Abstract
OBJECTIVE
In diabetes, it remains unclear whether the coronary artery calcium (CAC) score provides additional information about total mortality risk beyond traditional risk factors.
RESEARCH DESIGN AND METHODS
A total of 1,051 participants, aged 34–86 years, in the Diabetes Heart Study (DHS) were followed for 7.4 years. Subjects were separated into five groups using baseline computed tomography scans and CAC scores (0–9, 10–99, 100–299, 300–999, and ≥1,000). Logistic regression was performed adjusting for age, sex, race, smoking, and LDL cholesterol to examine the association between CAC and all-cause mortality. Areas under the curve with and without CAC were compared. Natural splines using continuous measures of CAC were fitted to estimate the relationship between observed CAC and mortality risk.
RESULTS
A total of 17% (178 of 1,051) of participants died during the follow-up. In multivariate analysis, the odds ratios (95% CIs) for all-cause mortality, using CAC 0–9 as the reference group, were CAC 10–99: 1.40 (0.57–3.74); CAC 100–299: 2.87 (1.17–7.77); CAC 300–999: 3.04 (1.32–7.90); and CAC ≥1,000: 6.71 (3.09–16.87). The area under the curve without CAC was 0.68 (95% CI 0.66–0.70), and the area under the curve with CAC was 0.72 (0.70–0.74) (P = 0.0001). Using splines, the estimated risk (95% CI) of mortality for a CAC of 0 was 6.7% (4.6–9.7), and the risk increased nearly linearly, plateauing at CAC ≥1,000 (20.0% [15.7–25.2]).
CONCLUSIONS
In diabetes, CAC was shown to be an independent predictor of mortality. Participants with CAC (0–9) were at lower risk (0.9% annual mortality). The risk of mortality increased with increasing levels of CAC, plateauing at approximately CAC ≥1,000 (2.7% annual mortality). More research is warranted to determine the potential utility of CAC scans in diabetes.
Diabetes is a coronary heart disease risk equivalent. The associated high overall mortality is largely attributable to increased cardiovascular deaths (1–3). Most of the morbidity and mortality in this high-risk condition are driven by accelerated atherosclerosis (4), characterized by increased amounts of connective tissue, glycoproteins, and calcified plaque in the blood vessels (5,6). Imaging by computed tomography (CT) reveals that individuals afflicted with diabetes have extensive calcification of their vascular beds (7–9).
Several studies have shown that subclinical atherosclerosis, as measured by coronary artery calcium (CAC), predicts future cardiovascular disease (CVD) events and death, independent of conventional risk factors (10–12), in the general population. Whether higher CAC scores are associated with adverse clinical outcomes, in particular all-cause mortality, in diabetes has not been studied extensively. Because individuals with diabetes represent a group at high risk for cardiovascular events and death, risk stratification may be particularly useful in this population. This study examines the risk of death in participants with diabetes across higher levels of CAC scores.
RESEARCH DESIGN AND METHODS
The Diabetes Heart Study (DHS) is a family study in which siblings concordant for type 2 diabetes, as well as unaffected family members, were recruited from internal medicine and endocrinology clinics in western North Carolina. The DHS design has been described in detail previously (5). To summarize, entry criteria required index case subjects to be diagnosed with type 2 diabetes after the age of 34 years and no historical evidence of diabetic ketoacidosis. At least one type 2 diabetic sibling was recruited for each index case subject enrolled. Additional nondiabetic and diabetic family members also were enrolled, when possible. The study was approved by the institutional review board at the Wake Forest University School of Medicine, and all subjects gave informed consent for all study protocols.
