Abstract
Hump-nosed pit vipers (Genus Hypnale) are venomous snakes from South India and Sri Lanka. Envenoming by Hypnale species may cause significant morbidity and is characterized by local envenoming and less commonly coagulopathy and acute renal failure. Currently there are three nominal species of this genus: H. hypnale, H. zara and H. nepa. This study investigates the biochemical and pharmacological properties of the venoms from the three Hypnale species in Sri Lanka. The three Hypnale venoms had similar chromatographic profiles using reverse phase high performance liquid chromatography and fractions with procoagulant activity were identified. Hypnale venoms had potent cytotoxicity in cultured rat aorta smooth muscle cells with similar IC50 values. The venoms had weak neurotoxic and myotoxic activity in the isolated chick biventer muscle preparation. They had mild procoagulant activity with close MCC5 values and also phospholipase activity. Locally available polyvalent antivenom did not neutralise any venom effects. The study demonstrates that the three Hypnale venoms are similar and cytotoxicity appears to be the most potent effect, although they have mild procoagulant activity. These findings are consistent with clinical reports.
INTRODUCTION
Hump-nosed pit-vipers of the genus Hypnale are medically important snakes and cause between 22 and 77% of all snake bites in India and Sri Lanka (De Silva, 1981; Seneviratne et al. 2000). Although death is rare (Ariaratnam et al. 2008), H. hypnale is listed as a snake of category 1 of medical importance by the World Health Organisation (World Health Organisation, 2010). This is because it is common, widespread and responsible for snakebite resulting in significant morbidity, disability and rarely mortality. However, little is known about the venom of this snake and no antivenom has been developed to treat envenoming by Hypnale species.
Recent taxonomic revision has led to changes in the species comprising the genus. Maduwage et al. (2009) showed H. hypnale to be widespread throughout the lowlands (<600m a.s.l.) of Sri Lanka and the Western Ghats mountains that border the west coast of the Indian peninsula. However, two other species in the genus are restricted to Sri Lanka: H. nepa to the central highlands above 1,250m a.s.l, and H. zara to the rainforests of the island's south-western lowlands. Previously, it was thought that all cases of Hypnale envenoming were from a single species and it is unclear if there are differences between the species in their venom composition and clinical manifestations.
The clinical effects of Hypnale envenoming have been studied only for the single species, H. hypnale. The most commonly reported effects include local pain and swelling, local haemorrhagic blistering, regional lymphadenopathy and rarely severe local necrosis and gangrene (Kularatne and Ratnatunga, 1999; Joseph et al. 2007; Ariaratnam et al. 2008; Ariaratnam et al. 2009). Systemic effects are less common and include non-specific symptoms (headache, nausea, vomiting and abdominal pain), nephrotoxicity, coagulopathy, thrombocytopenia and spontaneous haemorrhage (Kularatne and Ratnatunga, 1999; Joseph et al. 2007; Ariaratnam et al. 2008). There is one report of ptosis from envenoming by H. hypnale and no reports of myotoxicity.
H. hypnale was one the first snakes for which the pathophysiology of envenoming was scientifically studied (Davy, 1821). Davy reported local swelling, deep ulcerations and bloody stool based on envenoming in dogs, chickens and a frog. He concluded that the action of the venom was mainly local and inflammatory, which are observations that have been confirmed in more recent studies (Joseph et al. 2007). However, there have been few further attempts to characterize Hypnale venom. De Silva et al. (1994) showed the venom of H. hypnale had procoagulant, fibrinolytic and weak platelet-aggregation activity. Wang et al. (1999) purified a phospholipase A2 from H. hypnale venom and isolated several isoforms of which W6a and W6b showed strong heparin-binding affinity and caused oedema in rat paws.
The aim of the current study was to investigate the biochemical and pharmacological properties of the venoms from the three Hypnale species - H. hypnale, H. nepa and H. zara. In addition, it examined the effect of Indian polyvalent antivenom against the bioactivity of the venoms.
MATERIALS AND METHODS
Venom collection, preparation and storage
Venom was collected from each Hypnale species in Sri Lanka as follows: H. hypnale (five specimens) from Galle (06º03’N, 80º12’ E), H. nepa (ten specimens) from Agrapathana (06º50’N, 80º40’ E) and H. zara (four specimens) from Kottawa (06º06’N, 80º20’ E). The crude venom was desiccated using dehydrated silica gel with the venom being allowed to come into contact only with polyethylene materials. For cell proliferation assays and chick biventer cervicis nerve-muscle preparations stock solutions of venom prepared as 1 or 2mg/ml in Milli-Q water were stored at -20°C until required. For high performance liquid chromatography (HPLC), in-vitro coagulation and phospholipase A2 assays stock venom solutions were prepared as 1 or 2mg/ml in 50% glycerol, and stored at −20°C.
