Abstract
OBJECTIVE
We tested whether long-term treatment with the angiotensin II receptor antagonist irbesartan reduces nucleic acid oxidation in patients with type 2 diabetes and microalbuminuria.
RESEARCH DESIGN AND METHODS
The Irbesartan in Patients With Type 2 Diabetes and Microalbuminuria (IRMA 2) study was a 2-year multicenter randomized double-blind trial comparing irbesartan (150 and 300 mg once daily) with placebo. We studied a subgroup of 50 patients where urine samples were available for analysis of albumin and the oxidatively modified guanine nucleosides 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-oxo-7,8-dihydroguanosine (8-oxoGuo).
RESULTS
During the 2-year trial, no significant differences in 8-oxodG and 8-oxoGuo excretions between placebo and irbesartan treatment were seen. 8-oxodG and albumin excretion decreased with time (P = 0.0004 and P < 0.0001, respectively), whereas treatment-related differences were shown for albumin excretion (P = 0.0008) only, as previously reported. Important secondary findings were significant associations between changes in 8-oxodG excretion and changes in albumin excretion and glycated hemoglobin (HbA1c). During the study period, 8-oxodG excretion decreased by 3 and 26% in smokers and nonsmokers, respectively (P = 0.015), and urinary albumin excretion decreased 22% in smokers and 58% in nonsmokers (P = 0.011).
CONCLUSIONS
Irbesartan treatment was not significantly more effective than placebo in reducing nucleic acid oxidation. The results indicate that DNA oxidation in diabetes patients is reduced by various components in the treatment of diabetes where glycemic control seems to be important and addition of angiotensin II receptor antagonists does not lead to any substantial additional reduction. Furthermore, the reductions in DNA oxidation and albumin excretion seem to be counteracted by smoking.
Clinical trials have consistently shown that pharmacological blockade of the renin-angiotensin-aldosterone system reduces the development of cardiovascular morbidity and the risk of death in diabetic patients (1,2). Excessive oxidative stress, which arises as a result of an imbalance between generation and elimination of reactive oxygen species, has been suggested to be a common pathogenic mechanism in several complications of diabetes, and it has been shown that treatment with inhibitors of renin-angiotensin-aldosterone system reduces markers of oxidative stress in patients with diabetes (3).
The easiest and least invasive method to assess oxidative stress in patients is the measurement of oxidation products in urine. Together with the F2-isoprostanes, which measures lipid peroxidation, the oxidized deoxyribonucleoside 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) is the most frequently measured urinary marker of oxidative stress. 8-oxodG is considered a valid biomarker of DNA oxidation. Its propensity to mispair with adenine and mutagenic properties are well described. A specific, sensitive, and reproducible methodology has been developed. Elevated levels have been demonstrated in many diseases and from many hazardous influences, and 8-oxodG is regarded as the gold standard in this area (4).
The ribonucleoside counterpart 8-oxo-7,8-dihydroguanosine (8-oxoGuo) is one of the most extensively used biomarkers for assessment of RNA oxidation, and in the current study, we measured the urinary excretion of both 8-oxodG and 8-oxoGuo to assess the total systemic oxidative stress to DNA and RNA, respectively.
Studies have found that treatment with angiotensin II receptor blockers reduce urinary excretion of 8-oxodG in both patients with hypertension and diabetes (3,5,6), but evidence from placebo-controlled studies of the effect of long-term angiotensin II receptor blocker treatment on DNA or RNA oxidation are missing.
Diabetic patients with microalbuminuria have a 10–20 times increased risk of nephropathy compared with diabetic patients with normoalbuminuria, and treatment with the angiotensin II receptor blocker irbesartan reduces the rate of progression to macroalbuminuria, the hallmark of overt diabetic nephropathy in type 2 diabetic patients (7). Whether this renoprotective effect of irbesartan involves a reduction in oxidative stress is unknown. In the current study, we investigated this further.
Because other studies have shown differences in the rate of oxidative DNA damage between smokers and nonsmokers (8,9) and associations between smoking and progression of diabetic nephropathy (10–12), we chose to assess the effect of smoking post hoc.
