Abstract
Objectives To follow up a UK national cohort of women admitted to hospital with confirmed 2009/H1N1 influenza in pregnancy in order to obtain a complete picture of pregnancy outcomes and estimate the risks of adverse fetal and infant outcomes.
Design National cohort study.
Setting 221 hospitals with obstetrician led maternity units in the UK.
Participants 256 women admitted to hospital with confirmed 2009/H1N1 in pregnancy during the second wave of pandemic infection between September 2009 and January 2010; 1220 pregnant women for comparison.
Main outcome measures Rates of stillbirth, perinatal mortality, and neonatal mortality; odds ratios for infected versus comparison women.
Results Perinatal mortality was higher in infants born to infected women (10 deaths among 256 infants; rate 39 (95% confidence interval 19 to 71) per 1000 total births) than in infants of uninfected women (9 deaths among 1233 infants; rate 7 (3 to 13) per 1000 total births) (P<0.001). This was principally explained by an increase in the rate of stillbirth (27 per 1000 total births v 6 per 1000 total births; P=0.001). Infants of infected women were also more likely to be born prematurely than were infants of comparison women (adjusted odds ratio 4.0, 95% confidence interval 2.7 to 5.9). Infected women who delivered preterm were more likely to be infected in their third trimester (P=0.046), to have been admitted to an intensive care unit (P<0.001), and to have a secondary pneumonia (P=0.001) than were those who delivered at term.
Conclusions This study suggests an increase in the risk of poor outcomes of pregnancy in women infected with 2009/H1N1, which reinforces the message from studies of maternal risk alone. The health of pregnant women is an important public health priority in future waves of this and other influenza pandemics.
Introduction
When the influenza A/H1N1 2009 virus (2009/H1N1) reached pandemic status in June 2009, particular concerns were voiced about the effect of infection on women during pregnancy after initial reports from the United States.1 Further investigations, including through the UK Obstetric Surveillance System (UKOSS),2 highlighted specific groups of women who were at higher risk of morbidity after infection with 2009/H1N1 in pregnancy. Factors associated with admission to hospital with 2009/H1N1 in pregnancy include maternal obesity, asthma, multiparity, multiple pregnancy, black or other minority group ethnicity, and smoking among women younger than 25 years.2 3 4 Admission to an intensive care unit, taken as a proxy for severe morbidity, was associated with a delay in starting treatment with antiviral drugs (more than two days after the onset of symptoms) and maternal obesity.2 3 4 5 6
Analyses of the effects of 2009/H1N1 in pregnancy have so far focused primarily on maternal morbidity and mortality, partly because of the immediacy of the pandemic and the rapidity with which descriptions of case series were published. Documented follow-up of women after their original admission to hospital is uncommon, so the effect of infection on the outcome of pregnancy has not been fully investigated. Some evidence from previous pandemics suggests that pregnancies after influenza infection are more likely to end in a stillbirth or an early neonatal death,7 8 and women with a secondary pneumonia infection are more likely to deliver preterm.9 Studies of the effects of seasonal influenza have not been conclusive, but some evidence exists of an increase in the risk of congenital anomalies.10
The aim of this study was to follow up women admitted to hospital with confirmed 2009/H1N1 during the second wave of pandemic infection between September 2009 and January 2010 to obtain a complete picture of their pregnancy outcomes and assess the risk of adverse fetal and infant outcomes in association with possible causative factors.
Methods
We compared the outcomes of pregnancy among a cohort of women admitted to hospital with 2009/H1N1 infection with those of an unexposed cohort of women who delivered in the United Kingdom before the start of the pandemic, and, where available, with the most recent national data. In addition, we analysed the infected cohort to identify characteristics of infection associated with preterm birth (gestational age at delivery less than 37 weeks) after 2009/H1N1 infection in pregnancy.
Data sources and definitions
We defined the exposed cohort as pregnant women admitted to hospital with 2009/H1N1 infection between 1 September 2009 and 31 January 2010. We identified the exposed cohort prospectively through the UKOSS network of collaborating clinicians,11 using a web based reporting system for rapid response. The UKOSS network of clinicians includes between one and four nominated reporting clinicians (obstetricians, midwives, and anaesthetists) in each hospital in the UK with an obstetrician led maternity unit. The UKOSS network was set up to provide a routine system through which to conduct a programme of parallel studies of severe and uncommon complications of pregnancy throughout the UK. The nominated reporting clinicians coordinate case reports from all clinicians in their unit. For this study, we asked clinicians to report all pregnant women with confirmed 2009/H1N1 infection admitted to their unit, using a web based rapid reporting system, as soon as possible after the woman’s admission and whether or not the woman had delivered. In response to a report of a case on the web based system, clinicians were able to download a data collection form with a unique UKOSS identification number, asking for further detailed information about diagnosis, management, and outcomes. If a completed data collection form was not returned, up to three reminders were sent.
In addition, every two weeks, we sent nominated UKOSS reporting clinicians a summary detailing the cases that had been reported from their unit and asked them to confirm that no additional cases existed. We also asked clinicians to return a “nil report” indicating that no women had been admitted, so that we could monitor participation and confirm the denominator population for the study.
We double entered all data into a customised database. We checked cases to confirm that they met the case definition and to exclude duplicate reports. We excluded from the analysis women who did not have laboratory confirmed 2009/H1N1 or who delivered before infection. Where data were missing or the response invalid, we contacted the reporting clinician by email and asked for the correct information. If the woman had an ongoing pregnancy at the time of discharge after her 2009/H1N1 infection, we sent a copy of the initial data collection form to the reporting clinician two weeks after the expected date of delivery to obtain the pregnancy outcome data. We collected 48% of outcome information prospectively in this manner. All information collected was de-identified.
