Abstract
OBJECTIVE
Sympathetic vasoconstriction is blunted in contracting human skeletal muscles (functional sympatholysis). In young subjects, infusion of adenosine and ATP increases blood flow, and the latter compound also attenuates α-adrenergic vasoconstriction. In patients with type 2 diabetes and age-matched healthy subjects, we tested 1) the sympatholytic capacity during one-legged exercise, 2) the vasodilatory capacity of adenosine and ATP, and 3) the ability to blunt α-adrenergic vasoconstriction during ATP infusion.
RESEARCH DESIGN AND METHODS
In 10 control subjects and 10 patients with diabetes and normal endothelial function, determined by leg blood flow (LBF) response to acetylcholine infusion, we measured LBF and venous NA, with and without tyramine-induced sympathetic vasoconstriction, during adenosine-, ATP-, and exercise-induced hyperemia.
RESULTS
LBF during acetylcholine did not differ significantly. LBF increased ninefold during exercise and during adenosine- and ATP-induced hyperemia. Infusion of tyramine during exercise did not reduce LBF in either the control or the patient group. During combined ATP and tyramine infusions, LBF decreased by 30% in both groups. Adenosine had no sympatholytic effect.
CONCLUSIONS
In patients with type 2 diabetes and normal endothelial function, functional sympatholysis was intact during moderate exercise. The vasodilatory response for adenosine and ATP did not differ between the patients with diabetes and the control subjects; however, the vasodilatory effect of adenosine and ATP and the sympatholytic effect of ATP seem to decline with age.
In healthy people, blood flow regulation during exercise is well matched to metabolic demands; vasodilation occurs in active muscles, brought about by a complex interaction of various factors regulating the vascular tone in the microcirculation. One such factor is to overcome the sympathetically mediated vasoconstriction. Direct recordings of muscle sympathetic nerve activity (MSNA) show that exercise-induced increase in sympathetic discharge is targeted to both inactive and active skeletal muscles (1). In the latter, this vasoconstrictor activity is opposed by an inhibition termed “functional sympatholysis” (2).
Prostaglandins do not seem to be mandatory for functional sympatholysis, and exogenous nitric oxide cannot oppose sympathetic vasoconstriction (3). Combined inhibition of nitric oxide and prostaglandins augments sympathetic α-adrenergic vasoconstriction, yet the vasoconstrictor responses were substantially blunted compared with resting conditions, indicating that other signals compensate to maintain blood flow to the contracting muscle (4).
ATP and adenosine are present in both the systemic circulation and the microcirculation and may be part of cardiovascular regulation because of a direct vasodilatory effect on purinergic endothelial receptors (5). Circulating ATP, but not adenosine, was demonstrated to cause a concomitant vasodilation and inhibition of sympathetic vasoconstriction, indicating an involvement of ATP in functional sympatholysis (6). These versatile actions of ATP on vascular tone may therefore have clinical implications for the pathogenesis of hypertension and atherosclerosis and in the reduced exercise capacity observed in patients with diabetes (7), in whom the ability to increase leg blood flow (LBF) during exercise may be attenuated (8) and the vasoconstrictor responsiveness, as reflected in MSNA, is increased (9).
We have recently demonstrated that the vasodilatory effect of the purinergic system is attenuated in patients with type 2 diabetes (10). The current study focused on the capacity of functional sympatholysis in skeletal muscles during moderate exercise in patients with type 2 diabetes. The patients were selected to have a preserved endothelial function, based on their response to infusions with acetylcholine. Moreover, we evaluated the capacity of ATP as a sympatholytic agent and the vasodilatory potency of infusions of ATP and adenosine.
RESEARCH DESIGN AND METHODS
Ten subjects with type 2 diabetes and 10 age-matched healthy control subjects participated, all fully informed of the risks and discomforts associated with the experiments. The Ethics Committee for the Copenhagen County approved the study.
Patients were recruited from Steno Diabetes Center, an outpatient clinic specialized in treating patients with a history of poor glucose control or diabetes complications; subject characteristics are provided in Table 1. The patients continued their usual medication because antidiabetic, antihypertensive, and lipid-lowering drugs exhibit long-term effects without acute affection of cardiovascular regulation. Only insulin (four participants) was stopped on the day of the study. Patients with nephropathy or history of angina, acute myocardial infarction, or claudication were excluded.
