Abstract
Background. Despite an increase in the number of molecular epidemiological studies conducted in recent years to evaluate the association between human papillomavirus (HPV) infection and risk of bladder cancer, the studies remain inconclusive.
Methods. The prevalence of HPV in bladder cancer was estimated by pooling data from 52 studies, taking into consideration the heterogeneity from major related parameters including study region, histological type, HPV DNA specimen, publication calendar period, and detection method. Moreover, the association of HPV infection with bladder cancer was tested by a meta-analysis with 19 case-control studies.
Results. An HPV prevalence of 16.88% (95% confidence interval [CI], 15.53%–18.31%) among the bladder cancer cases was revealed, most of whom were high-risk HPV types (15.82% [95% CI, 14.37%–17.36%]). The prevalence varied by region, types of HPV DNA specimen, and polymerase chain reaction primers used. A significantly increased risk of bladder cancer was shown for the positivity of overall HPV (odds ratio, 2.84 [95% CI, 1.39–5.80]), which was also infuenced by HPV type, study region, HPV DNA specimen, and detection method.
Conclusions. Infection of high-risk HPV types, especially HPV16, may play a role in bladder carcinogenesis.
Carcinogenic types of human papillomavirus (HPV), such as HPV-16 and -18, have been shown to be the necessary cause of cervical cancer [1]. There has also been investigation of the possibility that HPV may cause cancers of other sites, such as head and neck, anus, vulva, and so on, because the virus is epithelium-tropic [2]. However, the association between HPV infection and some other cancers, for example, breast cancer [3] and bladder cancer [2], is still inconclusive.
It is certainly true that the epithelia of the bladder can also be infected by HPV, especially the condyloma acuminatum, which is characteristic of HPV infection and has been reported in the bladder [4, 5]. For the majority of bladder cancer originating from epithelium, the question whether bladder cancer is related to HPV infection has been raised. Since 1990, a series of descriptive studies have investigated the HPV prevalence in various histological types of bladder cancers, mainly in transitional cell carcinoma (TCC). Meanwhile, a growing number of case-control studies have detected HPV DNA in both malignant and benign or normal bladder tissues [2]. However, the study designs involved populations and HPV detection techniques that were diverse across studies, and the results were controversial rather than consistent.
Thus, our present objective was to collect published information on HPV prevalence in bladder tissues to explore the prevalence of HPV in bladder cancer cases and to test the association between HPV infection and risk of bladder cancer. This systematic analysis could directly help future research.
MATERIALS AND METHODS
Study Selection
MEDLINE was used to search for articles published from January 1989 through August 2010 using the MeSH terms “papillomavirus,” “human,” and “bladder cancer.” Additional relevant references cited in retrieved articles were also evaluated. Inclusion and exclusion criteria were as follows: (1) HPV DNA was detected in bladder tissues; (2) if data subsets were published in more than one article, only the latest one was included [6]; (3) immunosuppressed patients—for example, patients who had undergone renal or cardiac transplantation—were excluded; (4) patients with secondary malignant tumors of the bladder and cervical lesions simultaneously were excluded; (5) case reports were excluded; (6) publications not in English were excluded; (7) and if data could not be extracted or calculated from the original article, the study was excluded [7]. The work flow is shown in Supplementary Figure 1 (available online).
Data Extraction
Two investigators (N.L. and L.Y.) independently extracted the data and reached a consensus on all items. For each included study, the following information was extracted: first author’s name, journal and year of publication, country of origin, sample size, HPV DNA source, detection method, HPV prevalence (overall and type-specific: HPV-6, -11, -16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -68) in bladder tissues by disease status (case or control), and matching criteria if control participants were present. Furthermore, when HPV prevalence was assessed by both polymerase chain reaction (PCR) and non-PCR methods in the same study, only the prevalence obtained using the PCR method was included [8–17]. Detailed information on all included studies is presented in Supplementary Table 1 (available online).
Statistical Analyses
This meta-analysis was an epidemiological description of the overall, clade-specific (HPV-18 and -39 were classified into clade A7; HPV-16, -31, -33, -35, -52, -58 into clade A9; and HPV-6 and -11 into clade A10) and type-specific HPV prevalence (13 HPV types in the high-risk phylogenetic clade, namely, HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68, as well as 2 low-risk HPV types, namely, HPV-6 and -11) in bladder cancer cases [18]. In addition, we also performed a statistical pooling of estimates of HPV infection and bladder cancer risk with a case (bladder cancer tissues)–control (normal bladder tissues or benign bladder lesions, such as bladder cystitis) comparison.