We derived a comparison cohort of women from data, previously collected between February 2005 and February 2006 using the UKOSS methods, on women who delivered in UK hospitals between those dates, before the 2009/H1N1 pandemic, and who therefore represented an uninfected cohort. UKOSS reporters identified the comparison women as the two women delivering in the same hospital immediately before women delivering with other conditions (antenatal pulmonary embolism, eclampsia, and peripartum hysterectomy) under study through UKOSS at the time.12 We chose this cohort for pragmatic reasons to facilitate rapid comparisons during the epidemic and, as a historical cohort, to ensure that none of the women could have been infected with 2009/H1N1. For both exposed and unexposed women, we collected data on maternal demographic characteristics and characteristics of pregnancy, as well as maternal and infant outcomes. For the infected cohort, we collected additional data on disease presentation and management.
National comparison information came from routinely collected national data sources for England, Wales, Scotland, and Northern Ireland for 2008.13 14 15 When routine UK data were unavailable—for example, gestational age or congenital anomaly statistics—we used data from the maternity hospital episode statistics for England or the British Isles Network of Congenital Anomalies Registers (BINOCAR).16 17
We defined total births as stillbirths plus live births, perinatal mortality as stillbirths plus deaths after 24 weeks’ gestation and up to one week after delivery, and neonatal mortality as deaths up to one month after delivery. We classified terminations of pregnancy, fetal deaths, and miscarriages up to 24 weeks together as losses up to 24 weeks’ gestation. We calculated rates of stillbirth, perinatal mortality, and congenital anomaly per 1000 total births. We calculated neonatal mortality rates per 1000 live births. We calculated gestational age and delivery statistics per maternity (that is, a twin pregnancy equals one maternity); otherwise, the unit of interest was a birth.
Statistical analysis
We used Stata 11SE software for all analyses. We compared outcomes of pregnancy in women after 2009/H1N1 infection with those in unexposed women. For binary outcomes, we used logistic regression to estimate odds ratios as a measure of association between maternal 2009/H1N1 infection and outcome. For birth weight, we used linear regression to measure the change in mean value associated with being infected. In view of the rarity of the pregnancy outcomes considered, we used national data where available as a comparator to improve the precision of our estimated odds ratios and to gauge the representativeness of the comparison group data. We used Wald statistics to calculate 95% confidence intervals and P values. We compared characteristics between women infected before 37 weeks who delivered preterm and at term by using the χ2 test for association, Fisher’s exact test, or the Wilcoxon rank sum test as appropriate.
We made adjustments in the regression models comparing the exposed and unexposed cohorts for the a priori confounders of socioeconomic status (professional/unemployed/other), ethnicity (white/other), parity (primiparous/multiparous), smoking (during pregnancy/not during pregnancy), age of mother, body mass index, and multiple pregnancy. We included in the model variables previously identified as risk factors for admission with 2009/H1N1 in pregnancy and those known to be associated with adverse outcomes of pregnancy. To allow for the non-independence of infants from multiple births, all models included an option to specify that the calculated standard errors allow for within group correlation.
Outcome data were complete for 98% of women, but 25% of women had information missing for one or more covariates (table 1). Data were mostly missing for the single variable of socioeconomic status. Therefore, we used multiple imputation methods to incorporate all available data and reduce potential bias. We generated 20 imputed datasets by using the Stata ICE procedure,18 using all the a priori confounders listed above as well as exposure status, birth weight, preterm birth, and delivery by caesarean section to generate imputed values. We joined the datasets by using Rubin’s rules to obtain combined imputation estimates and standard errors.19 We compared the estimated odds ratios obtained through multiple imputation analysis with estimates obtained by using only information from women with complete data (“complete case” method). In addition, we did a sensitivity analysis to see how far the odds ratios might deviate towards a null finding under a missing not at random mechanism, by assuming that all infants born to infected women not followed up were born at term, with normal birth weight (2500 g or over), after vaginal delivery, and alive after one month. For simplicity, we have reported adjusted results after multiple imputation analysis throughout in the text but included in tabular format a comparison of the results from complete case, multiple imputation, and multiple imputation with sensitivity analysis methods.
Table 1.