Table 1.
Demographic data for diabetic subjects (n = 10) and control subjects (n = 10)
| Control subjects | Diabetic subjects | |
|---|---|---|
| Male/female | 6/4 | 6/4 |
| Age (years) | 55 ± 2 | 55 ± 2 |
| Height (m) | 1.76 ± 0.02 | 1.74 ± 0.03 |
| Weight (kg) | 83.2 ± 3 | 88.9 ± 5 |
| BMI (kg/m2) | 26.5 ± 1.0 | 29.1 ± 1.4 |
| Leg total mass (kg) | 14.5 ± 0.8 | 15.2 ± 0.4 |
| Leg muscle mass (kg) | 9.9 ± 0.6 | 10.2 ± 0.6 |
| P-glucose (mmol/L) | 5.9 ± 0.2 | 10 ± 1* |
| C-peptide (pmol/L) | 1,230 ± 283 | |
| HbA1c (%) | 5.4 ± 0.2 | 7.6 ± 1.2* |
| Range | 5.2–5.7 | 5.4–8.8 |
| Duration of diabetes (years) | 6 | |
| Range (years) | 2–13 | |
| P-cholesterol (mmol/L) | 5.8 ± 0.8 | 3.9 ± 0.6 |
| P-HDL (mmol/L) | 1.4 ± 0.4 | 1.1 ± 0.5 |
| P-LDL (mmol/L) | 3.6 ± 0.9 | 1.9 ± 1.1 |
| P-triglycerides (mmol/L) | 1.7 ± 0.9 | 3.2 ± 1.6 |
Data are presented as means ± SE, with the exception of years of diabetes (median value).
P-glucose sampled at the beginning of the first intervention because study participants were not fasting. All participants with diabetes were treated with lipid-lowering medication; one participant was only treated with sulfonyl, nine participants were treated with metformin (in combination with sulfonyl in four of these). All male patients received antihypertensive treatment with ACE inhibitors or AT-II antagonist (in combination with thiazide in three of these and with addition of Ca-antagonist in one of these). None of the control subjects received medication.
*P < 0.001.
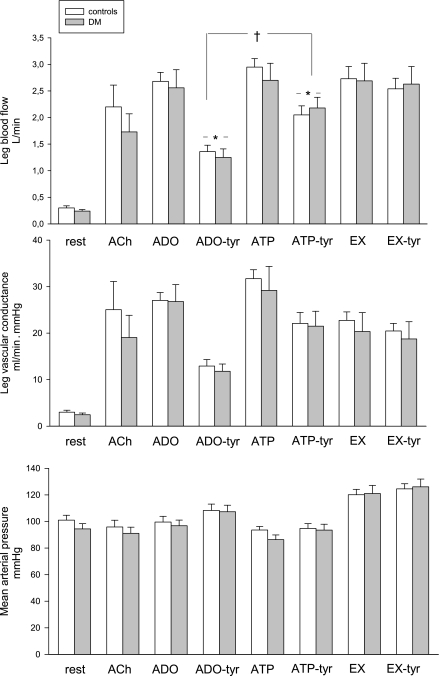
As a marker of muscle endothelial function, the elevation in LBF during acetylcholine infusion was evaluated. All 10 subjects had an increase in LBF within the normal range (Fig. 1).
Figure 1.
LBF, LVC, and MAP at baseline and during all hyperemic interventions. There were no significant differences between the groups at baseline or during the interventions. ACh, acetylcholine; ADO, adenosine; DM, diabetes mellitus; EX, exercise; tyr, tyramine. *Different from the previous intervention. †Different from ADO-tyr.
Control subjects were recruited through advertising in local newspapers, had no history of impaired glucose tolerance, and received no medications.
On the day of the experiment, participants reported to the laboratory at 8:00 a.m. after a light breakfast. BMI and leg mass were calculated from whole-body dual–energy X-ray absorptiometry scanning (GE Medical Systems, Fairfield, CT). With the participant resting in a supine position, three catheters were placed under local anesthesia in the femoral artery and vein of the right leg and in the femoral artery of the left leg using the Seldinger technique. Catheters were inserted 2 to 3 cm distal from the inguinal ligament.