Laboratory measurements
The participant examinations were conducted in the general clinical research center of the Wake Forest University Baptist Medical Center and included interviews for medical history and health behaviors and anthropometric measurements. Participants were questioned about smoking history. Measurements of weight and height to the nearest 0.1 kg and 0.5 cm, respectively, were obtained during the visits, and resting blood pressure was recorded. Hypertension was defined as the current use of any antihypertensive medications or a resting systolic blood pressure >140 mmHg or resting diastolic blood pressure >90 mmHg. Fasting laboratory assays for total cholesterol, HDL cholesterol, triglycerides, glucose, and hemoglobin A1c were obtained. LDL cholesterol was calculated with the Friedewald equation (13). Diabetes was defined by self-reported history of adult-onset of diabetes, fasting glucose ≥126 mg/dL, or use of insulin or oral glucose-lowering medications. The National Cholesterol Education Program Adult Treatment Panel (14) definition was used to classify participants having metabolic syndrome in the DHS cohort. Three of five of the following components are required for diagnosis: 1) waist circumference ≥102 cm for men and ≥88 cm for women; 2) ≥130 mmHg systolic or ≥85 mmHg diastolic blood pressure or self-reported use of medications for hypertension; 3) fasting blood glucose ≥100 mg/dL or treatment for impaired fasting glucose; 4) triglycerides ≥150 mg/dL or specific treatment; and 5) HDL cholesterol ≤40 mg/dL in men and ≤50 mg/dL in women.
CAC
CAC was measured using fast-gated helical CT. All scans were performed on two single-slice subsecond helical CT scanners equipped for retrospective cardiac gating and capable of 500-ms temporal resolution (HiSpeed LX with the Smart Score Cardiac Scan Package; General Electric Medical Systems, Milwaukee, WI). Participants were placed in the supine position on the CT couch over a quality-control calibration phantom (Image Analysis, Columbia, KY). After obtaining a scout image of the chest, a helical volume of the entire heart during suspended respiration at end inspiration was obtained with the following parameters: 3-mm slice thickness; 26-cm display field of view; retrospective cardiac gating, 120 kv, 240 mA; and CT scan pitch adjusted to heart rate, as described previously (15). To further improve the precision of the calcium score, a replicated scan was performed immediately after the initial scan so that the average of the two scores could be calculated. The reproducibility of the calcium score was high (r = 0.98, Spearman correlation coefficient) between the first and second CAC scores. The amount of coronary calcium was scored using a modified Agatston method with the traditional 130-Henry U threshold and a minimal lesion definition of 0.52 mm2. In apreviously published work (15), this method has very high correlation with the electron-beam CT–derived measure of coronary calcium (r = 0.98) and high agreement when categorizing individuals based on their coronary calcium score.
All-cause mortality
The primary outcome, all-cause mortality from any cause both cardiovascular and noncardiovascular, was recorded for an average of 7.4 years (range 4–12) after CAC screening. At intervals of 9–12 months, an interviewer contacted each participant or a family member by telephone to inquire about well being and death. For participants who had died, interviews were conducted with the next-of-kin, and copies of death certificates were obtained. In addition, a search by social security number in the national death index also was performed to determine the status of subjects lost to follow-up. Cardiovascular death was defined as death as a result of myocardial infarction, congestive heart failure, cardiac arrhythmia, sudden cardiac death, peripheral vascular disease, and stroke. Noncardiovascular death was defined as death due to cancer, infection, end-stage renal disease, accident, Alzheimer’s dementia, and others, such as chronic obstructive pulmonary disease, pulmonary fibrosis, and liver failure.
Statistical analysis
We used χ2 tests for discrete variables and one-way ANOVA for continuous variables to test for differences in demographic and risk factors between surviving and deceased participants. The coronary calcium score was examined both as a stratified variable and as a continuous variable. Subjects were separated into five groups on the basis of CAC scores derived from baseline CT scans (CAC scores 0–9, 10–99, 100–299, 300–999, and ≥1,000).