Drugs and reagents
The following drugs and chemicals were purchased from Sigma (St Louis, MO, USA) acetylcholine chloride, carbamylcholine chloride (carbachol), Dulbecco's modified Eagles Medium, high glucose (DMEM), Dulbecco's phosphate buffered saline (magnesium, calcium free), foetal bovine serum, penicillin/streptomycin and p-bromophenacyl bromide (pBPB). Trypsin was purchased from SAFC biosciences (Victoria, Australia). Tubocurarine chloride was purchased from Calbiochem (San Diego, CA, USA). All stock solutions were made up in distilled Milli Q water. Fresh frozen plasma was obtained from the Australian Red Cross and aliquots of 10ml were thawed at 37°C. Tris-buffered saline (TBS) consists of Tris (hydroxymethyl) methylamine (25mM) with NaCl (137mM) and KCl (3.4mM), adjusted to pH 7.4 with HCl and filtered through a 0.45µm membrane. 50µl calcium chloride (0.4M) was added per ml of plasma immediately prior to clotting studies for experiments using re-calcified plasma. Innovin was obtained from Siemens. For phospholipase assay, the Cayman kit #765001 was used. Polyvalent antivenom (Bharat Serums and Vaccines Limited, India [Lot # LY62/05]) containing lyophilised polyvalent immunoglobulins [F(ab’)2] raised against Indian Cobra (Naja naja), Russell's viper (Daboia russellii), Indian krait (Bungarus caeruleus) and Saw-scaled viper (Echis carinatus) was used for antivenom experiments because this is one of the antivenoms available in regions where these snakes occur. The amount of antivenom was based on that required to neutralize Russell's viper venom because it is a large viper which has procoagulant effects. The packaging states that 1ml of reconstituted solution neutralizes 0.60mg of Russell's viper venom.
Protein assay
Stock venom solutions were filter-sterilized using a 0.22μm Millipore membrane and the protein content determined using a BCA protein assay kit (Pierce; Illinois, USA), according to manufacturer's instructions. Briefly, 25μl of venom diluted 2 and 5 fold in Milli-Q was put in triplicate on a 96-well micro-titre plate. BSA solutions diluted from 1 to 0.025mg/ml were used as reference standards and Milli-Q used as the blank. Absorbance was measured at 562nm utilizing the fusion α-plate reader (PerkinElmer., Massachusetts, USA).
Treatment of venom with the phospholipase A2 inhibitor, p-Bromophenacyl bromide (pBPB)
A solution of H. hypnale venom (400µg) was prepared in TBS (4ml). pBPB (100µg) in ethanol (20µl) was added to half of the solution and ethanol (i.e., vehicle control) was added to the remaining portion. The venom solutions (with and without pBPB) were allowed to stand at room temperature for 20hr and then transferred to dialysis tubing (mwco 12000). The dialysis tubes were placed in TBS (200ml) and stirred for 90min and the TBS buffer was replaced once during that time. These solutions were then utilized in the clotting and sPLA2 assays (see below). TBS (the buffer) was replaced once during that time.
Reverse phase high performance liquid chromatography (RP-HPLC)
RP-HPLC profiles for the three venoms were obtained using a Phenomenex Jupiter C18 column (5u 300A 250x4.6mm) with mobile phase 15% acetonitrile containing 0.1% trifluoracetic acid, increasing to 70% acetonitrile by t=100min, at a flow rate of 0.5ml/min.