Frequencies (percentages) of missing data, for participants with outcome data
| Variable | Infected cohort (n=256) | Uninfected comparison cohort (n=1220) | Total (n=1476) |
|---|---|---|---|
| Socioeconomic group: | |||
| Managerial and professional occupations | 50 (20) | 333 (27) | 383 (26) |
| Other employed | 101 (39) | 608 (50) | 709 (48) |
| Unemployed/student | 28 (11) | 156 (13) | 184 (12) |
| Missing | 77 (30) | 123 (10) | 200 (14) |
| Booking body mass index (kg/m2): | |||
| <30 | 178 (70) | 867 (71) | 1045 (71) |
| ≥30 | 63 (25) | 202 (17) | 265 (18) |
| Missing | 15 (6) | 151 (12) | 166 (11) |
| Ethnicity: | |||
| White | 197 (77) | 973 (80) | 1170 (79) |
| Other | 57 (22) | 217 (18) | 274 (19) |
| Missing | 2 (1) | 30 (2) | 32 (2) |
| Smoking status: | |||
| Did not smoke during pregnancy | 195 (76) | 937 (77) | 1132 (77) |
| Smoked during pregnancy | 59 (23) | 257 (21) | 316 (21) |
| Missing | 2 (1) | 26 (2) | 28 (2) |
| Parity: | |||
| Primiparous | 96 (38) | 523 (43) | 619 (42) |
| Multiparous | 158 (62) | 694 (57) | 852 (58) |
| Missing | 2 (1) | 3 (0.3) | 5 (0.3) |
| Age (years): | |||
| <20 | 26 (10) | 61 (5) | 87 (6) |
| 20-34 | 198 (77) | 894 (73) | 1092 (74) |
| ≥35 | 32 (13) | 264 (22) | 296 (20) |
| Missing | 0 (0) | 1 (0.1) | 1 (0.1) |
| Multiple pregnancy: | |||
| No | 251 (98) | 1207 (99) | 1458 (99) |
| Yes | 5 (2) | 13 (1) | 18 (1) |
| Missing | 0 (0) | 0 (0) | 0 (0) |
| Any item of data missing: | |||
| No | 163 (64) | 947 (78) | 1110 (75) |
| Yes | 93 (36) | 273 (22) | 366 (25) |
Results
Of 223 UK hospitals with specialist obstetrician led maternity units, 221 (99%) participated in the study. In total, 272 women were admitted to hospital with confirmed 2009/H1N1; outcome data were reported for 256 (94%) infected women. These women had 249 live births, including five pairs of twins (table 2). In addition, five pregnancies were lost or terminated before 24 weeks’ gestation.
Table 2.
Outcome of pregnancy for women admitted to hospital with 2009/H1N1 infection (infected cohort) and uninfected women (comparison cohort)
| Outcome | No (%) | Odds ratio (95% CI) | National data, 2008 | |||||
|---|---|---|---|---|---|---|---|---|
| Infected cohort (n=256) | Comparison cohort (n=1220) | Unadjusted | Adjusted* | No (%) | Unadjusted odds ratio (95% CI) | |||
| Outcome of pregnancy: | ||||||||
| Live birth† | 249 (95) | 1226 (99) | 1 | 1 | 795 004 (99) | 1 | ||
| Stillbirth | 7 (3) | 7 (1) | 4.9 (1.7 to 14.2) | 4.2 (1.4 to 12.4) | 4 043 (1) | 5.5 (2.6 to 11.7) | ||
| Loss of pregnancy before 24 weeks | 5 (2) | NA | NA | NA | NA | NA | ||
| Neonatal death: | ||||||||
| Yes | 3 (1) | 2 (0) | 7.4 (1.2 to 44.7) | 5.6 (0.5 to 64.2) | 2 557 (0) | 3.8 (1.2 to 11.8) | ||
| No | 246 (99) | 1218 (100) | 1 | 1 | 792 487 (100) | 1 | ||
| Perinatal death: | ||||||||
| Yes | 10 (4) | 8 (1) | 6.2 (2.4 to 15.9) | 5.7 (2.2 to 15.1) | 6 025 (1) | 5.4 (2.8 to 10.1) | ||
| No | 246 (96) | 1219 (99) | 1 | 1 | 793 022 (99) | 1 | ||
| Mean (SD) birth weight (kg) | 3073 (774) | 3342 (614) | −270 (−356 to −183)‡ | −255 (−353 to −156)‡ | NA | NA | ||
| Low birth weight (<2500 g): | ||||||||
| Yes | 50 (20) | 94 (8) | 2.9 (2.0 to 4.3) | 3.2 (2.1 to 4.9) | 57 072 (7)§ | 3.0 (2.2 to 4.1) | ||
| No | 206 (80) | 1137 (92) | 1 | 1 | 713 201 (93) | 1 | ||
| Very low birth weight (<1500 g): | ||||||||
| Yes | 14 (5) | 22 (2) | 3.2 (1.6 to 6.3) | 2.9 (1.3 to 6.4) | 10 955 (1)§ | 4.0 (2.3 to 6.9) | ||
| No | 242 (95) | 1209 (98) | 1 | 1 | 759 318 (99) | 1 | ||
| Preterm (<37 weeks): | ||||||||
| Yes | 59 (24) | 89 (7) | 3.9 (2.7 to 5.6) | 4.0 (2.7 to 5.9) | 36 283 (8) | 3.6 (2.7 to 4.8) | ||
| No | 192 (76) | 1129 (93) | 1 | 1 | 423 475 (92) | 1 | ||
| Very preterm (<32 weeks): | ||||||||
| Yes | 18 (7) | 18 (1) | 5.2 (2.6 to 10.0) | 4.9 (2.4 to 10.0) | 10 932 (2) | 3.2 (2.0 to 5.1) | ||
| No | 233 (93) | 1200 (99) | 1 | 1 | 449 101 (98) | 1 | ||
| Delivered by caesarean section: | ||||||||
| Yes | 100 (40) | 299 (25) | 2.1 (1.5 to 2.7) | 2.3 (1.7 to 3.2) | 139 449 (24) | 2.2 (1.7 to 2.8) | ||
| No | 150 (60) | 921 (75) | 1 | 1 | 453 951 (76) | 1 | ||
| Congenital anomalies: | ||||||||
| Yes | 8 (3) | NA | NA | NA | 4 308 (2) | 1.9 (0.9 to 3.8) | ||
| No | 243 (97) | 248 644 (100) | 1 | |||||
NA=not available.
