Abstract
Data from a randomized trial of oral periodic presumptive treatment (PPT) to reduce vaginal infections were analyzed to assess the effect of the intervention on a healthy vaginal environment (normal flora confirmed by Gram stain with no candidiasis or trichomoniasis). The incidence of a healthy vaginal environment was 608 cases per 100 person-years in the intervention arm and 454 cases per 100 person-years in the placebo arm (hazard ratio [HR], 1.36; 95% confidence interval [CI], 1.17–1.58). Sustained vaginal health (healthy vaginal environment for ≥3 consecutive visits) was also more frequent in the intervention arm (HR, 1.69; 95% CI, 1.23–2.33). PPT is effective at establishing and sustaining a healthy vaginal environment.
Vaginal conditions including bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), Trichomonas vaginalis, and abnormal vaginal flora are highly prevalent among reproductive-aged women. Mixed infections, where >1 vaginal condition is present at the same time, are also common. Disturbances in the vaginal environment due to abnormal vaginal flora and vaginal infections have been associated with increased risk of sexually transmitted infections (STIs), including human immunodeficiency virus type 1 (HIV-1) [1–5]. Conversely, the presence of normal vaginal flora has been associated with the lowest risk of HIV-1 and STI acquisition [3, 6, 7].
Interventions that improve vaginal health by establishing and sustaining a healthy vaginal environment, defined as the absence of any vaginal infections or abnormal vaginal flora, may reduce susceptibility to HIV-1 and other STIs. Recently, a randomized controlled trial (RCT) assessed the effect of monthly periodic presumptive treatment (PPT) with 2 g of oral metronidazole plus 150 mg of fluconazole versus placebo on the incidence of BV, VVC, and T. vaginalis (each analyzed as a separate outcome) among Kenyan women [8]. The intervention reduced the incidence of BV and increased colonization with Lactobacillus species. In this secondary analysis, we take an additional step, assessing the effect of PPT on establishing and sustaining a healthy vaginal environment.
METHODS
Detailed methods for the RCT were published previously [8]. We conducted the trial in Mombasa, Kenya, between May 2003 and December 2006. Female sex workers who were enrolled in an open cohort study of risk factors for HIV-1 acquisition [9] were eligible to participate if they were HIV-1 seronegative, aged 18–45 years, and nonpregnant. To avoid open-label treatment at enrollment, women with abnormal vaginal discharge or itching were ineligible to enroll. The study was approved by the institutional review boards at Kenyatta National Hospital (Nairobi, Kenya) and the University of Washington (Seattle). All participants provided written, informed consent.
At enrollment and at each of 12 monthly follow-up visits, we conducted a brief face-to-face interview to collect information on medical and sexual history. A physical examination, including speculum-assisted pelvic examination, was performed, and we collected specimens to diagnose genital tract infections. We collected blood for HIV-1 testing and performed a urine pregnancy test. Participants were randomized to receive 2 g of metronidazole plus 150 mg of fluconazole or identical placebo monthly. At monthly visits, we administered study product orally as directly observed treatment. We syndromically treated women who reported abnormal vaginal discharge or vulvovaginal itching with a single 2 g dose of oral metronidazole plus clotrimazole 200 mg vaginal suppositories nightly for 3 nights. When this treatment was dispensed, we withheld study product. We treated other genital infections according to World Health Organization guidelines [10].
We performed all laboratory procedures in Mombasa. A Gram stain of vaginal fluid was evaluated for diagnosis of BV by Nugent criteria [11]. A vaginal saline wet mount was examined microscopically for the presence of motile trichomonads and fungal elements. A drop of 10% potassium hydroxide was added to the slide and evaluated again for the presence of yeast buds or hyphae. T. vaginalis culture was performed in Diamond’s modified medium. HIV-1 testing was performed using an enzyme-linked immunosorbent assay (ELISA, Detect-HIV [BioChem ImmunoSystems]). A positive ELISA result was confirmed using a second ELISA (Recombigen [Cambridge Biotech] or Vironostika [bioMerieux]).