Logistic regression was used to estimate odds ratios (ORs) for all-cause mortality or death from any cause according to the coronary calcium score. We constructed receiver-operating characteristic (ROC) curves and compared the areas under the ROC curves with and without CAC in the model. Spline regression was used in which calcium score was treated as a continuous variable to estimate the relationship between observed CAC and the risk of mortality. Natural splines were used to try to detect any nonlinear relationship between CAC and risk for all-cause mortality. The need for spline fitting (i.e., deviation from linearity) was tested by the significance of the spline term in the logistic model using two knots. Three knots were used if the addition of another spline term significantly improved the fit of the model. Annual mortality rates were calculated by dividing the predicted mortality by total follow-up time (in years). Additional analyses were performed to test for an interaction between CAC and all-cause mortality by race and sex.
In a separate analysis that used coronary calcium score as a continuous variable, the base 2 logarithm of the sum of the coronary calcium score plus 1 (log2 [CAC + 1]) was used. The choice of base 2 for the logarithm allowed us to examine how a doubling of the calcium score affects all-cause mortality, because each unit difference in the log-transformed calcium score represents a doubling of the score. The addition of one to the calcium score before logarithmic transformation allowed us to include in the analysis participants with a calcium score of zero. Additional multivariable analyses with ordinal coronary calcium categories to predict all-cause mortality also were performed.
All models were adjusted for age, sex, race, smoking, and LDL cholesterol. In additional analyses, adjustment for duration of diabetes, hemoglobin A1c, systolic blood pressure, and antihypertensive medications also was undertaken. All statistical analyses were performed using JMP version 8 (SAS Institute, Cary, NC).
RESULTS
Participant characteristics
Among 1,443 participants in the DHS cohort, we excluded 224 nondiabetic subjects and 168 subjects with missing data (n = 61 for calcium score, n = 100 for LDL cholesterol, and n = 7 for smoking). The baseline characteristics of 1,051 participants are shown in Table 1. The mean age of participants was 61 years, >50% of participants were female, and 17% of participants were black. A total of 85% of the participants had a CAC score >10. Risk factors were equally distributed in the two categories of mortality and no mortality, with a slightly higher number of smokers in the mortality group. Significant differences were found between the two categories, with higher mean CAC scores and higher prevalence of CAC scores ≥1,000 in the mortality group (Table 1).
Table 1.
Baseline demographic characteristics and risk factors stratified by all-cause mortality in the DHS cohort
| Variable | All participants | No death | Death | P |
|---|---|---|---|---|
| Age (years) | 61.7 ± 9.1 | 60.6 ± 8.9 | 65.9 ± 9.5 | 0.0001 |
| Male sex (%) | 480 (46) | 383 (44) | 97 (54) | 0.01 |
| Whites (%) | 876 (83) | 732 (84) | 144 (81) | 0.33 |
| Blacks (%) | 175 (17) | 141 (16) | 34 (19) | |
| Current smokers (%) | 174 (17) | 134 (15) | 40 (22) | 0.02 |
| Metabolic syndrome (%) | 924 (88) | 772 (88) | 152 (85) | 0.27 |
| Hypertension (%) | 930 (88) | 769 (88) | 161 (90) | 0.36 |
| Systolic blood pressure (mmHg) | 140.0 (18.9) | 140.0 (18.1) | 140.4 (22.3) | 0.79 |
| Fasting glucose (mg/dL) | 147.2 (58.0) | 146.2 (54.4) | 152.1 (73.2) | 0.22 |
| Waist circumference (cm) | 107.3 (16.9) | 107.6 (16.8) | 105.7 (17.4) | 0.18 |
| HDL cholesterol (mg/dL) | 44.1 (12.6) | 44.1 (12.2) | 43.8 (14.5) | 0.76 |
| LDL cholesterol (mg/dL) | 104.5 (33.2) | 104.0 (33.4) | 106.7 (32.3) | 0.33 |
| Triglycerides (mg/dL) | 170.0 (83.3) | 169.3 (83.2) | 173.2 (84.1) | 0.57 |
| Average CAC score | 1,706.1 (3,248.8) | 1,405.1 (3,059.3) | 3,182.5 (3,723.7) | 0.0001 |
| Prevalence of CAC | ||||
| CAC <10 | 143 (14) | 136 (16) | 7 (4) | 0.0001 |
| CAC 10–99 | 223 (21) | 207 (24) | 16 (9) | 0.0001 |
| CAC 100–299 | 125 (12) | 108 (12) | 17 (10) | 0.31 |
| CAC 300–999 | 186 (18) | 157 (18) | 29 (16) | 0.67 |
| CAC ≥1,000 | 371 (35) | 262 (30) | 109 (61) | 0.0001 |
Data are n (%), or continuous measures are means ± SD. P values were obtained by one-way ANOVA and χ2 test. Subjects were divided into five groups based on the CAC score derived from baseline CT scans (CAC 0–9, 10–99, 100–299, 300–999, and ≥1,000).