The participants underwent the following protocol: a pretest in which the individual target LBF was determined during ∼2 min of knee-extensor exercise at 15 W (11). LBF was increased in a dose-response manner by infusion of adenosine or ATP until LBF matched that obtained during the pre-exercise test. Adenosine (18.7 μmol/mL; Item Development AB, Stockholm, Sweden) was infused at rates of ∼0.8 in control subjects and ∼1.1 μmol/min in patients (P = 0.38), whereas ATP (1 μmol/mL, Sigma A7699; Sigma-Aldrich Co., St. Louis, MO) was infused at rates of ∼0.8 and ∼0.9 μmol/min in control subjects and patients (P = 0.62), respectively, to increase blood flow to target LBF (∼2.8 L/min). This amount of infused ATP, sufficient to increase plasma content by an estimated 500 nmol, is within physiologic range (12).
The vasoconstrictor effect of tyramine (5.9 μmol/mL, Sigma T-2879; Sigma-Aldrich Co.), which evokes endogenous noradrenaline (NA) release from sympathetic nerve endings and subsequent postjunctional α-adrenergic vasoconstriction, was examined during adenosine (control), ATP, and exercise-induced hyperemia; the latter two were randomized (13). Tyramine was coinfused during adenosine at rates of ∼5.4 and ∼7.4 μmol/min in control subjects and patients, respectively (P = 0.12), to reduce LBF by ∼50% without affecting arterial blood pressure (6). The individual infusion rate of tyramine, resulting in 50% reduction of LBF during adenosine, was used in the following tyramine trials.
LBF was calculated from measurements of diameter and blood velocity using the Doppler ultrasound method: probe 8C (Vivid 7; GE Healthcare, Little Chalfont, Buckinghamshire, U.K.) (10,14). LBF represents the average of three measurements obtained at baseline, 4 min after the start of exercise, or 4 min after reaching steady state under infusion of ATP, adenosine, or coinfusion of tyramine.
Pressure transducers (Pressure Monitoring Kit; Baxter, Deerfield, IL) monitored mean arterial pressure (MAP); heart rate was determined from an electrocardiogram, with all data continuously recorded using a Powerlab system (ADInstruments, Sydney, Australia).
Statistical analyses were performed with SigmaPlot 11. Difference of baseline values and subject characteristics was tested using a Student t test. Δ-values of all hemodynamic variables were calculated as the difference between baselines immediately before the intervention and steady state during the intervention and analyzed by two-way ANOVA repeated measurements with nucleotides as within-subject factors and control/type 2 diabetes as between-subject factors. The Student-Newman-Keuls method was used to correct for multiple comparisons. Significance level was set at P < 0.05. Data are presented as mean ± SE unless otherwise stated.
RESULTS
Hemodynamic variables
The vasodilatory potency of adenosine and ATP was similar in control subjects and patients (309 ± 54 vs. 250 ± 81 mL/μmol ATP⋅kg [P = 0.48] and 13.3 ± 1.7 vs. 12.5 ± 4 mL/μmol adenosine⋅kg [P = 0.38]).
During adenosine and ATP infusions, LBF increased ninefold in both control subjects and patients to similar levels as during the exercise intervention (2.7 ± 0.2 L/min, Fig. 1). In both groups, tyramine infusion reduced LBF during coinfusion with adenosine from 2.6 ± 0.2 to 1.4 ± 0.1 L/min, whereas infusion of the same amount of tyramine during exercise did not reduce LBF in either group (2.6 ± 0.25 L/min).
Coinfusion with tyramine during ATP infusion reduced LBF (from 2.9 ± 0.2 to 2.0 ± 0.2 L/min in control subjects and from 2.7 ± 0.3 to 2.2 ± 0.2 L/min in patients; P = 0.55 for control subjects vs. patients) (Fig. 1).
Leg vascular conductance (LVC) increased similarly from baseline values of 3 ± 0.4 to 27 ± 2 mL/min⋅mmHg during adenosine and 30 ± 4 mL/min⋅mmHg during ATP and decreased similarly during coinfusion of tyramine (12 ± 2 during adenosine and 22 ± 3 mL/min⋅mmHg during ATP). During exercise, LVC increased in both groups to 21 ± 4 mL/min⋅mmHg and was not affected by tyramine coinfusion.