An unconditional logistic regression model was used to compare the HPV prevalence according to the influential parameters and adjusted, where appropriate, for histological type, HPV DNA specimen, and publication calendar period.
A fixed-effect or random-effect model was used to pool the case-control data, based on the Mantel-Haenszel method [19] and the DerSimonian and Laird method [20], respectively. These 2 models provide similar results when between-studies heterogeneity is absent; otherwise, the random-effect model is more appropriate. Between-group heterogeneity was tested by means of the -based Q test, and the heterogeneity was considered significant if P < .05. Publication bias was evaluated with the linear regression asymmetry test by Egger et al [21] and Begg et al [22]. All analyses were performed using Stata statistical software (version 11.0, StataCorp).
RESULTS
In total, 52 publications were included in the present study. Twenty-eight countries and/or areas from 4 continents presented their data on HPV in bladder cancer cases (Supplementary Table 1; available online).
There were 2855 cases of bladder cancer in total, and most of them came from Europe (42.21%) and Asia (24.55%) (Table 1). The prevalence of HPV ranged from 0% to 100% (Supplementary Table 1; available online) but yielded an overall HPV prevalence of 16.88% (95% confidence interval [CI], 15.53%–18.31%) (Table 1) and 14.98% (95% CI, 13.29%–16.85%) after being adjusted for region, histological type, HPV DNA source, and publication calendar period (data not shown). The HPV prevalence was highest in Asia (24.25% [95% CI, 21.12%–27.60%]), followed by Africa (19.40% [95% CI, 16.02%–23.14%]). Compared with Asia, North America (13.49% [95% CI, 10.21%–17.36%]) and Europe (13.11% [95% CI, 11.26%–15.15%]) had significantly lower HPV prevalence in bladder cancer cases (adjusted odds ratio [OR], 0.30 [95% CI, 0.20–0.44] for North American cases and 0.41 [95% CI, 0.32–0.52] for European cases) (Table 1). HPV prevalence was significantly higher (adjusted OR, 1.80 [95% CI, 1.42–2.27]) when HPV DNA was extracted from fresh tissue samples or a mixture of fresh and fixed tissues of bladder cancer cases (17.95% [95% CI, 15.66%–20.41%]) than specifically from fixed tissues (16.05% [95% CI, 14.39%–17.82%]) (Table 1).
Table 1.
Human Papillomavirus (HPV) Prevalence in Bladder Cancer Cases Across Region, Histological Type, HPV DNA Specimen, and Publication Calendar Period
| Variable | No. of studies | No. of cases | Proportion (%) | HPV prevalence (%) (95% CI) | P | OR (95% CI) | Adjusted ORa (95% CI) |
| Total | 52 | 2855 | 100.00 | 16.88 (15.53–18.31) | … | … | |
| Region | <.001 | ||||||
| Asia | 14 | 701 | 24.55 | 24.25 (21.12–27.60) | Ref. | Ref. | |
| Africa | 7 | 500 | 17.51 | 19.40 (16.02–23.14) | 0.75 (0.57–1.00) | 0.84 (0.59–1.20) | |
| North America | 7 | 378 | 13.24 | 13.49 (10.21–17.36) | 0.49 (0.35–0.69) | 0.30 (0.20–0.44) | |
| Europe | 21 | 1205 | 42.21 | 13.11 (11.26–15.15) | 0.47 (0.37–0.60) | 0.41 (0.32–0.52) | |
| Otherb | 3 | 71 | 2.49 | 2.82 (0.34–9.81) | 0.09 (0.02–0.37) | 0.06 (0.01–0.23) | |
| Histological type | .07 | ||||||
| TCC | 38 | 2068 | 72.43 | 16.92 (15.33–18.61) | Ref. | Ref. | |
| Otherc | 29 | 787 | 27.57 | 16.26 (13.75–19.03) | 0.95 (0.76–1.19) | 0.85 (0.65–1.10) | |
| HPV DNA specimen | .19 | ||||||
| Fixed tissue | 34 | 1813 | 63.50 | 16.05 (14.39–17.82) | Ref. | Ref. | |
| Fresh and mixed tissue | 18 | 1042 | 36.50 | 17.95 (15.66–20.41) | 1.14 (0.93–1.40) | 1.80 (1.42–2.27) | |
| Publication date | .61 | ||||||
| 1990–1999 | 37 | 1916 | 67.11 | 16.49 (14.86–18.23) | Ref. | Ref. | |
| 2000–2010 | 15 | 939 | 32.89 | 17.25 (14.89–19.82) | 1.06 (0.86–1.30) | 0.83 (0.61–1.12) |
NOTE. CI, confidence ratio; OR, odds ratio; Ref., reference; TCC, transitional cell carcinoma.