All-cause mortality
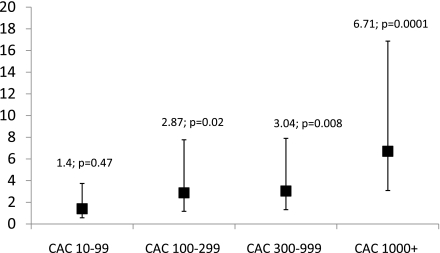
During 7.4 years of follow-up, 178 deaths were identified. All-cause mortality includes both cardiovascular and noncardiovascular deaths. One hundred fifty-one individuals had a documented cause of death, of which 82 participants died of cardiovascular causes. There is an increased incidence of all-cause mortality with higher categories of CAC, with a P trend of 0.0001 (Supplementary Fig. 1). In a univariate analysis, the ORs (95% CIs; P values) for all-cause mortality using CAC scores 0–9 as a reference group were CAC 10–99: 1.53 (0.64–4.08; 0.35); CAC 100–299: 3.13 (1.30–8.33; 0.01); CAC 300–999: 3.67 (1.64–9.34; 0.001); and CAC ≥1,000: 8.26 (4.01–20.00; 0.0001). When the models were further adjusted for age, sex, race, smoking, and LDL cholesterol, similar results were observed (CAC 10–99: 1.40 [0.57–3.74; 0.47]; CAC 100–299: 2.87 [1.17–7.77; 0.02]; CAC 300–999: 3.04 [1.32–7.90; 0.008]; and CAC ≥1,000: 6.71 [3.09–16.87; 0.0001]) (Fig. 1). In additional analyses, after further adjusting for duration of diabetes, hemoglobin A1c, systolic blood pressure, and antihypertensive medications, the results were qualitatively similar (data not shown).
Figure 1.
Subjects were separated into five groups based on CAC score derived from baseline CT scans, CAC (0-9, 10-99, 100-299, 300-999, and ≥1,000). CAC ORs for all-cause mortality for higher CAC scores in comparison to CAC score <10. Models adjusted for age, sex, race, smoking, and LDL cholesterol. ORs for all-cause mortality with higher CAC scores in the DHS cohort compared with CAC scores <10 in a full model.
The area under the ROC curve for the prediction of all-cause mortality was 0.68, using age, sex, race, smoking, and LDL cholesterol as predictors, and increased to 0.72 (P = 0.0001) with the addition of CAC in the model.
When CAC was used as a continuous measure using natural splines, the estimated risk (95% CI) of mortality for CAC = 0 was 6.7% (4.6–9.7) over 7.4 years, with an estimated annual mortality of 0.9%. The estimated risk of mortality for CAC ≥1,000 was 20.0% (15.7–25.2) over 7.4 years (Fig. 2). In addition, the risk of mortality increased nearly linearly up to the CAC level of ≥1,000, where it appeared to plateau and where additional increments of calcium may not be informative (Fig. 2).
Figure 2.
Spline regression estimating probability of all-cause mortality and continuous coronary calcium score: the DHS.