The primary aim of this study was to determine whether long-term treatment with the angiotensin II receptor antagonist irbesartan reduces DNA and RNA oxidation in patients with type 2 diabetes and microalbuminuria. In addition, post hoc analyses were carried out to examine whether smoking status had any influence on changes in nucleic acid oxidation and albumin excretion during the trial.
RESEARCH DESIGN AND METHODS
The Irbesartan in Patients With Type 2 Diabetes and Microalbuminuria (IRMA 2) study protocol has been described in detail elsewhere (7). In brief, 590 hypertensive type 2 diabetic patients with microalbuminuria were included in this multinational randomized double-blind placebo-controlled study of irbesartan (150 and 300 mg) and were followed for 24 months. The primary outcome was time to onset of diabetic nephropathy, defined as persistent albuminuria in overnight specimens with a urinary albumin excretion rate >200 μg/min and ≥30% increase from baseline level. Target trough blood pressure was <135/85 mmHg 3 months after randomization. Additional antihypertensive treatment used included diuretics, calcium-channel blockers (except dihydropyridines), and β-blockers. These agents were added if target blood pressure was not reached 3 months after randomization. The study was approved by the regional ethics committees and conducted in accordance with the Helsinki Declaration.
Data ascertained for this study were collected from a subgroup of 50 Danish patients from the original IRMA 2 study who were followed at the outpatient clinic at the Steno Diabetes Center. Measurements of blood pressure, weight, glomerular filtration rate (GFR), serum hemoglobin concentration, glycated hemoglobin (HbA1c) concentration, urinary albumin excretion, urinary 8-oxodG and 8-oxoGuo, and other laboratory evaluations were performed at baseline and at 3, 12, and 24 months.
Blood pressure was measured with a sphygmomanometer in the sitting position after at least 10 min of rest. HbA1c was measured by ion-exchange high-performance liquid chromatography. The hemoglobin, cholesterol, and triglyceride concentrations were analyzed with standard laboratory assays. GFR was measured after a single intravenous injection of 5 MBq 51Cr-EDTA at 8:00 a.m. by determining the radioactivity in venous blood samples taken 180, 200, 220, and 240 min after the injection, considering sex and body weight of the patient. The results were standardized for 1.73 m2 body surface area.
The urinary albumin concentration was determined by nephelometry and the creatinine concentration in serum and urine by the Jaffe reaction.
Spot urine samples, stored at −80°C until analysis, were assayed in 2009 for the oxidatively modified guanine nucleosides 8-oxodG and 8-oxoGuo using ultraperformance liquid chromatography and tandem mass spectrometry. 8-oxodG and 8-oxoGuo were normalized against urinary creatinine concentration. Chromatographic separation was performed on an Acquity UPLC system (Waters, Milford, MA). The column used was an Acquity UPLC BEH Shield RP18 column (1.7 μm, 2.1 × 100 mm) protected with an in-line filter (4 × 2 mm, 0.2 μm), both obtained from Waters.
The mass spectrometric detection was performed on an API 3000 triple quadrupole mass spectrometer (Sciex, Toronto, Canada) equipped with an electrospray ion source (Turbospray) operated in the positive mode. Details of the analysis are described elsewhere (13).
Statistical analysis
Baseline characteristics were compared using the χ2 test for categorical variables and ANOVA for continuous variables. Results are presented as mean ± SD for normally distributed variables or median (interquartile range) for nonnormally distributed variables. Pair-wise comparisons were performed using the paired or unpaired Student t test or Mann-Whitney test.
Repeated-measures ANOVA models were used to analyze the effect of intervention on nucleic acid oxidation and urinary albumin excretion. Repeated-measures ANOVA models were also used to analyze the effect of smoking, where comparison of changes in urinary 8-oxodG, 8-oxoGuo, and albumin excretion and other possible confounding factors (weight, blood pressure, HbA1c, GFR, and hemoglobin) between smokers and nonsmokers were made. Interaction terms were added to the models to assess interaction between smoking, time, and irbesartan treatment (time × smoking × treatment group effect).