Adjusted for region, histological type, HPV DNA specimen, and publication calendar period.
Others included 1 study from Oceania and 2 studies that did not specify clearly.
Others included adenocarcinoma, undifferentiated carcinoma, small-cell carcinoma, and mixed or unclear histological types.
With respect to HPV detection methods, the studies used broad-spectrum PCR primers, type-specific PCR primers, or a combination of both primers, as well as non-PCR methods, including in situ hybridization and Southern blot (Table 2). In general, the detection rate by means of PCR-based methods (16.66% [95% CI, 15.12%–18.29%]) was similar to that by means of non-PCR methods (17.01% [95% CI, 14.25%–20.08%]). However, PCR with type-specific primers was generally revealed to be more efficient at detecting HPV DNA in bladder tissues, with a detection rate of 33.19% (95% CI, 28.99%–37.61%). However, the detection rate was lower than <10.00% when PCR with broad-spectrum primers was used (9.41% [95% CI, 7.73%–11.32%]) (Table 2).
Table 2.
Human Papillomavirus (HPV) Prevalence in Bladder Cancer Cases by Detection Method
| Detection method | No. of studies | No. of cases | HPV prevalence (%) (95% CI) |
| PCR-based | 38 | 2185 | 16.66 (15.12–18.29) |
| Broad-spectrum primers | 15 | 1073 | 9.41 (7.73–11.32) |
| Type-specific primers | 10 | 479 | 33.19 (28.99–37.61) |
| Combination of both primers | 13 | 633 | 16.43 (13.63–19.55) |
| Non–PCR-based | 14 | 670 | 17.01 (14.25–20.08) |
| ISH | 12 | 594 | 18.52 (15.47–21.88) |
| Southern blot | 2 | 76 | 5.26 (1.45–12.93) |
NOTE. CI, confidence interval; ISH, in situ hybridization; PCR, polymerase chain reaction.
Fifteen HPV types (HPV-6, -11, -16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68) were analyzed in bladder cancer cases across studies. The prevalence of high-risk HPV types (15.82% [95% CI, 14.37%–17.36%]) was much higher than that of low-risk HPV types (1.58% [95% CI, 1.11%–2.17%]). Stratification by clade revealed that the prevalence of HPV clade A9 was higher (11.84% [95% CI, 10.57%–13.22%]) than those of HPV clade A7 (5.97% [95% CI, 5.03%–7.04%]) and clade A10 (2.30% [95% CI, 1.79%–2.91%]). The 5 most common HPV types identified, in order of decreasing prevalence, were HPV-16 (10.81% [95% CI, 9.59%–12.12%]), HPV-18 (5.91% [95% CI, 4.98%–6.95%]), HPV-33 (2.61% [95% CI, 1.93%–3.44%]), HPV-6 (2.06% [95% CI, 1.49%–2.76%]), and HPV-31 (1.85% [95% CI, 1.15%–2.81%]). The prevalences of HPV-11, -39, and -52 were <1.50%, and other types (HPV-35, -45, -51, -56, -58, -59, and -68) were absent in bladder cancer cases (Table 3).
Table 3.
Prevalence of Overall and Individual Human Papillomavirus (HPV) Types
| HPV type | No. of cases | No. HPV positive | HPV prevalence (%) (95% CI) |
| Total | 2855 | 482 | 16.88 (15.53–18.31) |
| High-risk | 2345 | 371 | 15.82 (14.37–17.36) |
| Low-risk | 2345 | 37 | 1.58 (1.11–2.17) |
| Presence of individual type in bladder cancer cases | |||
| Clade A7 | 2243 | 134 | 5.97 (5.03–7.04) |
| HPV-18 | 2318 | 137 | 5.91 (4.98–6.95) |
| HPV-39 | 358 | 1 | 0.28 (0.01–1.55) |
| Clade A9 | 2364 | 280 | 11.84 (10.57–13.22) |
| HPV-16 | 2397 | 259 | 10.81 (9.59–12.12) |
| HPV-31 | 1137 | 21 | 1.85 (1.15–2.81) |
| HPV-33 | 1840 | 48 | 2.61 (1.93–3.44) |
| HPV-52 | 533 | 1 | 0.19 (0.00–1.04) |
| Clade A10 | 2953 | 68 | 2.30 (1.79–2.91) |
| HPV-6 | 2088 | 43 | 2.06 (1.49–2.76) |
| HPV-11 | 1988 | 29 | 1.46 (0.98–2.09) |
NOTE. CI, confidence interval.