In a separate analysis, there was no significant interaction between CAC × race (P = 0.9) and CAC × sex (P = 0.7) in the prediction of all-cause mortality. In addition, in a separate sensitivity analysis, when using CAC as a continuous variable in a full model, CAC was a significant predictor of mortality (P = 0.0001). Supplementary Data Table 2 also shows the increase in the risk of all-cause mortality associated with a doubling of the coronary calcium score (a 1-unit increase in log2 [CAC + 1]). After adjustment for potential confounders, a doubling of the calcium score resulted in a 24% increase in the risk of all-cause mortality. In another multivariable analysis, the odds for death increased by 65% in patients with diabetes for every increase in CAC grouping from 10 to 99 to 100 to 299, 300 to 999, and >1,000 (P = 0.0001) (Supplementary Table 1).
CONCLUSIONS
We examined the predictive value of CAC for all-cause mortality in a biracial population with diabetes. In this cohort, we showed that the presence of any coronary artery calcification predicts risk for all-cause mortality compared with patients with diabetes with no or minimal (CAC = 0–9) calcium. In addition, the absence of CAC indicated a low risk of mortality in this high-risk population. CAC is an independent predictor of mortality even in diabetes, and the use of atherosclerosis imaging may help improve risk assessment in this high-risk condition.
The findings from this analysis corroborate and extend previous observations. In a multiethnic population free of CVD at baseline, the adjusted risk for coronary events was increased by a factor of 6.84 (95% CI 2.93–15.99) among participants with CAC ≥300 compared with participants with no CAC (10). Raggi et al. (11) analyzed a cohort of 10,377 asymptomatic individuals with 903 patients with diabetes referred for CAC imaging. The investigators found an increased risk of all-cause mortality in patients with diabetes with higher CAC scores with an OR (95% CI) of 5.76 (4.61–10.39) in CAC ≥1,000 compared with CAC 0–10, which are similar to our findings and a comparable mortality between patients with diabetes with no calcium and nondiabetic subjects. We compared the characteristics of patients with diabetes with CAC 0–9 versus CAC ≥10 to ascertain the cause of this increased risk of mortality (Table 2) and found that patients with diabetes with higher coronary scores had longer duration of disease, were older, were more likely to be male, and were hypertensive with higher mean systolic blood pressure. However, even after adjustment for diabetes duration, hemoglobin A1c, systolic blood pressure, and antihypertensive medications, the results were qualitatively similar, highlighting the significance of CAC as an independent risk predictor.
Table 2.
Characteristics of patients with diabetes with CAC 0–9 versus CAC ≥10: the DHS
| Variable | All participants | CAC 0–9 | CAC ≥10 | P |
|---|---|---|---|---|
| Age (years) | 61.7 ± 9.1 | 56.5 ± 8.4 | 62.5 ± 8.9 | 0.0001 |
| Male sex (%) | 480 (46) | 26 (18) | 454 (50) | 0.0001 |
| Whites (%) | 876 (83) | 103 (72) | 773 (85) | 0.0001 |
| Blacks (%) | 175 (17) | 40 (28) | 135 (15) | |
| Current smokers (%) | 174 (17) | 21 (15) | 153 (17) | 0.63 |
| Metabolic syndrome (%) | 924 (88) | 129 (90) | 795 (88) | 0.41 |
| Hypertension (%) | 930 (88) | 116 (81) | 814 (90) | 0.005 |
| Systolic blood pressure (mmHg) | 140.0 (18.9) | 136.7 (17.8) | 140.6 (19.0) | 0.02 |
| Fasting glucose (mg/dL) | 147.2 (58.0) | 154.5 (66.3) | 146.1 (56.5) | 0.11 |
| Waist circumference (cm) | 107.3 (16.9) | 106.2 (18.1) | 107.4 (16.8) | 0.43 |
| HDL cholesterol (mg/dL) | 44.1 (12.6) | 45.8 (13.2) | 43.8 (12.5) | 0.07 |
| LDL cholesterol (mg/dL) | 104.5 (33.2) | 108.0 (32.0) | 104.0 (33.4) | 0.17 |
| Triglycerides (mg/dL) | 170.0 (83.3) | 172.0 (92.0) | 170.0 (82.0) | 0.75 |
| HgbA1c (mg/dL) | 7.5 (1.9) | 7.9 (2.1) | 7.6 (1.8) | 0.08 |
| Diabetes duration (years) | 10.4 (7.2) | 8.2 (5.6) | 11.0 (7.6) | 0.0001 |
Data are n (%) or continuous measures are means ± SD. P values were obtained by one-way ANOVA and χ2 test.