Linear regression models were used to assess the relationship between changes in urinary 8-oxodG/8-oxoGuo and changes in diabetes-related variables and possible confounders of oxidative stress (albumin excretion, weight, blood pressure, HbA1c, GFR, and hemoglobin). Interactions between treatment group and changes in albumin excretion, weight, blood pressure, HbA1c, GFR, and hemoglobin were assessed by adding interaction terms to the models. Linear regression analysis was used to determine the relationship between baseline variables and changes in urinary 8-oxodG and 8-oxoGuo as well. Because of deviation from normal distribution, the variables 8-oxodG, 8-oxoGuo, and urinary albumin excretion were log-transformed before calculation.
All statistical analyses were performed using the SAS software version 9.1 (SAS Institute, Cary, NC). Statistical significance was defined as P < 0.05. All statistical tests were two-sided.
RESULTS
Treatment groups were balanced with respect to baseline demographic, clinical, and biochemical characteristics (Table 1). There were no statistically significant differences in the use of additional hypertensive and cholesterol-lowering treatment between the groups (Supplementary Table 1).
Table 1.
Baseline characteristics of the patients
| Placebo | 150 mg irbesartan | 300 mg irbesartan | |
|---|---|---|---|
| n | 17 | 16 | 17 |
| Sex (M/F) | 13/4 | 15/1 | 14/3 |
| Age (years) | 60 (54–66) | 58 (53–67) | 55 (50–62) |
| Smokers (%) | 6 (35) | 9 (56) | 5 (29) |
| Weight (kg) | 84.8 ± 14.1 | 91.8 ± 9.5 | 87.5 ± 14.7 |
| BMI (kg/m2) | 27.2 (26.8–29.6) | 28.4 (27.1–30.4) | 29.6 (25.1–31.4) |
| Known diabetes duration (years) | 6 (3–13) | 8.5 (6–12) | 9 (4–12) |
| Systolic blood pressure (mmHg) | 155 ± 15 | 156 ± 15 | 156 ± 19 |
| Diastolic blood pressure (mmHg) | 88 ± 7 | 91 ± 11 | 89 ± 9 |
| Urinary albumin excretion (μg/min) | 63 (39–81) | 45 (25.5–85.5) | 51 (39–109) |
| GFR (mL/min per 1.73 m2) | 107.4 ± 27.7 | 115.6 ± 20.5 | 116.6 ± 21.2 |
| HbA1c (%) | 7.1 ± 1.5 | 6.9 ± 1.3 | 6.8 ± 1.5 |
| Hemoglobin (mmol/L) | 8.9 (8.6–9.2) | 9.2 (9.0–9.6) | 9.1 (8.4–9.3) |
| Cholesterol (mmol/L) | 5.56 (5.04–5.90) | 5.61 (5.02–6.31) | 5.59 (5.04–5.92) |
| LDL cholesterol (mmol/L) | 3.21 (3.08–3.57) | 3.44 (2.48–3.67) | 3.18 (2.77–3.70) |
| HDL cholesterol (mmol/L) | 1.06 (0.96–1.22) | 1.03 (0.83–1.32) | 1.11 (1.01–1.40) |
| Triglycerides (mmol/L) | 1.78 (1.46–2.86) | 2.04 (1.46–3.78) | 2.31 (1.24–3.21) |
| Serum creatinine (μmol/L) | 97 (88–106) | 97 (88–102) | 88 (80–97) |
| 8-oxodG (nmol/mmol creatinine) | 2.10 (1.76–3.05) | 1.92 (1.47–2.20) | 2.59 (1.64–3.14) |
| 8-oxoGuo (nmol/mmol creatinine) | 3.63 (2.70–4.38) | 2.86 (2.45–3.84) | 4.01 (2.99–5.08) |
Data are means ± SD or median (interquartile range) unless otherwise indicated. NS between all treatment groups.