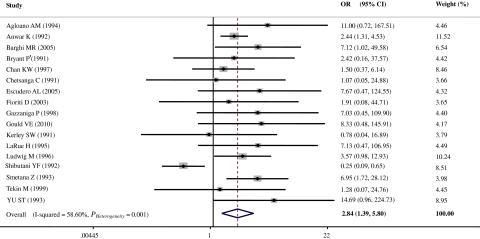
Nineteen case-control studies [12, 13, 23–39] with 926 bladder cancer cases and 219 controls were included in the estimation of HPV infection and bladder cancer risk. Two of the 19 studies were automatically excluded after data pooling because of the absence of HPV in both cases and controls [12, 28]. According to the result of the heterogeneity test, the random-effect model was chosen to evaluate the pooled OR (Figure 1). Overall, there was a significant 2.84-fold (OR, 2.84 [95% CI, 1.39–5.80]) increased bladder cancer risk with infection by any type of HPV (Figure 1). Moreover, when 1 study [37], which uniquely showed a significantly opposite association (OR, 0.25 [95% CI, 0.09–0.65]) (Figure 1), was excluded, a more significant 4.11-fold (OR, 4.11 [95% CI, 2.66–6.35]) increase in bladder cancer risk was shown for HPV infection without between-studies heterogeneity (Q = 10.46, P = .79). The Egger and Begg tests, which were designed to indicate publication bias, yielded insignificant results (P = .48 and .21, respectively).
Figure 1.
Overall odds ratios (ORs) of bladder cancer associated with human papillomavirus (HPV) (cases vs controls). CI, confidence interval.
As shown in Table 4, the pooled ORs of bladder cancer showed values of 3.48 (95% CI, 1.28–9.44) for the patients with high-risk HPV types and 5.74 (95% CI, 2.59–12.71) for the patients with specific HPV-16 infection. When the ORs were pooled by region, a significantly increased bladder cancer risk was shown in Europe and Asia (OR, 5.19 [95% CI, 2.01–13.40] for Europe and OR, 3.79 [95% CI, 2.26–6.35] for Asia) without between-studies heterogeneity (P = .92 and .29 for Europe and Asia, respectively). There was a significant 3.17-fold (95% CI, 1.27–7.98) increase in risk for HPV infection in TCC and a 2.28-fold (95% CI, 1.05–4.94) increase in risk for HPV infection in other histological types (including squamous cell carcinoma, adenocarcinoma, undifferentiated carcinoma, small-cell carcinoma, and mixed or unclear histological types). Similarly, an increased bladder cancer risk with HPV infection was revealed in fixed tissue samples (OR, 3.79 [95% CI, 2.32–6.20]) and in fresh tissue samples (OR, 6.35 [95% CI, 1.83–21.99]). A significantly increased bladder cancer risk was shown in HPV infection when HPV DNA was detected using PCR-based methods (OR, 4.28 [95% CI, 2.74–6.70]), specifically for PCR with type-specific primers (OR, 4.34 [95% CI, 2.59–7.27]), but not for non-PCR methods (OR, 0.64 [95% CI, 0.23–1.75]). Notably, a significantly increased risk was shown in both publication date periods, 1990–1999 (OR, 2.44 [95% CI, 1.07–5.75]) and 2000-2010 (OR, 6.41 [95% CI, 1.77–23.16) (Table 4).
Table 4.