The objectives of this analysis were to assess the effect of the intervention on the presence of a healthy vaginal environment (defined as a Nugent score of 0–3 with no yeast on wet mount and no T. vaginalis on wet mount or culture) and the presence of sustained vaginal health (defined as the presence of a healthy vaginal environment for ≥3 consecutive visits). Women were included in the analysis if they were randomized and returned for at least 1 follow-up visit. We conducted an intent-to-treat analysis using an Andersen–Gill proportional hazards model that allows for recurrent events to estimate the effect of the intervention versus placebo on the frequency of a healthy vaginal environment during follow-up. Participants were censored if they became pregnant or HIV-1 seropositive, or at 420 days following the date of enrollment (administrative censoring).
Because the outcome for the sustained vaginal health analysis was a composite variable based on vaginal health status at 3 consecutive visits, this analysis was restricted to women who were returned for ≥3 follow-up visits. We compared baseline characteristics by study arm using chi-squared tests for categorical outcomes and Wilcoxon rank sum tests for continuous outcomes. We used Kaplan–Meier survival analysis, the log-rank test, and a Cox proportional hazards model to estimate the effect of the intervention on the incidence of sustained vaginal health during follow-up. We conducted sensitivity analyses in which we repeated our analysis using the definitions of ≥2 and ≥4 consecutive follow-up visits with a healthy vaginal environment. All statistical tests used a 2-sided α of 0.05. Analyses were conducted using Stata version 11.0 (StataCorp).
RESULTS
This trial screened 378 women, of whom 310 were enrolled. Demographic and clinical characteristics of enrolled participants were presented previously, and were similar between study arms [8]. The median age, duration of sex work, and number of sexual episodes in the past week were: 32 years (interquartile range [IQR], 27–38 y), 4 years (IQR, 1–9 y), and 1 episode (IQR, 0–2 episodes), respectively. Condom use was high, with a median of 100% use (IQR, 0%–100%) among those who reported sex in the past week. Most women (266 women [94%]) reported vaginal washing in the past week, 88 (29%) reported using hormonal contraception, and 106 (35%) had a healthy vaginal environment at enrollment. Three hundred and two women returned for ≥1 follow-up visit (151 women in each arm). The number of follow-up visits attended was also similar between study arms (median [IQR] in the intervention arm = 12 [7–12] versus 12 [9–12] in the placebo arm; P = .7). The intervention was well tolerated with similar rates of adverse events reported by arm [8].
The frequency of a healthy vaginal environment is presented in Table 1. The proportion of women who had a healthy vaginal environment at every visit was identical between the study arms (6 [4%] women in each arm, P = 1.0). Conversely, the proportion of women who never had a healthy vaginal environment was lower in the intervention arm than in the placebo (9 [6%] vs 24 [16%]; P = .006). Women in the intervention arm were more likely to have a healthy vaginal environment at any visit than were women in the placebo arm (hazard ratio [HR], 1.36, 95% confidence interval [CI] 1.17–1.58) (Table 1).
Table 1.
Frequency of a Healthy Vaginal Environment and Sustained Vaginal Health by Study Arm
| Occurrences | Rate per 100 person-years (95% CI) | Hazard ratio (95% CI) | |
| Healthy vaginal environmenta | |||
| All women | |||
| Intervention (n = 151) | 815 | 608 (568–652) | 1.36 (1.17–1.58) |
| Placebo (n = 151) | 635 | 454 (420–491) | 1.0 (Reference) |
| Women with a healthy vaginal environment at enrollment | |||
| Intervention (n = 59) | 365 | 676 (610–749) | 0.99 (0.84–1.17) |
| Placebo (n = 47) | 293 | 679 (605–761) | 1.0 (Reference) |
| Women without a healthy vaginal environment at enrollment | |||
| Intervention (n = 92) | 450 | 563 (513–617) | 1.63 (1.32–2.02) |
| Placebo (n = 104) | 342 | 354 (318–393) | 1.0 (Reference) |
| Sustained vaginal health b | |||
| Intervention (n = 142) | 86 | 102 (83–126) | 1.69 (1.23–2.33) |
| Placebo (n = 142) | 65 | 63 (49–80) | 1.0 (Reference) |
NOTE. CI, confidence interval.