MAP at baseline was slightly higher in the control group, yet not significant and potentially reflecting antihypertensive treatment in the group with diabetes. MAP increased from baseline (101 ± 4 in control subjects vs. 94 ± 4 mmHg in patients) to similar values during exercise, 120 ± 4 mmHg in both groups. During adenosine infusion, MAP was unaltered, but during coinfusion with tyramine, MAP increased in both groups to 107 ± 3 mmHg. ATP infusion lowered MAP to 94 ± 3 in control subjects and 86 ± 4 mmHg in patients. Although coinfusion of ATP and tyramine did not affect MAP in control subjects (95 ± 4 mmHg), MAP increased (to 94 ± 4 mmHg) in patients (P < 0.05).
The arteriovenous differences of O2 content did not differ at baseline (92 ± 7 in control subjects vs. 77 ± 9 mL/L in patients, P = 0.24) or during the different interventions. During exercise, the arteriovenous difference increased similarly (130 ± 6 mL/L) and coinfusion with tyramine did not alter the values. Reflecting the alterations in LBF, with combined infusion of adenosine and tyramine, leg arteriovenous O2 difference increased from 11 ± 2 mL/L with adenosine infusion to 19 ± 3 mL/L with combined adenosine and tyramine and from 14 ± 3 to 18 ± 4 mL/L during ATP and ATP-tyramine infusion, respectively, with no differences in the groups.
O2 delivery was proportional to LBF, and a small difference in baseline LBF was reflected in a baseline difference of O2 delivery (P = 0.14). However, there were no differences in O2 delivery or uptake during the infusions of adenosine, ATP, exercise, or coinfusion of tyramine.
Heart rate at baseline tended to be higher in patients (68 ± 4 in control subjects vs. 79 ± 4 bpm in patients, P = 0.06), but during the interventions, δ values were similar. In addition, cardiac output differed at baseline (4.0 ± 0.4 in control subjects vs. 6.0 ± 0.5 L/min in patients, P = 0.005), but during ATP infusion and exercise, δ values were similar. In contrast, cardiac output increased more in the group with diabetes during adenosine infusion, both with and without tyramine, P = 0.03 (control subjects vs. patients, both adenosine and coinfusion of adenosine and tyramine).
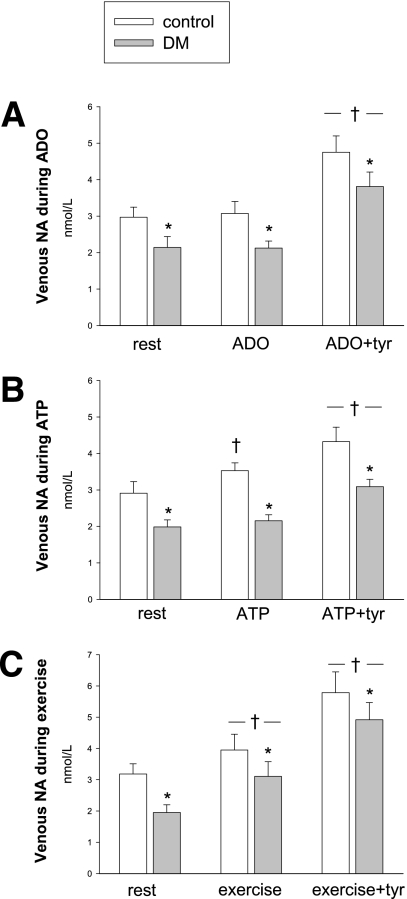
Venous NA
At baseline, femoral venous NA was different (2.6 ± 0.2 in control subjects vs. 1.8 ± 0.2 nmol/L in patients, P = 0.001), but NA increased similarly during exercise (1.3 ± 0.5 nmol/L, P = 0.98, Fig. 2). The increases during tyramine coinfusion also were similar in the two groups (2.2 ± 0.4 for adenosine, 1.5 ± 0.3 for ATP, and 3.2 ± 0.5 nmol/L during exercise P = 0.4 to 0.6), as were the increases in venous NA adjusted for the individual infusion rates of tyramine (0.38 ± 0.08 during adenosine infusion, 0.25 ± 0.04 during ATP infusion, and 0.56 ± 0.11 nmol/L during exercise per micromole of tyramine). During adenosine infusions, venous NA did not change in the two groups, whereas during ATP infusion, NA increased in the control group (P = 0.003). This difference was also reflected during combined ATP and tyramine infusions, where venous NA in the group with diabetes tended to be lower compared with adenosine (P = 0.053).