SDS-Polyacrylamide gel electrophoresis (SDS-PAGE)
Twenty-five μg venom was diluted in Laemmli's sample buffer (50%, v/v, glycerol; 0.5%, w/v, bromophenol blue; 3.1%, v/v, Tris-HCl pH 6.8, 0.05%, w/v, b-mercaptoethanol) at equal volumes [1:1 (v/v)] before heating for 5 min at 95°C. Proteins were loaded on a 12% SDS-PAGE gels [40% (v/v)] of 30% acrylamide monomer solution 375mM Tris-HCl pH 8.8; 0.1% (w/v) SDS, 0.1% (v/v) TEMED; 0.05% (w/v) ammonium persulfate with 4% stacking gel 13.2% (v/v) of 30% acrylamide monomer, 125mM Tris-HCl pH 6.8; 0.1% (w/v) SDS; 0.05% (v/v) TEMED 0.05% (w/v) ammonium persulfate; Gels were placed in a Mini- PROTEAN 3 system (Bio-Rad, CA, USA) containing running buffer (25mM Tris-base, 192mM Glycine, 0.1% (w/v) SDS and run for 10min at room temperature at 60V and then for 2hr at 100V. The molecular weight standard (Precision Plus Protein Standards, Dual color) was also run in parallel. Gels were transferred into fixative solution (40%, v/v, ethanol and 10%, v/v, acetic acid) for 1hr at room temperature on an orbital shaker. Gels were then placed in SYPRO™ Ruby Protein Gel Stain and allowed to incubate for 18hr at room temperature on an orbital shaker, after which they were washed in rinsing solution (10%, v/v, methanol and 7%, v/v, acetic acid) thrice for 20min. Gels were scanned on Typhoon Trio Scanner (GE Healthcare, Uppsala, Sweden).
Cell culture: A7r5
Rat aorta smooth muscle cells (A7r5 cells) were cultured in 75cm2 flasks in DMEM media supplemented with 5% (v/v) foetal bovine serum and 1% (w/v) penicillin/streptomycin (culture media) incubated at 37°C in an atmosphere of 5% (v/v) CO2. The medium was replaced every second day until cells were approximately 80% confluent (assessed by eye using a light microscope). Cells were then lifted using 1ml trypsin and combined with 4ml media and pelleted by centrifugation at 1000rpm for 5min. The cells were resuspended and counted using a haemocytometer. Cells were subsequently seeded at 50,000 cells per well in 68-wells, in a 96-well cell culture plate with a final volume per well of 100µl. The plates were incubated for 48hr at 37°C with 5%, v/v, CO2.
Cell proliferation assay
Cell proliferation assay was performed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) (Promega, Wisconsin, USA) according to manufacturer's instructions. Briefly, media was removed from wells in A7r5 cell culture plates and the wells were washed once with pre-warmed PBS. H. hypnale, H. nepa and H. zara venom stock solutions were diluted in culture media to a final concentration of 10µg/ml and subsequently serially diluted 1.2 fold, fifteen times (10 to 0.65µg/ml). Dilutions (100µl per well) were added to wells in cell culture plate in quadruplicate. Culture media controls (cells and media with no venom) and media blanks (no cells) were also run in parallel. The plates were incubated at 37°C with 5% (v/v) CO2 for 24hr. After 24hr the cell culture plates were removed from the incubator and washed with pre-warmed PBS three times. 50µl of fresh culture media and 10µl of MTS solution were added to each well. The plates were further incubated at 37°C with 5% (v/v) CO2 for 3hr. Absorbance was measured at 492nm utilizing fusion α plate reader (PerkinElmer). Cell viability was measured as a percentage of the control cells absorbance.
For studies with antivenom cells were grown and seeded at 50,000 cells per well as described above, but only 28-wells were seeded. Plates were incubated for 48hr at 37°C with 5% (v/v) CO2. Cell proliferation assays were performed as described above, however a constant concentration on venom (5µg/ml) was added to the culture media. The culture media was further supplemented with antivenom at concentrations ranging from 4 to 0.5 times the manufacturer's recommended dose for Russell's viper or cobra venom, and each antivenom concentration was added in quadruplicate to the wells. Control wells (no antivenom) and media blanks (no cells) were also run in parallel. After 24hr, wells were washed three times with pre-warmed PBS and 50µl of fresh culture media and 10µl of MTS solution were added to each well. The plates were further incubated at 37°C with 5% (v/v) CO2 for 3hr. Absorbance was measured at 492nm utilizing fusion α plate reader (PerkinElmer) as described above. Culture media supplemented with antivenom at the varying concentrations was also incubated with MTS, similar to media blanks, to ensure the antivenom does not elicit a change in absorbance readings.