*Adjusted for socioeconomic status (managerial/other/unemployed), ethnicity (white/other), parity, maternal age, smoking (during pregnancy/not during pregnancy), multiple birth, and body mass index; values taken from multiple imputation model.
†Including 5 pairs of twins in infected group, 13 in comparison group.
‡Change in mean value associated with being in exposed cohort rather than comparison cohort.
§Missing data on stillbirths in Scotland and all births in Northern Ireland.
Among women with 2009/H1N1, seven stillbirths occurred out of 256 infants, representing an estimated rate of 27 (95% confidence interval 11 to 56) per 1000 total births, compared with seven stillbirths out of 1233 infants in the comparison cohort: a stillbirth rate of 6 (2 to 12) per 1000 total births (P=0.001). The stillborn infants were delivered a median of 12 (range 1-53) days after the onset of symptoms of influenza. In addition, three neonatal deaths occurred among the 249 liveborn infants of women with 2009/H1N1, representing an increase in the odds of neonatal mortality compared with the national rate (odds ratio 3.8, 95% confidence interval 1.2 to 11.8). The perinatal mortality rate among infected women (10 deaths among 256 infants) was 39 (19 to 71) per 1000 total births, compared with 7 (3 to 13) per 1000 total births (nine deaths among 1233 infants) among uninfected women (P<0.001). After adjustment for known confounders, evidence still existed of an association between maternal 2009/H1N1 infection and perinatal mortality (adjusted odds ratio 5.7, 2.2 to 15.1).
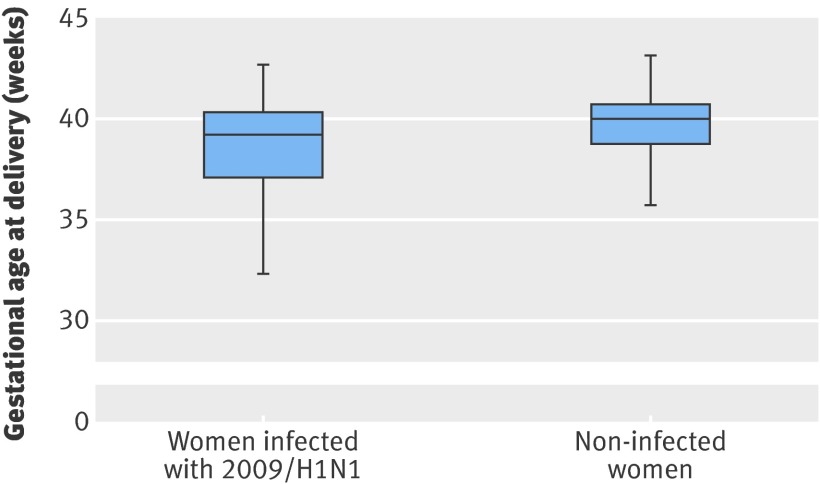
Women with 2009/H1N1 were more likely to deliver before 37 weeks’ gestation (adjusted odds ratio 4.0, 2.7 to 5.9) and before 32 weeks’ gestation (4.9, 2.4 to 10.0) than were comparison women. The difference in median gestational age at delivery between infected and comparison women was five days, although the lower quartile of infected women was almost two weeks lower than that of comparison women, reflecting a skewed distribution (figure; P<0.001). Of 58 preterm deliveries with delivery information recorded, 42 (72%) were by caesarean section; the indication reported for 22 (52%) of these was poor maternal condition due to influenza infection. After exclusion of these iatrogenic preterm caesarean deliveries for maternal influenza, a statistically significant association remained between preterm birth and infection with 2009/H1N1 (adjusted odds ratio 2.5, 1.6 to 3.9).
Box plot of gestational age of delivery by infection status, showing 10th, 25th, 50th, 75th, and 90th centiles
The mean birth weight after adjustment for potential confounders was 255 (95% confidence interval 156 to 353) grams lower among the infants of infected women than among those of comparison women. This was reflected in an increased likelihood of having a very low birthweight infant among the infected cohort compared with national data (odds ratio 4.0, 2.3 to 6.9). However, after adjustment for gestational age at delivery, we found no evidence of a difference in mean birth weight between infected and comparison women (P=0.55), suggesting that 2009/H1N1 infection had no effect on fetal growth.
Estimates after multiple imputation were more precise than those from a complete case analysis (table 3). For some outcomes, such as very low birth weight and very preterm birth, the estimates seem to differ, indicating confounding by missing values. When we did a sensitivity analysis assuming that all mothers in the infected cohort who were lost to follow-up had a normal delivery of a liveborn infant at term, we still found evidence of an association between 2009/H1N1 infection and all adverse outcomes.
Table 3.