Figure 2.
Venous NA at rest and during hyperemia. A: Adenosine. B: ATP. C: Exercise. *Different from control. †Different from the previous intervention.
During combined adenosine and tyramine infusion, the flow reduction per micromole of tyramine was similar in the two groups (245 ± 40 mL/μmol tyramine), whereas during ATP infusion, the sensitivity to tyramine in terms of flow reduction was lower in the group with diabetes (182 ± 27 vs. 92 ± 34 mL/μmol tyramine, P = 0.042).
CONCLUSIONS
The main findings of the current study are that patients with type 2 diabetes and intact endothelial function have a similar capacity to blunt sympathetic vasoconstriction during moderate exercise as healthy age- and BMI-matched control subjects. However, both groups were only partially capable of blunting α-adrenergic vasoconstriction during ATP-induced hyperemia. In addition, the vasodilatory potency of adenosine and ATP did not differ between patients and control subjects. Finally, the subjects with diabetes had a lower venous content of NA but a similar elevation of venous NA during the adenosine infusions, exercise, and tyramine interventions.
Functional sympatholysis and sympatholytic effect of ATP
Consistent with a study on young healthy subjects (6), exercise fully blunted sympathetic vasoconstriction in both groups, indicating that the ability of functional sympatholysis during moderate exercise was not reduced in the group with diabetes. This may be clinically relevant given that pathology, affecting the vasodilatory function, could be expected to limit skeletal muscle blood flow because of enhanced sympathetic vasoconstriction in the active muscles, thereby potentially reducing exercise capacity.
A study on elderly healthy humans (>65 years) demonstrated that aging is associated with a greater vasoconstrictor tone in active muscles during exercise compared with young adults (15). The magnitude of functional sympatholysis was significantly lower in older persons compared with young persons in the presence of tyramine, leading the authors to conclude that aging is associated with impaired functional sympatholysis in the vascular beds of contracting forearm muscle. The present finding of intact functional sympatholysis in middle-aged healthy subjects and patients with diabetes could demonstrate that the phenomenon may be age-dependent; however, limb differences should also be kept in mind (16). Moreover, a component of systemic limitation to peripheral blood flow during exercise may explain the observed low blood flows in some studies of patients with type 2 diabetes; the knee-extensor model eliminates this risk and therefore allows studies of the local microcirculation and may not be translated to all skeletal muscles or applied to high-intensity exercise with a large muscle mass but is likely to represent the leg muscles, which accounts for the largest part of vascular resistance in the body and is of particular interest because insulin resistance is primarily present in leg muscles (17).
In young subjects, luminal ATP was shown to abolish tyramine-induced sympathetic vasoconstriction, indicating that ATP is contributing to functional sympatholysis via activation of the P2Y2 receptor (6). In contrast with this finding, both groups in the current study had a reduction in LBF during combined ATP and tyramine infusion. The sympatholytic effect of ATP is graded and dose-dependent; during very modest ATP infusions, ATP had no sympatholytic effect (18). However, the present infusion rates of ATP increased LBF ninefold, most likely increasing arterial ATP to levels sufficient to limit α-adrenergic vasoconstriction in young subjects. Therefore, the sympatholytic effect of ATP may be affected by age.
In contrast with ATP, adenosine has minor sympatholytic effect (6,13). The amount of tyramine leading to 50% reduction in LVC and LBF during adenosine infusion resulted in a ∼30% reduction during ATP; thus, ATP has a role in functional sympatholysis in the elderly, but other factors must be of importance to offset sympathetic vasoconstriction, because there were no changes in LVC or LBF during exercise with coinfusion of tyramine. Functional sympatholysis in middle-aged persons could gradually be more dependent on factors other than ATP, such as KATP channel activation, which opposes α-adrenergic vasoconstriction during muscle contraction (19).
Regardless of the compounds responsible for functional sympatholysis, endothelial function also could be of importance. The present findings do not exclude that endothelial dysfunction affects the ability of sympatholysis during exercise.
Vasodilatory action of adenosine and ATP
The amount of ATP and adenosine needed to increase LBF to levels matching exercise was similar in the two groups. In contrast, in a previous study on patients with diabetes and age-matched control subjects, we found a 50% reduction in the LBF response to ATP and adenosine in the group with diabetes (10). The LBF response in control groups was identical, whereas the response in the present group with diabetes was higher in regard to both ATP and adenosine. When comparing the two groups of patients with diabetes, there were no differences in demographic variables or medication. Only the LBF response to acetylcholine infusion was significantly different, indicating that the reduction in effect of purinergic agonist may be proportional to the extent of endothelial dysfunction.