Chick biventer cervicis nerve-muscle preparation
Chicks (4-10 days old) were killed by CO2 and exsanguination. The biventer cervicis nerve-muscle was dissected and mounted under 1g tension in 5ml organ baths containing physiological salt solution (NaCl 118.4mM; KCl 4.7mM; MgSO4 1.2mM; KH2PO4 1.2mM; CaCl2 2.5mM; NaHCO3 25mM and glucose 11.1mM) at 34°C, bubbled with carbogen (95%, v/v, O2; 5%, v/v, CO2). Indirect twitches of the preparation were evoked by electrical stimulation of the motor nerves using a Grass S88 stimulator (0.2ms, 0.1 Hz, supramaximal V). Blockade of twitches by the addition of d-tubocurarine (10μM) confirmed the selective stimulation of the motor nerves. The preparation was then washed until twitches were re-established. In the absence of electrical stimulation, responses to acetylcholine (ACh, 1mM for 30sec), carbachol (CCh, 20µM for 60sec) and KCl (40mM for 30sec) were obtained. The preparation was washed thoroughly, electrical stimulation recommenced and the preparation allowed to equilibrate for 30min. Venoms (10µg) were added to the organ bath and twitch height was recorded for 1hr or until twitches were abolished. Contractile responses to ACh, CCh and KCl were obtained (as described above) at the conclusion of the experiment. In experiments examining the myotoxic effects of venom, the biventer cervicis muscle was directly stimulated every 10sec with pulses of 2ms duration at supramaximal voltage. In these experiments the electrodes were placed around the belly of the muscle and d-tubocurarine (10µM) remained in the organ bath for the duration of the experiment. Venom was left in contact with the preparation until twitch blockade occurred, or for a 3hr period. Venom was considered to be myotoxic if it inhibited direct twitches or caused a contracture of the skeletal muscle.
In vitro coagulation assay
In vitro coagulation was performed using a turbidimetric assay as previously described (O'Leary and Isbister, 2010). In brief, venom dilutions in TBS (100µl) were placed in microtitre plate wells at 37°C and then re-calcified plasma (100µl) at 37°C was added simultaneously to each well. After shaking for 5sec to mix, the optical density was monitored at 340nm every 30sec up to 30min in a BioTek ELx808 plate reader. The clotting time is taken as the time when the absorbance becomes 0.02U greater than the average of the first two absorbance measurements (O'Leary and Isbister, 2010). The minimum clotting concentration (MCC5) was taken as the concentration of venom required to cause clotting in 5 min and was used to assess procoagulant activity. Anticoagulant activity was assessed by adding thromboplastin (Innovin) and venom to re-calcified plasma to determine the anticoagulant effect of venom on thromboplastin triggered clotting time compared to thromboplastin alone clotting time.
Solutions of the Hypnale venoms (10µg/ml) in 50µl TBS were added to a solution of antivenom (4mg/ml) in 50µl TBS and the mixtures were incubated for 30min at 37°C. Re-calcified plasma (100µl) was then added, and the procedure followed as above. Clotting studies were also repeated using venom treated with pBPB.
Phospholipase A2 assay
Phospholipase A2 activity of the venoms was measured using the sPLA2 assay kit according to the manufacturer's instructions. Activities were measured at a minimum of two concentrations of venom.
Analysis
One-way analysis of variance (ANOVA) followed by a Bonferroni's mulitple comparisons test was used to compare samples for both cell culture assays and in vitro neurotoxicity and myotoxicity. Statistical significance was indicated by P<0.05. All analyses and graphics were done in GraphPad Prism version 5.03 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com.
RESULTS
Reverse-Phase High Performance Liquid Chromatography (rpHPLC)
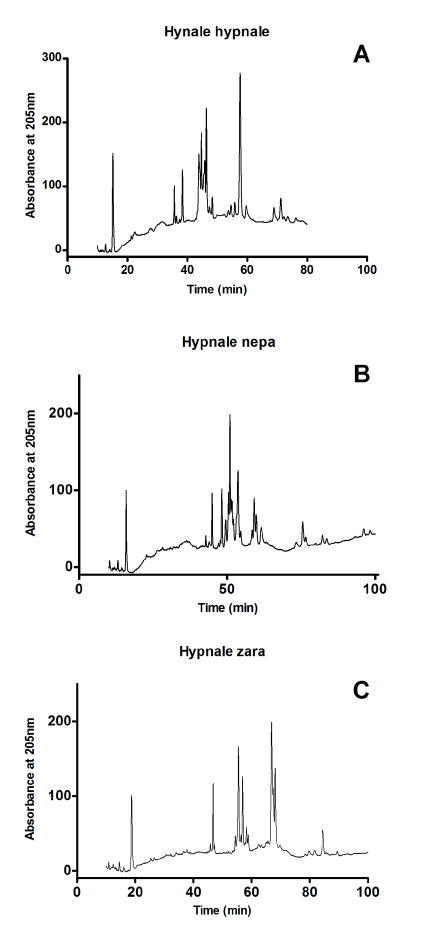
RP-HPLC analysis of the venoms produced similar profiles for H. hypnale and H. nepa venoms while the profile for H. zara was different. There were six groups of peaks for H. hypnale and H. nepa venoms and five for H. zara venom (Figure 1) with the venoms eluting between 45 and 60min. Fractions in the regions designated A, B, C, D and E (Figure 1) were collected and tested for clotting activity.