Nugent score of 0–3 with no yeast on wet mount and no Trichomonas vaginalis on wet mount or culture.
Healthy vaginal environment at ≥3 consecutive follow-up visits.
We also conducted an exploratory analysis, stratifying by vaginal conditions present at enrollment (Table 1). Among women who had a healthy vaginal environment at enrollment, the intervention had no effect on the presence of a healthy vaginal environment during follow-up. However, among women who did not have a healthy vaginal environment at enrollment, those in the intervention arm had an increased incidence of a healthy vaginal environment during follow-up compared with placebo.
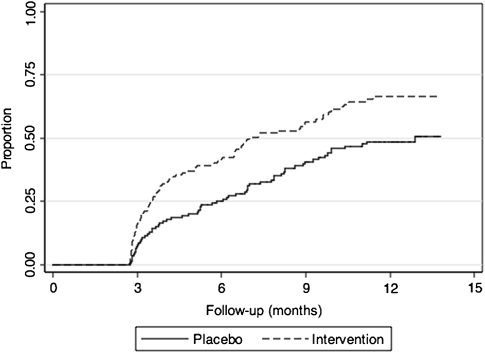
The analysis of sustained vaginal health included 284 women who attended ≥3 follow-up visits, comprising 142 women in each arm. Women included in this analysis were slightly older (median age, 32 y vs 28 y; P = .002) and reported a longer duration of sex work (median duration, 4 y vs 2 y; P < .001) compared with women who were excluded due to <3 follow-up visits. Baseline characteristics were similar by study arm among the subgroup who attended ≥3 follow-up visits (data not shown). There were 151 episodes of sustained vaginal health (Table 1). Median time to sustained vaginal health was 30 weeks in the intervention arm versus 56 weeks in the placebo arm (log-rank test P = .001) (Figure 1). Women in the intervention arm were more likely to have sustained vaginal health than women in the placebo arm (HR, 1.69; 95% CI, 1.23–2.33). Results were similar in sensitivity analyses that assessed the effect of the intervention using shorter (≥2 mo) or longer (≥4 mo) periods to define sustained vaginal health (data not shown).
Figure 1.
Cumulative proportion of sustained vaginal health by study arm.
DISCUSSION
In this secondary analysis, monthly oral treatment with metronidazole and fluconazole increased both the frequency of having a healthy vaginal environment and the incidence of sustained vaginal health compared with placebo. In addition, we observed that the impact of the intervention was greatest among women lacking a healthy vaginal environment at enrollment. This analysis complements the primary RCT analysis that reported a decrease in the incidence of BV and an increase in Lactobacillus colonization [8]. Interventions that promote the establishment and maintenance of a healthy vaginal environment are important for potentially reducing susceptibility to HIV-1 and STIs.
The issue of sustained vaginal health is of critical importance given the prevalence of vaginal infections and the frequency with which they recur. A prospective study of 121 women treated for symptomatic BV reported that 23% experienced another episode of symptomatic BV within 1 month and 58% experienced a recurrence by month 12 [12]. In addition, vaginal infections can occur in combination (mixed infections) or sequentially. T. vaginalis and BV are frequently detected together [13], and several studies have reported an association between BV treatment with metronidazole and subsequent symptomatic VVC [12, 14]. Finally, when considering the question of vaginal health, the absence of vaginal infections does not guarantee the presence of healthy vaginal flora; abnormal vaginal flora (Nugent score 4–6) has also been associated with increased risk of HIV-1 acquisition [7].
In both arms of the study, we observed equal proportions of women who experienced a healthy vaginal environment at every follow-up visit. It is possible that microbiologic, immunologic, behavioral, or other host factors that are unique to these women may contribute to reduced susceptibility to abnormal flora and vaginal infections. The identification of factors that are associated with prolonged vaginal health could inform the development of future vaginal health interventions.
