, Bruder GE, Polan HJ, Gilliam TC. DRD2 C957T polymorphism interacts with the COMT Val158Met polymorphism in human working memory ability. Schizophrenia Research. 2007;90(1–3):104–107. doi: 10.1016/j.schres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, Schmitz N, van Amelsvoort T, de Win M, van den Brink W, Baas F, Linszen D. The COMT val158met polymorphism and brain morphometry in healthy young adults. Neuroscience Letters. 2006;405(1–2):34–39. doi: 10.1016/j.neulet.2006.06.034. [DOI] [PubMed] [Google Scholar]