Effects of irbesartan treatment
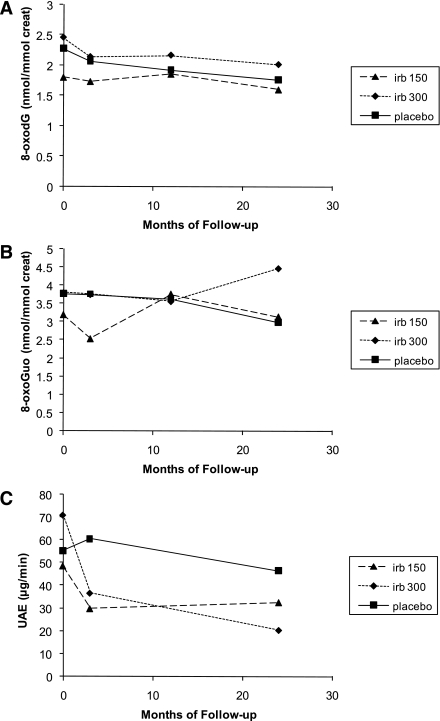
Changes in 8-oxodG, 8-oxoGuo, and albumin excretion according to treatment group during the study are shown in Fig. 1. No significant differences in 8-oxodG and 8-oxoGuo excretions between placebo and irbesartan treatment were seen during the trial.
Figure 1.
Change in 8-oxodG (A), 8-oxoGuo (B), and albumin excretion (C) in patients (n = 50) according to treatment group (placebo [n = 17], 150 mg irbesartan [irb] [n = 16], and 300 mg irbesartan [n = 17]). A: Time effect: P = 0.0004; treatment × time effect: P = 0.46. B: Time effect: P = 0.87; treatment × time effect: P = 0.11. C: Time effect: P < 0.0001; treatment × time effect: P = 0.0008. Values are geometric means. creat, creatinine; UAE, urinary albumin excretion.
Urinary 8-oxodG excretion decreased by 23% (P = 0.06), 12% (P = 0.1), and 18% (P = 0.01) in the placebo, 150 mg irbesartan, and 300 mg irbesartan groups, respectively.
Analysis by repeated-measures ANOVA showed that 8-oxodG excretion decreased with time (time effect, P = 0.0004), whereas no significant treatment × time interaction was seen (treatment × time effect, P = 0.46), reflecting no treatment-related differences in the rate of change over time (Fig. 1A). No effect of time or treatment on 8-oxoGuo excretion was shown (Fig. 1B).
Albumin excretion decreased during the study period (time effect, P < 0.0001) and a significant treatment × time interaction was shown for urinary albumin excretion (treatment × time effect, P = 0.0008), reflecting treatment-related differences in the rate of change during the trial, where the albumin excretion decreased by 16, 33, and 71% in the placebo, 150 mg irbesartan, and 300 mg irbesartan groups, respectively (Fig. 1C).
Post hoc analyses
Associations between nucleic acid oxidation and other variables.
Simple regression analysis was used to evaluate the relationship between changes in nucleic acid oxidation and changes in urinary albumin excretion, weight, blood pressure, HbA1c, GFR, and hemoglobin (Supplementary Table 2).
The combined data of all the subjects showed significant positive association (R2 = 0.18, P = 0.014) between percentage changes in 8-oxodG excretion and urinary albumin excretion, significant positive association (R2 = 0.18, P = 0.015) between the percentage changes in 8-oxodG excretion and HbA1c, and significant negative association (R2 = 0.13, P = 0.04) between percentage changes in 8-oxodG excretion and weight over the 2-year study period. There were no significant associations between changes in 8-oxoGuo and changes in urinary albumin excretion, weight, blood pressure, HbA1c, GFR, and hemoglobin.
Possible effect modification by the variables urinary albumin excretion, weight, blood pressure, HbA1c, GFR, and hemoglobin were assessed in the interaction analyses. For changes in 8-oxodG and 8-oxoGuo excretion, no interactions between treatment group and changes in the above-mentioned variables were found (data not shown).
Significant associations between percentage changes in 8-oxodG excretion and the baseline values of 8-oxodG, HDL cholesterol, and age were found. Greater 8-oxodG excretion (R2 = 0.26, P = 0.002), HDL cholesterol (R2 = 0.31, P = 0.0008), and age (R2 = 0.14, P = 0.03) at baseline were related to greater reduction in 8-oxodG excretion during the trial (Supplementary Table 2).
Effects of smoking.