Meta-analysis of 19 Case-Control Studies on the Risk of Bladder Cancer With Human Papillomavirus (HPV) Infection, Stratified by HPV Type, Region, Histological Type, Detection Method, HPV DNA Specimen, and Publication Calendar Period
| Variable | No. of studies | for heterogeneity | P for heterogeneity | Model selected | OR (95% CI) |
| Total | 19 | 38.66 | .001 | Random | 2.84 (1.39–5.80 ) |
| HPV type | |||||
| High-risk typea | 16 | 27.27 | .004 | Random | 3.48 (1.28–9.44) |
| Low-risk typeb | 16 | 12.75 | .03 | Random | 1.04 (0.33–3.24) |
| Related to HPV-16 | |||||
| Yes | 15 | 8.02 | .53 | Fixed | 5.74 (2.59–12.71) |
| No | 15 | 3.62 | .94 | Fixed | 2.93 (1.74–4.93) |
| Region | |||||
| Europe | 8 | 1.42 | .92 | Fixed | 5.19 (2.01–13.40) |
| North America | 4 | 18.11 | <.001 | Random | 1.64 (0.10–26.57) |
| Asia | 6 | 6.19 | .29 | Fixed | 3.79 (2.26–6.35) |
| Histological type | |||||
| TCC | 15 | 36.71 | <.001 | Random | 3.17 (1.27–7.98) |
| Otherc | 9 | 4.50 | .61 | Fixed | 2.28 (1.05–4.94) |
| HPV DNA specimen | |||||
| Fixed tissue | 10 | 6.98 | .54 | Fixed | 3.79 (2.32–6.20) |
| Fresh tissue | 6 | 2.73 | .60 | Fixed | 6.35 (1.83–21.99) |
| HPV DNA detection method | |||||
| PCR-based | 16 | 9.62 | .7 | Fixed | 4.28 (2.74–6.70) |
| Broad-spectrum primers | 4 | 1.12 | .57 | Fixed | 5.12 (0.97–26.98) |
| Type-specific primers | 8 | 7.53 | .28 | Fixed | 4.34 (2.59–7.27) |
| Combination of both primers | 4 | 0.91 | .82 | Fixed | 3.64 (1.26–10.50) |
| Non–PCR-based | 3 | 4.69 | .10 | Fixed | 0.64 (0.23–1.75) |
| Publication date | |||||
| 1990–1999 | 15 | 34.73 | .001 | Random | 2.44 (1.07–5.57) |
| 2000–2010 | 4 | 0.63 | .89 | Fixed | 6.41 (1.77–23.16) |
NOTE. CI, confidence ratio; OR, odds ratio; PCR, polymerase chain reaction; TCC, transitional cell carcinoma.
High-risk types included HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68.
Low-risk types included HPV-6 and -11.
Others included squamous cell carcinoma, adenocarcinoma, undifferentiated carcinoma, small-cell carcinoma, and mixed or unclear histological types.
DISCUSSION
Although 2 meta-analyses were published in 2005 [40, 41] on the correlation between HPV infection and the risk of bladder cancer, the results are still obscure because of the relatively few publications analyzed and small sample sizes used. Moreover, several questions have not been answered yet. First, is there a geographic variation of risk of bladder cancer with HPV infection? Second, is the effect of HPV infection similar in different histological types of bladder cancer? Third, since different techniques were used for HPV detection with varying specificity and sensitivity, do the changes in HPV testing protocols over time affect the estimation of the association of HPV infection with bladder cancer risk? Thus, the present study evaluated the overall effect of HPV infection on bladder cancer by considering the heterogeneity of major related parameters, such as study region, histological type, detection method, and so on. In addition, this study updated the previous meta-analyses with a larger quantity of new data on HPV infection in bladder cancer tissues published during the period 2006–2010 and attempted to answer the questions above.
Overall, there was an increased risk of bladder cancer associated with HPV infection, which was consistent with the results of previous meta-analyses [40, 41]. Moreover, our study also showed a greater risk in the patients with high-risk HPV types, specifically HPV-16. The fact that HPV-16 was the most prevalent HPV type detected in bladder cancer tissues showed that HPV-16 had the strongest carcinogenicity in bladder tissues. Although low-risk HPV types could also be detected in bladder tissues, this seems less related to the carcinogenesis of the bladder.
Our study suggested a moderate geographical variation in HPV prevalence. This might be due to the difference resulting from genetic background, environmental risk factors including sexual behavior, smoking, and other ethnic and cultural differences, as well as other unknown sources. Meta-analysis of the worldwide prevalence of cervical HPV DNA also showed a higher HPV detection rate in Africa and East Asia and a lower prevalence in North America and Europe [42]. It was also shown that the prevalence of HPV in breast cancer cases was highest in Asia, followed by North America and Europe [3]. However, although the HPV prevalence varied across continents, the risks of bladder cancer with HPV infection increased significantly in both Asia and Europe but not in North America (possibly due to the small sample size and high between-studies heterogeneity).