A number of researchers, such as Qu et al. (16), who studied 249 patients with diabetes did not report any statistically significant increase in adverse cardiovascular end points with increasing CAC scores. This divergence is probably a reflection of a smaller diabetic cohort compared with the DHS cohort and also the fact that the specific end points that were examined were cardiovascular end points and not all-cause mortality.
Diabetes is synonymous with CVD (3), as subsequent cardiovascular morbidity and mortality is similar to that of individuals with known coronary heart disease (1). This clinical observation also is reflected by the presence of extensive calcification in asymptomatic patients with diabetes, which is similar to nondiabetic subjects with known coronary heart disease on atherosclerosis imaging (9,17). However, among patients with diabetes, a subset of individuals without evidence of atherosclerosis has a lower short to intermediate risk for death, and here coronary imaging may serve as a worthwhile tool for risk stratification.
The mechanism for advanced atherosclerosis in patients with diabetes is almost certainly multifactorial. Hyperglycemia and glucosylated proteins lead to activation of genes and enzymes involved in calcification, atherogenesis and thrombosis (18,19), and medial artery calcification, which substantially increases the risk for coronary events (20).
There is an increasing prevalence of obesity, metabolic syndrome, and diabetes globally (21). Thus, timely diagnosis of atherosclerosis in this high-risk group is imperative to prevent future micro- and macrovascular complications. This risk-stratification strategy using CAC may lead to better, novel, and aggressive individualized therapy in this population, perhaps resulting in decreased mortality, which is not currently observed compared with the general population (22). Whether aggressive therapy needs to be directed at those with or without advanced calcification remains controversial in light of the recent Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, where intensive lowering of risk factors in patients with diabetes led to harm rather than benefit (23). Our results also suggest that atherosclerosis is an independent predictor of mortality in diabetes independent of diabetes duration and diabetes control and conventional cardiovascular risk factors.
The strength of this study is the inclusion of a large high-risk population of only patients with diabetes with metabolic syndrome with meticulous standardized measures of CAC and data collection leading to generalizability in the diabetic population. However, several limitations need to be acknowledged. We excluded insulin-dependent patients with diabetes from the analysis, but previous studies show that both forms of disease have increased coronary calcification compared with nondiabetic subjects (7,8). Thus, the results of the current analysis may be applicable in insulin-dependent patients with diabetes as well. Selection of participants from a single center may limit the generalizability of results. Finally, the choice of all-cause mortality rather than cardiovascular end points may result in different outcomes. However, the majority of fatal events in patients with diabetes are cardiovascular in nature, and it is now preferred as an outcome in cardiovascular research (24). In addition, in patients with diabetes, the risks for CVD-only mortality and all-cause mortality are similar (25).
In conclusion, coronary artery calcification can serve as a tool for risk stratification in a high-risk population of patients with diabetes. In addition, patients with diabetes free of coronary calcification remain at a lower risk for cardiovascular deaths. Whether implementation of aggressive medical management in patients with diabetes with advanced calcification leads to decreased mortality remains to be determined. Furthermore, whether aggressive management of patients with diabetes with absent coronary calcification leads to further risk reduction also remains to be seen.
Supplementary Material
Acknowledgments
S.A. was supported by a National Heart, Lung, and Blood Institute, National Institutes of Health T32 Training Grant (2T32-HL076132-06A1). In addition, this study was supported in part by the General Clinical Research Center of the Wake Forest University School of Medicine (grant M01-RR07122) and by grants R01-AR48797 (to J.J.C.), and R01-HL67348 and R01-HL092301 (to D.W.B.).