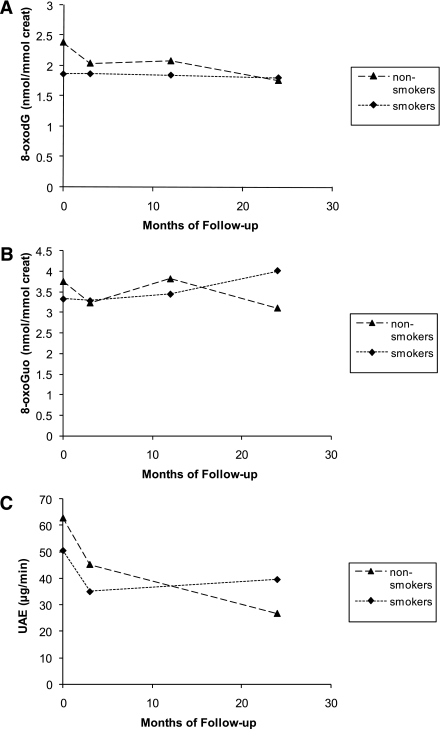
Effects of time and smoking on urinary 8-oxodG, 8-oxoGuo, and albumin excretions are shown in Fig. 2. At baseline, there were no significant differences in albumin (P = 0.35), 8-oxodG (P = 0.10), and 8-oxoGuo (P = 0.41) excretions between smokers and nonsmokers.
Figure 2.
Change in 8-oxodG (A), 8-oxoGuo (B), and albumin excretion (C) in patients (n = 50) according to smoking status (smokers [n = 20], nonsmokers [n = 30]). A: Time effect: P = 0.002; smoking group × time effect: P = 0.015. B: Time effect: P = 0.99; treatment × time effect: P = 0.10. C: Time effect: P < 0.0001; smoking group × time effect: P = 0.011. Values are geometric means. creat, creatinine; UAE, urinary albumin excretion.
Regardless of treatment regimen, both the excretion of 8-oxodG and urinary albumin showed significant group × time interactions, reflecting differences in the rate of change during the trial between smokers and nonsmokers. During the 2-year study period, 8-oxodG excretion decreased by 3% in smokers and 26% in nonsmokers (smoking group × time effect, P = 0.015) (Fig. 2A). Urinary albumin excretion decreased 22% in smokers and 58% in nonsmokers (smoking group × time effect, P = 0.011) (Fig. 2C).
No significant difference in change in urinary 8-oxoGuo between smokers and nonsmokers was shown. Figure 2B gives an impression of a difference between smokers and nonsmokers, where smokers had an increase and nonsmokers a decrease in 8-oxoGuo excretion during the trial, but a significant difference could not be shown, even when including only baseline and 2-year measurements in the analysis (smoking group × time effect, P = 0.10; baseline + 2-year smoking group × time effect, P = 0.08).
No interaction between smoking, time, and irbesartan treatment (time × smoking × treatment group effect) was shown for albumin, 8-oxoGuo, and 8-oxodG excretions (data not shown). When treatment interaction was included in the analysis, the differences between smokers and nonsmokers were still significant for both 8-oxodG (smoking group × time effect, P = 0.03) and albumin excretion (smoking group × time effect, P = 0.02).
There were no significant differences in any of the baseline variables between smokers and nonsmokers, and changes in weight, blood pressure, HbA1c, GFR, and hemoglobin during the trial did not differ between the two groups (data not shown).
CONCLUSIONS
Our study demonstrates a reduction of the urinary excretion of 8-oxodG during the 2-year trial period in 50 type 2 diabetic patients with microalbuminuria. However, there was no significant effect of treatment with irbesartan on 8-oxodG excretion compared with placebo treatment.
In previous studies, significant reductions in 8-oxodG excretion were shown after short-term treatment with candesartan for 12 weeks in 64 patients with essential hypertension (5) and with candesartan/valsartan for 8 weeks in 33 type 2 diabetic patients with nephropathy (3). Miyashita et al. (6) demonstrated a significant reduction of urinary excretion of 8-oxodG after 12 months of olmesartan treatment in 35 type 2 diabetic patients with hypertension.
Ogawa et al. (3), Dohi et al. (5), and Miyashita et al. (6) did not find significant reductions in 8-oxodG excretion in the comparison groups (calcium-channel blocker group, control group, and trichlormethiazide group), but none of the studies included comparison with a placebo group, and tests of significance of the differences between the groups were only performed in one study.