A majority of studies used paraffin-embedded tissue for the determination of HPV infection status. The sensitivity of HPV detection varied mainly from the differences in amplification efficiency between different types of HPV primers, especially when utilizing paraffin-embedded tissue samples. Therefore, the observed variation in HPV prevalence between HPV DNA specimens was understandable. The low detection rate of HPV DNA is known to occur with paraffin-embedded tissue and particularly when PCR amplification of long DNA fragments in the L1 gene was adopted. In contrast, HPV type–specific primers that are usually designed to amplify shorter sequences of HPV DNA might be more sensitive for detecting HPV DNA sequences [43]. Therefore, it is possible that the detection rate for HPV using HPV type–specific PCR primers may be higher than that with other PCR methods. In addition, a type-specific PCR method for HPV detection in bladder tissues might be more useful, partly as a result of the low number of copies of HPV DNA in bladder tissues.
Most bladder cancers occur in men. Among the patients with bladder cancer, males seemed to have more infections [44]. Because few studies presented HPV prevalence data in bladder cancer cases stratified by sex, it was difficult for us to estimate the sex differences in HPV prevalence and risk of bladder cancer. Additional research focusing more on the effect of sex differences is necessary.
HPV has been recognized as the necessary cause of cervical cancer [1], and the link between HPV infection and other cancers has been studied. The strength of association for HPV infection and bladder cancer was similar to those for oropharynx [45] and breast cancers [3], less than that for tonsil cancers [45], but greater than those for oral, larynx [45], and prostate cancer [46] based on previous meta-analyses. In conclusion, our study revealed a clear link between bladder cancer and HPV infection, although the risk estimates may vary by HPV type, study region, histological type, detection method, HPV DNA source, and so on. However, considering the great variation in HPV prevalence in bladder tissues and risk estimates among case-control studies, large-scale multicenter studies are needed. Our study also highlighted the importance of detecting HPV DNA in fresh tissues in samples from both case patients and well-matched control patients simultaneously with the use of more sensitive lab methods. In addition, more detailed information on HPV infection in bladder cancer, such as distribution by sex, clinical stage, and even association with survival, is also needed to find more authentic conclusions and potential clinical implications.
Supplementary Data
Supplementary figures and tables are available at The Journal of Infectious Diseases online.
Funding
This work was supported by the National Natural Science Fund 30901236 from the National Natural Science Foundation of China, the Research Fund for the Doctoral Program of Higher Education of China from the Chinese Ministry of Education (grant No. 20091106120030), and by a Fogarty Training Grant from the National Institutes of Health (NIH) (grant No. 1D43TW008323-01).
Supplementary Material
Acknowledgments
We gratefully thank A. A. Bahnassi, MD, from the Pathology Department and A. R. Zekri, PhD, from the Virology and Immunology Unit, Cancer Biology, National Cancer Institute, Cairo University, Cairo, Egypt, who made additional data available from their published study.
References
- 1.Munoz N. Human papillomavirus and cancer: the epidemiological evidence. J Clin Virol. 2000;19:1–5. doi: 10.1016/s1386-6532(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 2.Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 90. Geneva: WHO Press; 2007. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Bi X, Zhang Y, Zhao P, Zheng T, Dai M. Human papillomavirus infection and sporadic breast carcinoma risk: a meta-analysis. Breast Cancer Res Treat. 2011;126:515–20. doi: 10.1007/s10549-010-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasawa A, Kumamoto Y, Maruta H, et al. Presence of human papillomavirus 6/11 DNA in condyloma acuminatum of the urinary bladder. Urol Int. 1992;48:235–8. doi: 10.1159/000282342. [DOI] [PubMed] [Google Scholar]