Figure 1.
Reverse phase high-peformance liquid chromatogram (RP-HPLC) chromatogram of (A) H. hypnale, (B) H. nepa and (C) H. zara using a Phenomenex Jupiter C18 column (5μ 300A 250x4.6mm) with mobile phase 15% (v/v) acetonitrile containing 0.1% (v/v) trifluoracetic acid, increasing to 70% acetonitrile by t=100min, at a flow rate of 0.5ml/min.
SDS-PAGE
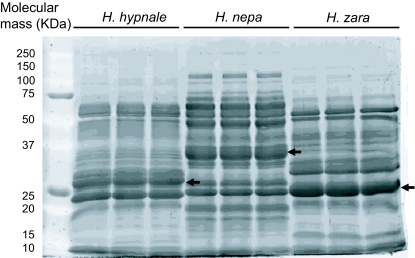
The electrophoretic profiles indicated that the three venoms studied contained proteins ranging from ~10kDa to ~100kDa, and were similar to each other, with the main differences being quantitative (in the region of ~30kDa to ~80kDa) rather than qualitative (indicated by arrows). All of the venoms showed a strongly staining band at ~25kDa and ~50kDa. In addition, H. nepa showed another strongly staining band at ~35kDa (indicated by arrow) (Figure 2).
Figure 2.
Electrophoretic profile of H. hypnale. H. nepa and H. zara venom. Molecular weight maker (7µg) where loaded in lane 1and venom (25µg) were loaded loaded onto lanes 2-10 of a 12% (w/v) polyacrylamide gel. The arrows indicate quantitative differences in band intensities among the venoms.
In vitro cytotoxicity study
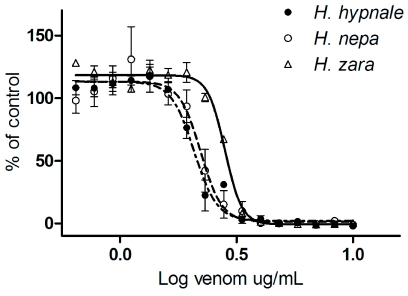
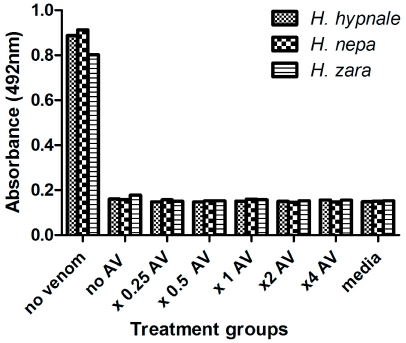
All three species of Hypnale inhibited cell proliferation in the A7r5 cell culture with similar IC50 values of 2.07µg/ml, 2.22µg/ml and 2.82 µg/ml, respectively for H. hypnale, H. nepa and H. zara venoms (Figure 3). The addition of antivenom, at concentrations ranging from 0.5 to 4 times the recommended dose failed to negate the inhibition of cell proliferation caused by 5µg/ml H. hypnale, H. nepa or H. zara venoms (Figure 4). Addition of antivenom to culture media did not cause a change in absorbance readings (data not shown).
Figure 3.
Sigmoidal growth curves of H. hypnale (filled circle), H. nepa (open circle) or H. zara (open triangle) venoms, displayed as percentage of maximum cell viability in A7r5 cells (n=3).
Figure 4.
Cell proliferation of A7r5 cells treated with 5µg/ml of H. hypnale (small cross hatching), H. nepa (large cross hatching) or H. zara (horizontal lines) venoms and varying polyvalent antivenom concentrations (n=4).