A comparison of data from our two studies of middle-aged subjects, both control subjects and patients with diabetes, and previous studies of young subjects (20,21) demonstrates a decline in LBF response to adenosine in particular, which was reduced fourfold. In regard to ATP, there was a clear decline in sensitivity only in the group with more developed endothelial dysfunction. Still, it is a possibility that the response to ATP also is diminished in an elderly population, possibly to a minor extent, because the dose-response in young healthy subjects may not be linear for the dose interval under study, hampering data extrapolation and direct comparison (6). The decline in LBF responses to ATP and adenosine may reflect age-related changes of the signaling pathways (20,21). However, physical inactivity and obesity also affect endothelial function and cardiovascular regulation and should be of note, because both groups in the current study had moderately elevated BMI and the majority of the subjects had a sedentary lifestyle.
Venous NA and sensitivity
The interpretation of plasma levels of NA and its correlation to sympathetic activity is complex; plasma NA elevation may not be a precise measure of the effect on MSNA but merely a reflection of direction of change. It should be noted that during infusion with adenosine alone, there was no increase in venous NA, despite a ninefold increase in LBF, indicating that the increase in NA was indeed due to tyramine infusion and not to hyperemia.
Δ-values of venous NA between the two groups were similar during all three tyramine interventions. Furthermore, during moderate exercise, both with and without tyramine, the venous NA increase in the groups was similar and additive, indicating that the stimulus of exercise to the sympathetic system also may have been uniform. These findings are consistent with previous studies in young subjects, showing that NA spillover is a function of active muscle mass and exercise intensity (22).
In young subjects (6), plasma NA increased from ∼1.7 nmol L−1 at baseline to ∼3 nmol L−1 during tyramine infusions with comparable infusion rates, thus lower than in the present middle-aged control subjects. However, the levels of NA in the present group of patients with diabetes were similar to those of young subjects.
Despite increases in sympathetic nerve activity in patients with diabetes, plasma NA level has been found to be reduced compared with control subjects (23). Therefore, it is of interest to investigate whether the presented differences are a result of antidiabetic treatment or a physiologic response to elevation in sympathetic nerve activity, leading to enhanced reuptake and thus a reduced spillover.
Circulating ATP mimics exercise hyperemia by its vasodilatory potency and by increasing MSNA and circulating NA (6). Measurements of interstitial NA in skeletal muscle during ATP infusion with the microdialysis technique showed a dose-dependent increase in interstitial NA, whereas interstitial NA increased to a larger extent during exercise, despite similar LBF, consistent with the results in the present control group (24). The lack of increase in venous NA during ATP infusion in the group with diabetes may be to the result of an impaired baroreceptor function (25) or altered function of the ATP inducement of NA exocytosis because the changes in MAP during ATP infusions were similar in the two groups.
Δ-values of venous NA during tyramine infusion and the changes in LVC were proportional and similar in the two groups, suggesting that the subjects with diabetes were equally sensitive to NA as the control subjects. Therefore, the present measurements of venous NA may reflect interstitial conditions, and the preserved functional sympatholysis in the group with diabetes could be partly attributed to a reduced level of NA, perhaps in combination with or attributed to a reduction in ATP-induced sympathetic activity.
Limitations
The primary limitation is that no truly selective human antagonists and ligands of P2 receptors are currently available, which hinders confirmatory studies of the role of the purinergic system.
Power calculations ahead were impeded because the current study is the first to investigate this particular area in patients with diabetes. In our previous study on 10 control subjects and 10 patients with diabetes and endothelial dysfunction, we demonstrated clear and significant differences in the relevant variables (10). However, patients with type 2 diabetes are a heterogenic group, and sample size for the current study does not necessarily reflect such heterogeneity.
Antidiabetic and antihypertensive treatment has been shown to improve endothelial function through several pathways, and studies of patients with diabetes have been carried out primarily during withdrawal of medications; however, in most studies analyzing risk profiles of different chronic diseases, the patients are usually taking medication. It may be more accurate to investigate patients in their “normal” functional status if the drugs do not affect the measurements directly, because little is known of the time course after withdrawal.