With repeated use of antibiotic regimens, it is important to consider the issue of antimicrobial resistance. A study of resistance patterns associated with BV treatment reported metronidazole resistance in <1% of anaerobic bacterial isolates [15]. While resistance was not measured in our trial, these results suggest that metronidazole resistance is likely to be infrequent.
The high rate of retention in the trial, combined with monthly measurement of biological outcomes, allowed us to gain a substantial level of precision in this longitudinal characterization of vaginal health. Nonetheless, these findings should be interpreted in the context of several limitations. First, this is a secondary analysis in which our sustained vaginal health analysis used a subgroup of participants from the RCT, potentially introducing bias. Because the endpoint of sustained vaginal health required 3 months of follow-up, the analysis necessarily restricted the population to women who had ≥3 follow-up evaluations. Second, our definition of sustained vaginal health was somewhat arbitrary. We hypothesized that 3 consecutive visits with a healthy vaginal environment would reflect a more substantial shift in the vaginal flora than would 2 visits. Although the clinical implications of having sustained vaginal health have not yet been explored, we are reassured by the consistency of the findings observed in the sensitivity analyses. Third, wet mount microscopy alone was used to diagnose VVC. The combination of wet mount with culture may provide greater sensitivity and specificity. Lastly, behavioral characteristics that are unique to the study population could limit generalizability. Women in our study reported high rates of vaginal washing and condom use. It is possible that the effect of the intervention may differ in other populations.
There is mounting evidence that disruptions in the vaginal environment due to vaginal infections and abnormal flora increase susceptibility to HIV-1 infection. Among women at risk for HIV-1 infection, PPT could be used to promote vaginal health and potentially reduce susceptibility to HIV-1. As antiretroviral-based HIV-1–prevention strategies such as Tenofovir gel [16] continue to be evaluated, interventions that promote or preserve a healthy vaginal environment could be used to augment the effect of these HIV-1–prevention strategies, especially if antiretroviral-based strategies provide only partial protection. Additional studies are needed to assess the effects of a healthy vaginal environment and sustained vaginal health on risk of HIV-1 and STI acquisition.
Funding
Funding for this study was provided by the National Institutes of Health (grant K23 AI52480); the University of Washington Center for AIDS and STDs (grant T32 AI007140-32, to J. E. B.); and the University of Washington Center for AIDS Research, an NIH funded program (grant P30 AI027757) that is supported by the following NIH Institutes and Centers: National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Child Health and Human Development, National Heart, Lung and Blood Institute, and National Center for Complementary and Alternative Medicine.
References
- 1.Van Der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–54. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 2.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: A meta-analysis of published studies. AIDS. 2008;22:1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Wijgert JH, Morrison CS, Cornelisse PG, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr. 2008;48:203–10. doi: 10.1097/QAI.0b013e3181743936. [DOI] [PubMed] [Google Scholar]
- 4.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;3:319–25. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 5.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol. 2007;196:517.e1–6. doi: 10.1016/j.ajog.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Wijgert JH, Morrison CS, Brown J, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African women. Sex Transm Dis. 2009;36:357–64. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 7.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 8.McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: Results of a randomized trial. J Infect Dis. 2008;197:1361–8. doi: 10.1086/587490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin HL, Jr, Jackson DJ, Mandaliya K, et al. Preparation for AIDS vaccine evaluation in Mombasa, Kenya: Establishment of seronegative cohorts of commercial sex workers and trucking company employees. AIDS Res Hum Retroviruses. 1994;10:S235–7. [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) Guideline for the management of sexually transmitted infections. Geneva: WHO, 2003. [Google Scholar]
- 11.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–86. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 13.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 14.Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283–9. doi: 10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Beigi RH, Austin MN, Meyn LA, Krohn MA, Hillier SL. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am J Obstet Gynecol. 2004;191:1124–9. doi: 10.1016/j.ajog.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]