In vitro neurotoxicity and myotoxicity in chick biventer cervicis preparations
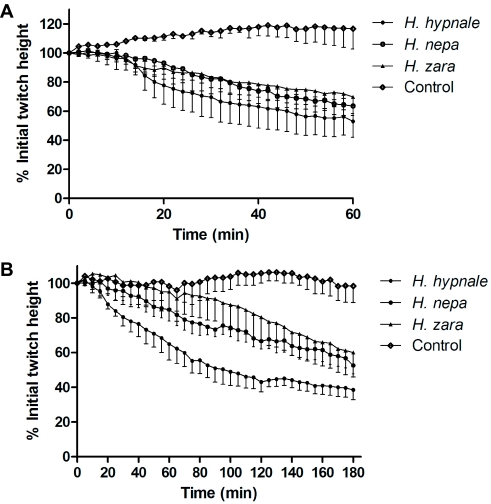
All three Hypnale venoms displayed neurotoxicity (Figure 5A) and myotoxicity (Figure 5B) as indicated by partial blockade of indirect and direct twitches, respectively, in the skeletal muscle preparation. Responses to exogenous ACh and CCh were significantly inhibited by all venoms indicating a post-synaptic site of action (data not shown).
Figure 5.
The effects of H. hypnale, H. nepa or H. zara venoms (10μg/ml; n=3) on (A) indirect (i.e., nerve mediated) twitches and, (B) direct (i.e., muscle mediated) twitches of the chick biventer cervicis nerve-muscle preparation.
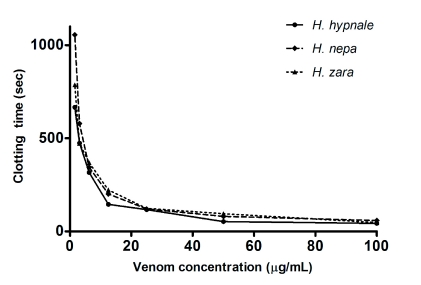
Figure 6.
Procoagulant effects of Hypnale venoms. Clotting times for increasing concentrations of H. hypnale, H. nepa or H. zara venoms in the absence of another triggering agent showing the procoagulant activity.
In vitro coagulation assay
All three Hypnale venoms had weak procoagulant activity with MCC5 of 4.4µg/ml, 5.6µg/ml and 5.5µg/ml of plasma, respectively for H. hypnale, H. nepa and H. zara venoms. The procoagulant activity of all 3 Hypnale venoms at 5µg/ml was not neutralised by pre-mixing with polyvalent antivenom at a concentration of 2000µg/ml of plasma. This amount of antivenom was sufficient to prevent clotting by Russell's viper venom at 250ng/ml of plasma. The addition of pBPB to H. hypnale did not neutralize procoagulant activity. Hypnale venoms were able to clot plasma that had not been re-calcified. Clotting activity was only found in the “C” region of peaks for all three species and in region “D” for H. hypnale and H. zara venoms.
Phospholipase A2 (PLA2) activity
PLA2 activity was detected in all venoms with values of 0.3, 0.2 and 0.2nmol of product/ml/min/ng being obtained for H. hypnale, H. nepa and H. zara, respectively. Treatment of H. hypnale venom with pBPB neutralised the PLA2 activity.
DISCUSSION
We have demonstrated that the three Hypnale venoms have similar chromatographic profiles and bioactivity based on a number of different assays with only minor differences between species. The Hypnale venoms all had cytotoxicity but less potent neurotoxicity, myotoxicity and procoagulant activity. The predominant cytotoxicity of the Hypnale venoms is consistent with Hypnale envenoming which causes local effects in 91% of cases (Ariaratnam et al. 2008; Ariaratnam et al. 2009). The lack of other toxicity is consistent with major systemic clinical effects being less common for Hypnale compared to other medically important snakes in Sri Lanka (Sellahewa and Kumararatne, 1994; Premawardena, 1998; Ariaratnam et al. 2008; Ariaratnam et al. 2009). Antivenom did not neutralize the procoagulant effects which is the commonest systemic effect reported (Ariaratnam et al. 2009).
The chromatographic profile of the three whole venoms demonstrates they have a similar composition with five well defined common regions (A, B, C, D and E) with similar retention times (Figure 1). The main difference is in region D which is less prominent in H. nepa. Clotting activity was found in regions C of all three venoms and region D of H. hypnale and H. zara showing the presence of more than one procoagulant.