No potential conflicts of interest relevant to this article were reported.
S.A. wrote the research proposal, performed statistical analysis, and wrote the manuscript. T.M. performed statistical analysis, researched the data, and reviewed and edited the manuscript. D.M.H. performed statistical analysis and reviewed and edited the manuscript. J.X. researched data and helped with statistical analysis. A.J.C. researched data and contributed to the discussion of the manuscript. B.I.F. reviewed and edited the manuscript. J.J.C. reviewed and edited the manuscript. D.W.B. contributed to the discussion and reviewed and edited the manuscript.
The authors had full access to the data and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
This study was presented orally at the Scientific Sessions of the American Heart Association, Chicago, Illinois, 13–17 November 2010.
The authors thank the other investigators, the staff, and the participants of the DHS study for their valuable contributions.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0008/-/DC1.
References
- 1.Wilson PW. Diabetes mellitus and coronary heart disease. Am J Kidney Dis 1998;32(Suppl. 3):S89–S100 [DOI] [PubMed] [Google Scholar]
- 2.Sarwar N, Gao P, Seshasai SR, et al. ; Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet . 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999;100:1134–1146 [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham Study. JAMA 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 5.Wagenknecht LE, Bowden DW, Carr JJ, Langefeld CD, Freedman BI, Rich SS. Familial aggregation of coronary artery calcium in families with type 2 diabetes. Diabetes 2001;50:861–866 [DOI] [PubMed] [Google Scholar]
- 6.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia 1988;31:16–23 [DOI] [PubMed] [Google Scholar]
- 7.Arad Y, Newstein D, Cadet F, Roth M, Guerci AD. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron-beam computed tomographic study. Arterioscler Thromb Vasc Biol 2001;21:2051–2058 [DOI] [PubMed] [Google Scholar]
- 8.Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care 2001;24:335–338 [DOI] [PubMed] [Google Scholar]
- 9.Hoff JA, Quinn L, Sevrukov A, et al. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J Am Coll Cardiol 2003;41:1008–1012 [DOI] [PubMed] [Google Scholar]
- 10.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345 [DOI] [PubMed] [Google Scholar]
- 11.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 2004;43:1663–1669 [DOI] [PubMed] [Google Scholar]
- 12.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008;168:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 14.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 15.Carr JJ, Crouse JR, 3rd, Goff DC, Jr, D’Agostino RB, Jr, Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol 2000;174:915–921 [DOI] [PubMed] [Google Scholar]
- 16.Qu W, Le TT, Azen SP, et al. Value of coronary artery calcium scanning by computed tomography for predicting coronary heart disease in diabetic subjects. Diabetes Care 2003;26:905–910 [DOI] [PubMed] [Google Scholar]
- 17.Mielke CH, Shields JP, Broemeling LD. Coronary artery calcium, coronary artery disease, and diabetes. Diabetes Res Clin Pract 2001;53:55–61 [DOI] [PubMed] [Google Scholar]
- 18.Mori S, Takemoto M, Yokote K, Asaumi S, Saito Y. Hyperglycemia-induced alteration of vascular smooth muscle phenotype. J Diabetes Complications 2002;16:65–68 [DOI] [PubMed] [Google Scholar]
- 19.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem 1998;273:30427–30434 [DOI] [PubMed] [Google Scholar]
- 20.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification: a neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 1996;16:978–983 [DOI] [PubMed] [Google Scholar]
- 21.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195–1200 [DOI] [PubMed] [Google Scholar]
- 22.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA 1999;281:1291–1297 [DOI] [PubMed] [Google Scholar]
- 23.Gerstein HC, Miller ME, Byington RP, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 1999;34:618–620 [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Stampfer MJ, Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med 2001;161:1717–1723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.