Comparison of results of complete case and multiple imputation (MI) models. Values are adjusted odds ratios (95% CI) for cases versus controls
| Variable | Complete case model (n=1109 maternities; n=1120 infants) | MI dataset (n=1476 maternities; n=1494 infants) | After MI and sensitivity analysis (n=1492 maternities; n=1510 infants) |
|---|---|---|---|
| Stillborn: | |||
| Yes | 4.1 (1.0 to 16.6) | 4.2 (1.4 to 12.4) | 3.8 (1.3 to 11.4) |
| No | 1 | 1 | 1 |
| Neonatal death: | |||
| Yes | 3.9 (0.2 to 64.6) | 5.6 (0.5 to 64.2) | 6.0 (0.8 to 43.5) |
| No | 1 | 1 | 1 |
| Perinatal death: | |||
| Yes | 5.6 (1.7 to 18.6) | 5.7 (2.2 to 15.1) | 4.9 (1.8 to 13.3) |
| No | 1 | 1 | 1 |
| Mean birth weight (kg) | −258 (−370 to −147) | −255 (−353 to 156) | – |
| Low birth weight (<2500 g): | |||
| Yes | 3.5 (2.1 to 5.7) | 3.2 (2.1 to 4.9) | 2.9 (2.0 to 4.4) |
| No | 1 | 1 | 1 |
| Very low birth weight (<1500 g): | |||
| Yes | 1.6 (0.4 to 6.2) | 2.9 (1.3 to 6.4) | 2.7 (1.3 to 5.5) |
| No | 1 | 1 | 1 |
| Preterm (<37 weeks): | |||
| Yes | 4.4 (2.7 to 7.2) | 4.0 (2.7 to 5.9) | 3.5 (2.4 to 5.1) |
| No | 1 | 1 | 1 |
| Very preterm (<32 weeks): | |||
| Yes | 3.3 (1.2 to 9.2) | 4.9 (2.4 to 10.0) | 4.3 (2.1 to 8.8) |
| No | 1 | 1 | 1 |
| Delivered by caesarean section: | |||
| Yes | 2.1 (1.5 to 3.0) | 2.3 (1.7 to 3.2) | 2.0 (1.5 to 2.7) |
| No | 1 | 1 | 1 |
Eight infants born to women after 2009/H1N1 infection had congenital anomalies diagnosed at birth, representing a birth prevalence of 32 (exact 95% confidence interval 14 to 62) per 1000 total births compared with the national rate of 17 per 1000 total births (P=0.08). Owing to the temporality of infection and type of anomaly, only one cephalic anomaly seemed to be potentially related to 2009/H1N1 infection. Twenty-three per cent (n=53) of infants born to infected women were admitted to neonatal intensive care units after birth for a median of 12 (range 1-61) days.
A higher proportion of women infected before 37 weeks’ gestation who delivered preterm were infected in their third trimester compared with women infected before 37 weeks who delivered at term (86% v 74%; table 4); all the women admitted to hospital who were infected before 12 weeks went on to deliver at term. Women who had a secondary pneumonia were more likely to deliver preterm than were those who did not (71% (n=12) v 27% (47); P=0.001), as were women admitted to intensive care units (67% (32) v 19% (27); P<0.001). However, the increased risk of preterm birth associated with 2009/H1N1 infection persisted even after exclusion of the women with secondary pneumonia (adjusted odds ratio 3.3, 2.2 to 5.0). We found no evidence of a difference in the proportion of women treated with antiviral drugs within two days of onset of symptoms between those who delivered at term and preterm.
Table 4.
Characteristics of infected women, for those who delivered preterm and those who delivered at term. Values are numbers (percentages) unless stated otherwise
| Characteristic | Preterm (n=59) | Not preterm (n=131)* | P value |
|---|---|---|---|
| Trimester of infection: | 0. 046† | ||
| First (0-11 weeks) | 0 (0) | 10 (8) | |
| Second (12-23 weeks) | 8 (14) | 24 (18) | |
| Third (≥24 weeks) | 51 (86) | 97 (74) | |
| Median (interquartile range) No of symptoms‡ at presentation | 4 (3-5) | 5 (3-6) | 0.09§ |
| Median (interquartile range) days before start of treatment | 3 (1-7) | 3 (1-6) | 0.46† |
| Treated within 2 days of infection: | |||
| Yes | 28 (53) | 69 (57) | 0.61 |
| No | 25 (47) | 52 (43) | |
| Immunised against 2009/H1N1: | |||
| Yes | 2 (4) | 7 (6) | 0.72§ |
| No | 54 (96) | 119 (94) | |
| Admitted to intensive care unit: | |||
| Yes | 32 (54) | 16 (12) | <0.001 |
| No | 27 (46) | 115 (88) | |
| Pneumonia as secondary infection: | |||
| Yes | 12 (20) | 5 (4) | 0.001† |
| No | 47 (80) | 126 (96) | |
| Asthma: | |||
| Yes | 9 (15) | 18 (14) | 0.78 |
| No | 50 (85) | 113 (86) | |
| Other comorbidity: | |||
| Yes | 15 (25) | 23 (18) | 0.21 |
| No | 44 (75) | 108 (82) | |
| Delivered by caesarean section: | |||
| Yes | 42 (72) | 39 (30) | <0.001 |
| No | 16 (28) | 92 (70) | |
| Indication for caesarean section: | |||
| Maternal influenza infection | 22 (52) | 3 (8) | <0.001† |
| Other | 20 (48) | 36 (92) |
*Considering only those women with birth after 37 weeks who had confirmed infection before 37 weeks.
†Fisher’s exact test.
‡Fever, cough, sore throat, headache, tiredness/lethargy, limb or joint pain, diarrhoea, breathlessness, vomiting, rhinorrhoea.
§Wilcoxon rank sum test.
Discussion
This study suggests that poor perinatal outcomes, in addition to poor maternal outcomes, are associated with 2009/H1N1 influenza infection in pregnancy. The risks of poor outcomes persist after adjustment for maternal and pregnancy related characteristics known to be associated with poor perinatal outcomes, although we were not able to adjust for all possible confounding factors as we were relying on information that is obtainable from medical records. Our study suggested an increased risk of perinatal mortality for women infected with 2009/H1N1 compared with the general population, which was explained almost entirely by an increased risk of stillbirth. Perinatal mortality, however, remains a rare outcome, and the statistical uncertainty that surrounds this estimated increased mortality risk should be noted, given that we have information on outcome for only 94% of the cohort and that adjusting for potential confounders may be ineffective in removing the confounding in a model with a small number of outcomes. In view of the increase in maternal mortality and morbidity previously shown to be associated with 2009/H1N1 influenza in pregnancy,2 an ongoing immunisation programme for pregnant women, together with actions to ensure rapid availability of antiviral drugs in the context of increased circulating levels of influenza in the community,20 remains important.