The Hypnale venoms had low IC50 values of between 2 and 3μg/ml compared to cytotoxic Naja mossambica with an IC50 of 7.9μg/ml and Australasian death adders with IC50 ranging from 47.7 to 107.4μg/ml (Kalam et al. 2010). Such low IC50 suggest that they contain more potent cytotoxins than other snake venoms. This is supported by a recent study demonstrating necrotic activity of Hypnale venom (Tan et al. 2011). However, Hypnale venoms were much less neurotoxic and myotoxic compared to elapid snakes known to cause clinically important neurotoxicity and myotoxicity, consistent with the lack of neurotoxicity and myotoxicity reported for Hypnale.
Although Hypnale venoms were procoagulant they were much less potent than Russell's viper venom or Australasian elapid venoms [MCC5 0.0005-0.02µg/ml] (Isbister et al. 2010; O'Leary and Isbister, 2010). Coagulopathy is reported less in Hypnale envenoming (Sellahewa and Kumararatne, 1994; Premawardena, 1998; Ariaratnam et al. 2009), which is consistent with the limited procoagulant effect demonstrated in this study. The procoagulant effect is not neutralized by antivenom at concentrations used for patients with envenoming from other snakes consistent with antivenom not being raised against Hypnale venom. In addition, it is not neutralized by pBPB treatment suggesting the procoagulant toxin in the venom is not a phospholipase. De Silva et al. (1994) demonstrated thrombin-like activity and weak fibrinolysis action of H. hypnale venom.
Previous studies of Hypnale venom have only investigated the PLA2 activity of H. hypnale venom with no previous studies on H. nepa and H. zara venom (De Silva et al. 1994; Wang et al. 1999). This study has demonstrated PLA2 activity in all three Hypnale venoms and shown that this activity is neutralized pBPB. Four PLA2 isoforms previously isolated from H. hypnale had strong heparin binding ability and induced oedema.
Another recent study investigated the efficacy of Indian polyvalent and monovalent and polyvalent Malayan Pit Viper (Calloselasma rhodostoma) antivenoms against the in vitro effects of H. hypnale venoms (Tan et al. 2011). Similar to our study they found that Bharat polyvalent antivenom did not protect mice from H. hypnale venom. However, they demonstrated cross-neutralisation with monovalent and polyvalent antivenoms from the Thai Red Cross Society raised against C. rhodostoma (Tan et al. 2011). This is most likely because C. rhodostoma is closely related to Hypnale and is regarded as a sister taxon.
The use of venom dried using silica gel may be associated with degradation of some components of the venom. However, studies of dessicated venom suggest this occurs for long periods of storage and in our study the venom was used within 12 months of collection (Schöttler, 1951).
This study demonstrates that cytotoxicity appears to be the most potent effect of Hypnale venoms and they have mild procoagulant activity, both consistent with their clinical effects. The study represents the first investigation and characterization of biochemical and biological properties of the venoms from H. nepa and H. zara. Finally the study shows that Indian polyvalent antivenom raised against Naja naja, Daboia russellii, Bungarus caeruleus and Echis carinatus did not neutralize the venom effects consistent with previous studies (Tan et al. 2011).
Acknowledgments
We would like to thank the following for their help: Andrew Hart (Monash Venom Group), Andrew Dawson (SACTRC), Rohan Pethiyagoda (Ichthyology Section, Australian Museum, Sydney), Anjana Silva (Rajarata University), SAM Kularatne, R Sivakaneshan (University of Peradeniya), Dharma Sri Kandamby (National Maritime Museum, Galle), Sudath Nanayakkara and Amal Wijesekara. Department of Wildlife Conservation, Sri Lanka is acknowledged for granting permission for this project. The study was supported in part by NHMRC Project Grant 631073 and SACTRC which is funded by the Wellcome trust and NHMRC international collaborative capacity building research grant no: GR071669MA. GKI is supported by an NHMRC Clinical Career Development Award ID605817.
LIST OF ABBREVIATIONS
- mwco
Molecular weight cut off
- m a.s.l.
Meters above sea level
COMPETING INTERESTS
None declared.