We assessed confounders by identifying associations between 8-oxodG and 8-oxoGuo excretions and other measured variables, and by performing analysis of interactions between treatment group and changes in the variables urinary albumin excretion, weight, blood pressure, HbA1c, GFR, and hemoglobin. No important confounders were identified in these analyses.
The fact that 8-oxodG excretion was reduced in a similar degree in irbesartan and placebo treatment indicates that the shown reduction in DNA oxidation is most likely induced by other factors in the pharmacological or nonpharmacological treatment of diabetes. We found a significant association between change in 8-oxodG excretion and change in HbA1c, which suggests that glycemic control is important regarding the reduction in DNA oxidation. Reduction in HbA1c was associated with a reduction in 8-oxodG excretion rate, reflecting diminished DNA oxidation, and this association was shown in other studies (14–17). To explain the association between hyperglycemia and DNA oxidation, several mechanisms have been suggested. One possible mechanism is hyperglycemia-induced mitochondrial reactive oxygen species production, which in turn leads to induction of DNA damage (18,19).
As previously shown in the original IRMA 2 study (7) and in the Microalbuminuria Reduction With Valsartan (MARVAL) study (20), treatment-related differences in the rate of change in urinary albumin excretion during the trial was demonstrated in this study, where irbesartan treatment led to a greater reduction in albumin excretion than placebo.
The reduction of albumin excretion rate was shown to be associated with a reduction in 8-oxodG excretion in this study, and this positive association was also shown by Ogawa et al. (3)
This relationship between albumin and 8-oxodG excretion is indeed noteworthy considering that albumin excretion is currently the best available noninvasive means of following the course of kidney disease in nonproteinuric diabetic patients (21) and is an independent predictor of increased risk for cardiovascular morbidity and mortality in patients with diabetes and hypertension, as well as in the general population (22).
Whether albumin excretion and excretion of 8-oxodG express the same, different, or overlapping pathophysiological mechanisms in diabetes is unknown. As albumin excretion is considered to primarily reflect renal damage and 8-oxodG excretion expresses the whole-body DNA oxidation, it seems likely that 8-oxodG excretion contains additional pathophysiological information. This leaves open the possibility that 8-oxodG excretion could be used as a supplement to albumin excretion in diabetes care. The main question in this context is whether patients experiencing both reductions in 8-oxodG and albumin excretion have a better prognosis than patients with reduction in albumin excretion but with no reduction in 8-oxodG excretion. This question must be addressed on a larger scale, which underlines the relevance of large-scale studies evaluating the predictive role of changes in 8-oxodG excretion for morbidity and mortality in diabetes.
Elevated 8-oxodG excretion, HDL cholesterol, and age at baseline were associated with greater reduction in 8-oxodG excretion during the trial. HDL cholesterol has been shown to be a predictor of progression to overt nephropathy, independently of the presence of microalbuminuria or hypertension, where higher levels of HDL cholesterol are associated with a lower risk of nephropathy (23). That high levels of HDL cholesterol are associated with greater reductions in 8-oxodG leaves open the possibility that DNA oxidation could play a role in the reduced risk of nephropathy at higher HDL cholesterol levels. This association should be investigated further for confirmation.
A significant difference in the change in 8-oxodG excretion and urinary albumin excretion between smokers and nonsmokers was seen. Nonsmokers had a greater reduction in both 8-oxodG excretion and albumin excretion during the trial period.
These results are in accordance with earlier studies of the relationship between smoking and development of nephropathy in type 2 diabetes, where smoking promotes the onset and progression of nephropathy (10–12). Smoking has also been shown to exacerbate markers of kidney failure, such as microalbuminuria (24,25), and smoking cessation has been shown to reduce the progressive damage to the kidneys in type 2 diabetes (11).
Our results indicate that smoking status is not only a predictor of nephropathy but also an important predictor of the change in DNA oxidation in type 2 diabetic patients with microalbuminuria.