- 5.Karim RZ, Rose BR, Brammah S, Scolyer RA. Condylomata acuminata of the urinary bladder with HPV 11. Pathology. 2005;37:176–8. doi: 10.1080/00313020500058615. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Beltran A, Munoz E. Transitional cell carcinoma of the bladder: low incidence of human papillomavirus DNA detected by the polymerase chain reaction and in situ hybridization. Histopathology. 1995;26:565–9. doi: 10.1111/j.1365-2559.1995.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 7.Badawi H, Ahmed H, Ismail A, et al. Role of human papillomavirus types 16, 18, and 52 in recurrent cystitis and urinary bladder cancer among Egyptian patients. Medscape J Med. 2008;10:232. [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Beltran A, Escudero AL, Carrasco-Aznar JC, Vicioso-Recio L. Human papillomavirus infection and transitional cell carcinoma of the bladder: immunohistochemistry and in situ hybridization. Pathol Res Pract. 1996;192:154–9. doi: 10.1016/S0344-0338(96)80210-X. [DOI] [PubMed] [Google Scholar]
- 9.Aynaud O, Tranbaloc P, Orth G. Lack of evidence for a role of human papillomaviruses in transitional cell carcinoma of the bladder. J Urol. 1998;159:86–9. doi: 10.1016/s0022-5347(01)64019-9. [DOI] [PubMed] [Google Scholar]
- 10.Youshya S, Purdie K, Breuer J, et al. Does human papillomavirus play a role in the development of bladder transitional cell carcinoma? A comparison of PCR and immunohistochemical analysis. J Clin Pathol. 2005;58:207–10. doi: 10.1136/jcp.2004.017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper K, Haffajee Z, Taylor L. Human papillomavirus and schistosomiasis associated bladder cancer. Mol Pathol. 1997;50:145–8. doi: 10.1136/mp.50.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowles MA. Human papillomavirus sequences are not detectable by Southern blotting or general primer-mediated polymerase chain reaction in transitional cell tumours of the bladder. Urol Res. 1992;20:297–301. doi: 10.1007/BF00300263. [DOI] [PubMed] [Google Scholar]
- 13.Smetana Z, Keller T, Leventon-Kriss S, et al. Presence of human papilloma virus in transitional cell carcinoma in Jewish population in Israel. Cell Mol Biol (Noisy-le-grand) 1995;41:1017–23. [PubMed] [Google Scholar]
- 14.Chang F, Lipponen P, Tervahauta A, Syrjanen S, Syrjanen K. Transitional cell carcinoma of the bladder: failure to demonstrate human papillomavirus deoxyribonucleic acid by in situ hybridization and polymerase chain reaction. J Urol. 1994;152:1429–33. doi: 10.1016/s0022-5347(17)32437-0. [DOI] [PubMed] [Google Scholar]
- 15.Sano T, Sakurai S, Fukuda T, Nakajima T. Unsuccessful effort to detect human papillomavirus DNA in urinary bladder cancers by the polymerase chain reaction and in situ hybridization. Pathol Int. 1995;45:506–12. doi: 10.1111/j.1440-1827.1995.tb03493.x. [DOI] [PubMed] [Google Scholar]
- 16.Sur M, Cooper K, Allard U. Investigation of human papillomavirus in transitional cell carcinomas of the urinary bladder in South Africa. Pathology. 2001;33:17–20. [PubMed] [Google Scholar]
- 17.Gopalkrishna V, Srivastava AN, Hedau S, Sharma JK, Das BC. Detection of human papillomavirus DNA sequences in cancer of the urinary bladder by in situ hybridisation and polymerase chain reaction. Genitourin Med. 1995;71:231–3. doi: 10.1136/sti.71.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;1:4–8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 20.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 23.Bryant P, Davies P, Wilson D. Detection of human papillomavirus DNA in cancer of the urinary bladder by in situ hybridisation. Br J Urol. 1991;68:49–52. doi: 10.1111/j.1464-410x.1991.tb15256.x. [DOI] [PubMed] [Google Scholar]
- 24.Barghi MR, Hajimohammadmehdiarbab A, Moghaddam SM, Kazemi B. Correlation between human papillomavirus infection and bladder transitional cell carcinoma. BMC Infect Dis. 2005;5:102–106. doi: 10.1186/1471-2334-5-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fioriti D, Pietropaolo V, Dal FS, Laurenti C, Chiarini F, Degener AM. Urothelial bladder carcinoma and viral infections: different association with human polyomaviruses and papillomaviruses. Int J Immunopathol Pharmacol. 2003;16:283–8. doi: 10.1177/039463200301600315. [DOI] [PubMed] [Google Scholar]
- 26.LaRue H, Simoneau M, Fradet Y. Human papillomavirus in transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 1995;1:435–40. [PubMed] [Google Scholar]