REFERENCES
- Ariaratnam CA, Thuraisingam V, Kularatne SAM et al. Frequent and potentially fatal envenoming by hump-nosed pit vipers (Hypnale hypnale and H. nepa) in Sri Lanka: lack of effective antivenom. Trans R Soc Trop Med Hyg. 2008;102:1120–1126. doi: 10.1016/j.trstmh.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Ariaratnam CA, Sheriff MHR, Arambepola C, Theakston RDG, Warrell DA. Syndromic Approach to Treatment of Snake Bite in Sri Lanka Based on Results of a Prospective National Hospital-Based Survey of Patients Envenomed by Identified Snakes. Am J Trop Med Hyg. 2009;81:725–731. doi: 10.4269/ajtmh.2009.09-0225. [DOI] [PubMed] [Google Scholar]
- Davy J. Longman, Hurst. Rees and Brown; London: 1821. An account of the interior of Ceylon. [Google Scholar]
- De Silva A. Snakebites in Anuradhapura District. The Snake. 1981;13:117–130. [Google Scholar]
- De Silva A, Wijekoon ASB, Jayasena L et al. Haemostatic dysfunction and acute renal failure following envenoming by Merrem's hump-nosed viper (Hypnale hypnale) in Sri Lanka: first authenticated case. Trans R Soc Trop Med Hyg. 1994;88:209–212. doi: 10.1016/0035-9203(94)90301-8. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Woods D Alley, O'Leary MA, Seldon M, Lincz LF. Endogenous thrombin potential as a novel method for the characterization of procoagulant snake venoms and the efficacy of antivenom. Toxicon. 2010;56:75–85. doi: 10.1016/j.toxicon.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Joseph JK, Simpson ID, Menon NCS et al. First authenticated cases of life-threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans R Soc Trop Med Hyg. 2007;101:85–90. doi: 10.1016/j.trstmh.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Kalam Y, Isbister GK, Hodgson WC, Konstantakopoulos N. Validation of a cell-based assay to differentiate between the cytotoxic effects of elapid snake venom. J Pharmacol Toxiocol Methods. 2010;63:137–142. doi: 10.1016/j.vascn.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Kularatne SA, Ratnatunga N. Severe systemic effects of Merrem's hump-nosed viper bite. Ceylon Med J. 1999;44:169–170. [PubMed] [Google Scholar]
- Maduwage K, Silva A, Manamendra-Arachchi K, Pethiyagoda R. A taxonomic revision of the South Asian hump-nosed vipers (Squamata: Viperidae: Crotalinae: Hypnale). Zootaxa. 2009;2232:1–28. [Google Scholar]
- O'Leary M, Isbister GK. A turbidimetric assay for the measurement of clotting times of procoagulant venoms in plasma. J Pharmacol Toxiocol Methods. 2010;61:27–31. doi: 10.1016/j.vascn.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Premawardena AP, Seneviratne SL, Gunatilake SB, De Silva HJ. Excessive fibrinolysis: The coagulopathy following Merrem's hump-nosed viper (HYPNALE HYPNALE) bites. Am J Trop Med Hyg. 1998;58:821–823. doi: 10.4269/ajtmh.1998.58.821. [DOI] [PubMed] [Google Scholar]
- Schöttler WHA. On the stability of desiccated snake venoms. J Immunol. 1951;67(4):299–304. [PubMed] [Google Scholar]
- Sellahewa KH, Kumararatne MP. Envenomation by the hump-nosed viper (HYPNALE HYPNALE). Am J Trop Med Hyg. 1994;51:823–825. doi: 10.4269/ajtmh.1994.51.823. [DOI] [PubMed] [Google Scholar]
- Seneviratne SL, Opanayaka CJ, Ratnayake NSL et al. Use of antivenom serum in snake bite: a prospective study of hospital practice in the Gampaha district. Ceylon Med J. 2000;45:65–68. doi: 10.4038/cmj.v45i2.8003. [DOI] [PubMed] [Google Scholar]
- Tan CH, Leong PK, Fung SY et al. Cross neutralization of Hypnale hypnale (hump-nosed pit viper) venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in a rodent model. Acta Trop. 2011;117:119–24. doi: 10.1016/j.actatropica.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Wang YM, Liew YF, Chang KY, Tsai IH. Purification and characterization of the venom phospholipases A2 from Asian monotypic Crotalinae snakes. J Nat Toxins. 1999;8:331–340. [PubMed] [Google Scholar]
- World Health Organisation . WHO Technical Series; 2010. WHO Guidelines for the production control and regulation of snake antivenom immunoglobulins; pp. 1–134. [Google Scholar]