CONCLUSIONS
Patients with well-diagnosed type 2 diabetes and only minor or no endothelial affection have an intact capacity of functional sympatholysis during moderate exercise. However, both the patients and the aging control subjects have a lower sympatholytic effect of ATP. Because this does not compromise functional sympatholysis, ATP is not mandatory for an adequate hyperemic response during exercise.
The LBF response to ATP and adenosine was similar in the two groups; thus, purinergic-induced LBF may not be affected by diabetes per se; attenuation of purinergic vasodilation in patients with diabetes could be a result of endothelial dysfunction.
When comparing the present findings with those of young subjects, the vasodilatory potency of adenosine in particular may be markedly reduced by aging and further aggravated with diabetes, in correlation to the grade of endothelial affection.
Acknowledgments
Novo Nordisk A/S provided financial support to this study. No other potential conflicts of interest relevant to this article were reported.
P.T. wrote the manuscript and researched data. L.T.B. and M.Z. researched data and contributed to the manuscript. B.S. and J.B.R. contributed to discussion and reviewed and edited the manuscript.
The authors thank Andreas Bornoe, Louise Bondo, and Christina Kjaer (all affiliated with CMRC) for technical assistance. The authors thank Steno Diabetes Center for help with patient recruiting.
References
- 1.Ray CA, Mark AL. Sympathetic nerve activity to nonactive muscle of the exercising and nonexercising limb. Med Sci Sports Exerc 1995;27:183–187 [PubMed] [Google Scholar]
- 2.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 1962;11:370–380 [DOI] [PubMed] [Google Scholar]
- 3.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol 2004;97:731–738 [DOI] [PubMed] [Google Scholar]
- 4.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 2004;287:H2576–H2584 [DOI] [PubMed] [Google Scholar]
- 5.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal 2008;4:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 2004;558:351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogarth DK, Sandbo N, Taurin S, Kolenko V, Miano JM, Dulin NO. Dual role of PKA in phenotypic modulation of vascular smooth muscle cells by extracellular ATP. Am J Physiol Cell Physiol 2004;287:C449–C456 [DOI] [PubMed] [Google Scholar]
- 8.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 2003;26:899–904 [DOI] [PubMed] [Google Scholar]
- 9.Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 2003;108:3097–3101 [DOI] [PubMed] [Google Scholar]
- 10.Thaning P, Bune LT, Hellsten Y, Pilegaard H, Saltin B, Rosenmeier JB. Attenuated purinergic receptor function in patients with type 2 diabetes. Diabetes 2010;59:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 1985;366:233–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 2002;91:1046–1055 [DOI] [PubMed] [Google Scholar]
- 13.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 2002;541:623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rådegran G, Saltin B. Human femoral artery diameter in relation to knee extensor muscle mass, peak blood flow, and oxygen uptake. Am J Physiol Heart Circ Physiol 2000;278:H162–H167 [DOI] [PubMed] [Google Scholar]
- 15.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 2005;567:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol 2004;556:1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen DB, Sacchetti M, Dela F, Ploug T, Saltin B. Glucose clearance is higher in arm than leg muscle in type 2 diabetes. J Physiol 2005;565:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 2008;586:4305–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest 1997;99:2602–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 2009;53:993–999 [DOI] [PubMed] [Google Scholar]
- 21.Mortensen SP, González-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 2009;296:R1140–R1148 [DOI] [PubMed] [Google Scholar]
- 22.Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. Am J Physiol 1989;257:H1812–H1818 [DOI] [PubMed] [Google Scholar]
- 23.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004;25:543–567 [DOI] [PubMed] [Google Scholar]
- 24.Mortensen SP, Gonzalez-Alonso J, Nielsen JJ, Saltin B, Hellsten Y. Muscle interstitial ATP and norepinephrine concentrations in the human leg during exercise and ATP infusion. J Appl Physiol 2009;107:1757–62. Epub 2009 Oct 1 [DOI] [PubMed]
- 25.Sanya EO, Brown CM, Dütsch M, Zikeli U, Neundörfer B, Hilz MJ. Impaired cardiovagal and vasomotor responses to baroreceptor stimulation in type II diabetes mellitus. Eur J Clin Invest 2003;33:582–588 [DOI] [PubMed] [Google Scholar]