- 27.Ludwig M, Kochel HG, Fischer C, Ringert RH, Weidner W. Human papillomavirus in tissue of bladder and bladder carcinoma specimens: a preliminary study. Eur Urol. 1996;30:96–102. doi: 10.1159/000474152. [DOI] [PubMed] [Google Scholar]
- 28.Noel JC, Thiry L, Verhest A, et al. Transitional cell carcinoma of the bladder: evaluation of the role of human papillomaviruses. Urology. 1994;44:671–5. doi: 10.1016/s0090-4295(94)80202-5. [DOI] [PubMed] [Google Scholar]
- 29.Tekin MI, Tuncer S, Aki FT, Bilen CY, Aygun C, Ozen H. Human papillomavirus associated with bladder carcinoma? Analysis by polymerase chain reaction. Int J Urol. 1999;6:184–6. doi: 10.1046/j.1442-2042.1999.06435.x. [DOI] [PubMed] [Google Scholar]
- 30.Anwar K, Naiki H, Nakakuki K, Inuzuka M. High frequency of human papillomavirus infection in carcinoma of the urinary bladder. Cancer. 1992;70:1967–73. doi: 10.1002/1097-0142(19921001)70:7<1967::aid-cncr2820700726>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Agliano AM, Gradilone A, Gazzaniga P, et al. High frequency of human papillomavirus detection in urinary bladder cancer. Urol Int. 1994;53:125–9. doi: 10.1159/000282652. [DOI] [PubMed] [Google Scholar]
- 32.Escudero AL, Luque RJ, Quintero A, et al. Association of human herpesvirus type 6 DNA with human bladder cancer. Cancer Lett. 2005;230:20–4. doi: 10.1016/j.canlet.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Yu ST, Wu MM, Li LM. Prevalence of human papillomaviruses 16 and 18 in transitional cell carcinoma of bladder. Chin Med J (Engl) 1993;106:494–6. [PubMed] [Google Scholar]
- 34.Chan KW, Wong KY, Srivastava G. Prevalence of six types of human papillomavirus in inverted papilloma and papillary transitional cell carcinoma of the bladder: an evaluation by polymerase chain reaction. J Clin Pathol. 1997;50:1018–21. doi: 10.1136/jcp.50.12.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chetsanga C, Malmstrom PU, Gyllensten U, Moreno-Lopez J, Dinter Z, Pettersson U. Low incidence of human papillomavirus type 16 DNA in bladder tumor detected by the polymerase chain reaction. Cancer. 1992;69:1208–11. doi: 10.1002/cncr.2820690523. [DOI] [PubMed] [Google Scholar]
- 36.Kerley SW, Persons DL, Fishback JL. Human papillomavirus and carcinoma of the urinary bladder. Mod Pathol. 1991;4:316–9. [PubMed] [Google Scholar]
- 37.Shibutani YF, Schoenberg MP, Carpiniello VL, Malloy TR. Human papillomavirus associated with bladder cancer. Urology. 1992;40:15–7. doi: 10.1016/0090-4295(92)90429-z. [DOI] [PubMed] [Google Scholar]
- 38.Gazzaniga P, Vercillo R, Gradilone A, et al. Prevalence of papillomavirus, Epstein-Barr virus, cytomegalovirus, and herpes simplex virus type 2 in urinary bladder cancer. J Med Virol. 1998;55:262–7. doi: 10.1002/(sici)1096-9071(199808)55:4<262::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Gould VE, Schmitt M, Vinokurova S, et al. Human papillomavirus and p16 expression in inverted papillomas of the urinary bladder. Cancer Lett. 2010;292:171–5. doi: 10.1016/j.canlet.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez J, Jimenez A, de Dios LJ, Soto MJ, Sorlozano A. Meta-analysis of studies analyzing the relationship between bladder cancer and infection by human papillomavirus. J Urol. 2006;176:2474–81. doi: 10.1016/j.juro.2006.07.157. [DOI] [PubMed] [Google Scholar]
- 41.Wiwanitkit V. Urinary bladder carcinoma and human papilloma virus infection, an appraisal of risk. Asian Pac J Cancer Prev. 2005;6:217–8. [PubMed] [Google Scholar]
- 42.de Sanjosé S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan M, Taioli E, Ragin CC. Human papillomavirus type 16 and 18 in primary lung cancers–a meta-analysis. Carcinogenesis. 2009;30:1722–8. doi: 10.1093/carcin/bgp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khaled HM, Bahnassi AA, Zekri AR, Kassem HA, Mokhtar N. Correlation between p53 mutations and HPV in bilharzial bladder cancer. Urol Oncol. 2003;21:334–41. doi: 10.1016/s1078-1439(03)00014-0. [DOI] [PubMed] [Google Scholar]
- 45.Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, Thomas SJ. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31:259–66. doi: 10.1111/j.1749-4486.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 46.Taylor ML, Mainous AG, III, Wells BJ. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam Med. 2005;37:506–12. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.