We found a statistically significant difference in the rate of preterm birth between infected women and comparison women; almost half of the infants delivered preterm were delivered early because of maternal compromise. Women are typically delivered during the third trimester to aid mechanical ventilation. However, emerging evidence indicates that when women are referred for management with extracorporeal membrane oxygenation, in the absence of fetal compromise, no indication to deliver the fetus early necessarily exists.21 This is noted particularly at gestations below 30-32 weeks, when the size of the uterus is unlikely to affect mechanical ventilation. Increased availability and use of extracorporeal membrane oxygenation may therefore have the potential to have a positive effect on outcomes for infants, even in the presence of critical illness in the mother.
Although we did not find a direct relation between delayed treatment with antiviral drugs and preterm delivery, delayed antiviral treatment has been clearly related to poorer maternal outcomes,2 3 4 and we believe that this study further highlights the importance of prompt treatment during seasonal peaks of influenza infection, epidemic, and pandemic situations. The risk of preterm delivery also highlights the need to consider the effect of additional requirements for neonatal intensive care services, as well as adult intensive care provision, in planning for future pandemics.
Comparison with other studies
Other studies of 2009/H1N1 in pregnancy have reported very incomplete outcomes or outcomes for only a subset of severely affected women (table 5).3 4 5 6 22 23 Half of outcome rates were calculated by using sub-samples of less than 50% of the study cohort. Most studies did not follow up women to the end of their pregnancy,3 5 6 22 or in some cases the follow-up time was too short for collection of information on outcomes in women infected at all gestations.6 23 This approach will bias any results towards reporting preterm births, which is likely to lead to overly pessimistic results. Nevertheless, we have followed up more than 90% of the UK cohort, thus minimising potential bias, and a statistically significant association between maternal 2009/H1N1 infection and preterm birth remains.
Table 5.
Previous studies of pregnancy outcomes among women infected with 2009/H1N1
| Study | Study period (2009) | Study population | No of pregnant women reported | No (%) women with outcome data | Pregnancy outcome | No (%) affected |
|---|---|---|---|---|---|---|
| Siston 20105 | 14 April to 21 August | Pregnant women with 2009/H1N1 influenza, USA | 788* | 169 (21) | Preterm delivery | 51 (30) |
| 200 (25) | Spontaneous abortion | 8 (4) | ||||
| Louie 20103 | 23 April to 11 August | Women with confirmed 2009/H1N1 needing intensive care, California, USA | 18 | 12 (67) | Preterm delivery | 10 (83) |
| Women admitted to hospital (>24 hrs) or died with confirmed 2009/H1N1, California, USA | 94 | 37 (39) | Spontaneous abortion | 2 (5) | ||
| Creanga 20106 | 01 May to 30 June† | Women admitted to hospital with H1N1v infection, New York, USA | 62 | 40 (65) | Preterm delivery | 6 (15) |
| Neonatal death | 2 (5) | |||||
| Hewagama 201022 | 20 May to 31 July | Pregnant women admitted to hospital with 2009/H1N1 infection, Victoria, Australia | 43 | 15 (35) | Preterm delivery | 6 (40) |
| 24 (56) | Stillbirth‡/neonatal death | 3 (13) | ||||
| ANZIC 20104 | 01 June to 31 August | Pregnant or recently postpartum women admitted to intensive care unit with 2009/H1N1, Australia and New Zealand | 64 | 61 (95) | Miscarriage§ | 2 (3) |
| Stillbirth‡ | 4 (7) | |||||
| Preterm delivery | 22 (37) | |||||
| Low birth weight | 18 (31) | |||||
| Dubar 201023 | 01 August to 31 December¶ | Pregnant women admitted to hospital with confirmed 2009/H1N1, France | 314 | 146 (46) | Stillbirth | 2 (1) |
| Loss of pregnancy before 24 weeks | 4 (2) | |||||
| Low birth weight | 22 (16) | |||||
| Preterm birth | 26 (19) |
*Including 509 women admitted to hospital.
†Followed up until 18 September 2009.
‡Defined as in utero death ≥20 weeks’ gestation.
§Defined as in utero death <20 weeks’ gestation.
¶Followed up until 30 April 2010.
In common with our study, a large study of the influenza pandemic of 1919 found an increased risk of preterm birth among women with secondary pneumonia9; however, investigations into the 1958 pandemic did not find any evidence of an increased risk of adverse fetal outcomes.24 An excess of stillbirths was found in data from the 1919 pandemic,7 but data from the 1970 pandemic indicated an increase only in neonatal deaths and not stillbirths.8 Previous studies into seasonal influenza in pregnancy have presented conflicting evidence on fetal risk; some have reported an increase in poor outcomes such as preterm delivery or stillbirth,25 26 whereas others found no increased risk.27 28 Studies of infants born to women infected in the 1958 pandemic found an increased risk of congenital anomalies,29 30 a finding supported by studies into fever and hyperthermia in pregnancy.31
These data suggest that women with 2009/H1N1 infection who had given birth preterm were more likely to have been infected in their third trimester. Secondary infection with pneumonia played an important role in preterm delivery in this 2009-10 cohort; secondary pneumonia was also associated with preterm birth in women with pandemic influenza in 1919.9 In our data, the risk of preterm birth associated with 2009/H1N1 infection persisted even after we accounted for the role of secondary pneumonia, which suggests that the excess risk cannot be explained by this factor alone.
Strengths and limitations of study
A limitation of our study is that we did not collect further information on the clinical characteristics of influenza, such as highest recorded temperature or viral load, which could lead to a better understanding of the reasons for adverse outcomes of pregnancy. In addition, we collected information only on women who presented in secondary care, most of whom were in the third trimester of pregnancy. We could not, therefore, investigate any potential association between early infection and risk of congenital anomaly. Furthermore, the outcomes we present are likely to represent the severe end of the disease spectrum, as we can estimate from previous work that up to 30 women presented with influenza-like illness in pregnancy for each one admitted to hospital with confirmed 2009/H1N1 infection.2 The UK Teratology Information Service has gathered information on pregnant women consulting in primary care with 2009/H1N1 infection and women who have been immunised.32 When published, these data may give additional indication of the severity of outcomes of illness presenting in primary care and the clinical effectiveness of immunisation. We do not have any sources of information with which to estimate the completeness of the cohort we identified; however, we used several methods to try to ensure that we identified the cohort as fully as possible, and, with the participation of 99% of hospital units in the data collection, we have no evidence of any systematic bias in reporting of infected women.
For pragmatic reasons and to ensure they were an uninfected cohort, the comparator cohort we used was an historical one, which may be regarded as a limitation of this study, as outcomes of pregnancy within the population may have changed over this time. National surveillance of perinatal mortality has not, however, identified any changes over time that are likely to affect the conclusions of the study.33 Additionally, some of the outcomes were collected retrospectively, which gives the potential for selective reporting bias; however, given the objective nature of these outcomes, the effect of any bias is likely to be minimal.
As we highlighted earlier in the discussion, although we have attempted to adjust the outcome estimates to account for known confounders, as this is an observational study persisting confounding that we have not been able to account for may remain. Other possible known confounders, such as previous delivery by caesarean section,34 inter-pregnancy interval,35 or quality or type of obstetric care,36 may contribute to the relation we found. In addition, the analysis had limited power to adjust fully even for known confounders when the number of outcomes was small, and the results should therefore be interpreted with caution.
Conclusions and policy implications
This study suggests an increase in the risk of poor outcomes of pregnancy in women infected with 2009/H1N1, which reinforces the message from studies on maternal risk alone. Almost half of the preterm deliveries were due to early delivery for maternal compromise, indicating that the health of pregnant women is an important public health priority in future waves of this and other influenza pandemics.
What is already known on this topic
2009/H1N1 is the predominant circulating strain of influenza in winter in the northern hemisphere
Pregnancy is known to be a risk factor for critical illness and death in pregnant women after 2009/H1N1 infection
Women at particular risk of admission to hospital with 2009/H1N1 include those with obesity, asthma, multiple pregnancy, and black or other minority group ethnicity and those who are multiparous or who smoke
What this study adds
Women infected with 2009/H1N1 influenza in pregnancy are at risk of poor pregnancy outcomes, with an increased risk of preterm and very preterm delivery and perinatal mortality
Almost half of the preterm deliveries were due to early delivery for maternal compromise, indicating that the health of pregnant women is an important public health priority in influenza pandemics
This study would not have been possible without the contribution and enthusiasm of the UKOSS reporting clinicians who notified cases and completed the data collection forms.
Contributors: MP assisted with coding of data, did the analysis, and wrote the first draft of the paper. JJK provided advice at every stage of the study and contributed to the writing and editing of the paper. PS assisted with coding of data, validated the data, and contributed to writing and editing the paper. PB provided advice at every stage of the study and contributed to the writing and editing of the paper. MK designed the study, coordinated data collection, coded data, supervised the analysis, and contributed to the writing and editing of the paper. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. MK is the guarantor.
Funding: The research presented here was funded by a grant from the National Institute for Health Research Health Technology Assessment Programme, grant reference 09/84/47. MK was funded by a personal award from the NIHR National Coordinating Centre for Research Capacity Development. All researchers are independent of the funders.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that none have support from any company for the submitted work; they have had no relationships with companies that might have an interest in the submitted work in the previous three years; and they have no non-financial interests that may be relevant to the submitted work.
Ethical approval: This study was approved by the County Durham and Tees Valley 1 Research Ethics Committee (study reference 09/H0905/66).
Data sharing: Data sharing is governed by the National Perinatal Epidemiology Unit data sharing policy, which may be obtained from the corresponding author at marian.knight@npeu.ox.ac.uk.
Cite this as: BMJ 2011;342:d3214
References
- 1.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374:451-8. [DOI] [PubMed] [Google Scholar]
- 2.Yates L, Pierce M, Stephens S, Mill AC, Spark P, Kurinczuk JJ, et al. Influenza A/H1N1v in pregnancy: an investigation of the characteristics and management of affected women and the relationship to pregnancy outcomes for mother and infant. Health Technol Assess 2010;14(34):109-82. [DOI] [PubMed] [Google Scholar]
- 3.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010;362:27-35. [DOI] [PubMed] [Google Scholar]
- 4.Australian and New Zealand Intensive Care Influenza Investigators, Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ 2010;340:c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010;303:1517-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol 2010;115:717-26. [DOI] [PubMed] [Google Scholar]
- 7.Nishiura H. Excess risk of stillbirth during the 1918-1920 influenza pandemic in Japan. Eur J Obstet Gynecol Reprod Biol 2009;147:115. [DOI] [PubMed] [Google Scholar]
- 8.Griffith GW, Adelstein AM, Lambert PM, Weatherall JA. Influenza and infant mortality. Br Med J 1972;3:553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris JW. Influenza occurring in pregnant women. JAMA 1919;72:978-80. [Google Scholar]
- 10.Laibl VR, Sheffield JS. Influenza and pneumonia in pregnancy. Clin Perinatol 2005;32:727-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight M, Kurinczuk JJ, Tuffnell D, Brocklehurst P. The UK obstetric surveillance system for rare disorders of pregnancy. BJOG 2005;112:263-5. [DOI] [PubMed] [Google Scholar]
- 12.Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Inequalities in maternal health: national cohort study of ethnic variation in severe maternal morbidities. BMJ 2009;338:b542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Office for National Statistics. Birth Statistics 2008 Series FM1 No.37. Office for National Statistics, 2010.
- 14.Centre for Maternal and Child Enquiries. Perinatal mortality 2008: United Kingdom. CMACE, 2010.
- 15.Information Services Division Scotland. Births in Scottish hospitals. 2010 www.isdscotland.org/isd/1022.html.
- 16.NHS Information Centre. Hospital Episode Statistics Maternity data archive. 2010 www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=1009.
- 17.Office for National Statistics. A statistical review of notifications of congenital anomalies received as part of the England and Wales National Congenital Anomaly System, 2008. MB3. Office for National Statistics, 2010.
- 18.Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on interval censoring. Stata J 2007;7:445-64. [Google Scholar]
- 19.Rubin DB. Multiple imputation for nonresponse in surveys. Wiley, 1987.
- 20.Royal College of Obstetricians and Gynaecologists. Update on influenza H1N1 (2009) for providers of maternity services. rcog.org.uk/print/news/update-influenza-h1n1-2009-providers-maternity-services.
- 21.Department of Health. Clinical management guidelines for pregnancy. Addendum: extracorporeal membrane oxygenation (ECMO) in pregnancy. 2010. www.rcog.org.uk/files/rcog-corp/CLINICAL%20MANAGEMENT%20GUIDELINES%20FOR%20PREGNANCY%20-%2020%20dec%2010%20amended.pdf.
- 22.Hewagama S, Walker SP, Stuart RL, Gordon C, Johnson PD, Friedman ND, et al. 2009 H1N1 influenza A and pregnancy outcomes in Victoria, Australia. Clin Infect Dis 2010;50:686-90. [DOI] [PubMed] [Google Scholar]
- 23.Dubar G, Azria E, Tesniere A, Dupont H, Le Ray C, Baugnon T, et al. French experience of 2009 A/H1N1v influenza in pregnant women. PLoS One 2010;5(10). [DOI] [PMC free article] [PubMed]
- 24.Hardy JM, Azarowicz EN, Mannini A, Medearis DN Jr, Cooke RE. The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957-1958. Am J Public Health Nations Health 1961;51:1182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox S, Posner SF, McPheeters M, Jamieson DJ, Kourtis AP, Meikle S. Hospitalizations with respiratory illness among pregnant women during influenza season. Obstet Gynecol 2006;107:1315-22. [DOI] [PubMed] [Google Scholar]
- 26.Stanwell-Smith R, Parker AM, Chakraverty P, Soltanpoor N, Simpson CN. Possible association of influenza A with fetal loss: investigation of a cluster of spontaneous abortions and stillbirths. Commun Dis Rep CDR Rev 1994;4(3):R28-32. [PubMed] [Google Scholar]
- 27.Hartert TV, Neuzil KM, Shintani AK, Mitchel EF Jr, Snowden MS, Wood LB, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol 2003;189:1705-12. [DOI] [PubMed] [Google Scholar]
- 28.Rogers VL, Sheffield JS, Roberts SW, McIntire DD, Luby JP, Trevino S, et al. Presentation of seasonal influenza A in pregnancy: 2003-2004 influenza season. Obstet Gynecol 2010;115:924-9. [DOI] [PubMed] [Google Scholar]
- 29.Coffey VP, Jessop WJ. Maternal influenza and congenital deformities: a prospective study. Lancet 1959;2:935-8. [DOI] [PubMed] [Google Scholar]
- 30.Doll R, Hill AB, Sakula J. Asian influenza in pregnancy and congenital defects. Br J Prev Soc Med 1960;14:167-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards MJ. Review: Hyperthermia and fever during pregnancy. Birth Defects Res A Clin Mol Teratol 2006;76:507-16. [DOI] [PubMed] [Google Scholar]
- 32.UK Teratology Information Service. www.uktis.org.
- 33.Centre for Maternal and Child Enquiries (CMACE). Perinatal mortality 2008: United Kingdom. CMACE, 2010.
- 34.Smith GC, Pell JP, Dobbie R. Caesarean section and risk of unexplained stillbirth in subsequent pregnancy. Lancet 2003;362:1779-84. [DOI] [PubMed] [Google Scholar]
- 35.Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study. BMJ 2003;327:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldenberg RL, McClure EM, Bann CM. The relationship of intrapartum and antepartum stillbirth rates to measures of obstetric care in developed and developing countries. Acta Obstet Gynecol Scand 2007;86:1303-9. [DOI] [PubMed] [Google Scholar]