The statistical power of our analysis is limited by the relatively small sample size. However, the size of our population is sufficient to show significant reductions in 8-oxodG and albumin excretion and to show the significant difference in albumin excretion between placebo and irbesartan treatment. Given the three-group design, our study had 93% power to detect an effect size of 0.56 in the change in albumin excretion, which is considered to be a large effect. The effect size of the change in 8-oxodG excretion was in our study estimated to be only 0.14, and with such a small effect size, it would require a total sample size of 450 to achieve 80% power. This means that if a difference in the change in 8-oxodG excretion between the groups actually exists, it is too small to be detected here, since our study was not designed to detect such small differences.
Another important limitation of our study is that no information regarding changes in smoking patterns was available. We cannot rule out that changes in smoking habits during the trial could be a confounder regarding treatment effects. In this study, we conclude that patients who were smokers at baseline had a smaller reduction in albumin and 8-oxodG excretion than nonsmokers, but the effect of smoking reduction/cessation could not be investigated.
Despite the limitations described above, strengths that distinguish the current evaluation from previous studies are that our study has the benefit of being a placebo-controlled trial and having a longer observation period than previous studies of the relationship between renin-angiotensin-aldosterone system inhibition and DNA oxidation.
In summary, in patients with type 2 diabetes and microalbuminuria, long-term treatment with the angiotensin II receptor antagonist irbesartan did not lead to a greater reduction in urinary excretion of nucleic acid oxidation markers than placebo treatment. Post hoc analyses showed significant associations between changes in 8-oxodG excretion and changes in albumin excretion and HbA1c and a greater reduction in both the urinary excretion of the DNA oxidation marker 8-oxodG and albumin in nonsmokers than in smokers.
These results indicate that DNA oxidation in diabetic patients is reduced by various factors in the diabetes treatment, where glycemic control seems to be important, and addition of angiotensin II receptor blockers does not have a significant effect on nucleic acid oxidation. Furthermore, the reductions in DNA oxidation and albumin excretion seem to be counteracted by smoking.
Supplementary Material
Acknowledgments
This study was supported by research funding from the Research Committee at Copenhagen University Hospital–Rigshospitalet (Rigshospitalets Forskningsudvalg), the Danish Medical Research Council, Aase and Ejnar Danielsen Foundation, and P. Carl Petersen Foundation.
No potential conflicts of interest relevant to this article were reported.
K.B. researched data, contributed to discussion, reviewed and edited the manuscript, and wrote the manuscript. T.H. and A.W. researched data and reviewed and edited the manuscript. M.P., J.T.A., S.A., and E.J.-S. researched data, contributed to discussion, and reviewed and edited the manuscript. F.P., H.-H.P., and P.R. researched data and reviewed and edited the manuscript. H.E.P. researched data, contributed to discussion, and reviewed and edited the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2214/-/DC1.
References
- 1.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–259 [PubMed] [Google Scholar]
- 2.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G, The Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000;342:145–153 [DOI] [PubMed] [Google Scholar]
- 3.Ogawa S, Mori T, Nako K, Kato T, Takeuchi K, Ito S. Angiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension 2006;47:699–705 [DOI] [PubMed] [Google Scholar]
- 4.Mistry V, Teichert F, Sandhu JK, et al. Non-invasive assessment of oxidatively damaged DNA: liquid chromatography-tandem mass spectrometry analysis of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Methods Mol Biol 2011;682:279–289 [DOI] [PubMed] [Google Scholar]
- 5.Dohi Y, Ohashi M, Sugiyama M, Takase H, Sato K, Ueda R. Candesartan reduces oxidative stress and inflammation in patients with essential hypertension. Hypertens Res 2003;26:691–697 [DOI] [PubMed] [Google Scholar]
- 6.Miyashita Y, Saiki A, Endo K, et al. Effects of olmesartan, an angiotensin II receptor blocker, and amlodipine, a calcium channel blocker, on Cardio-Ankle Vascular Index (CAVI) in type 2 diabetic patients with hypertension. J Atheroscler Thromb 2009;16:621–626 [DOI] [PubMed] [Google Scholar]
- 7.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870–878 [DOI] [PubMed] [Google Scholar]
- 8.Loft S, Vistisen K, Ewertz M, Tjønneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis 1992;13:2241–2247 [DOI] [PubMed] [Google Scholar]
- 9.Priemé H, Loft S, Klarlund M, Grønbaek K, Tønnesen P, Poulsen HE. Effect of smoking cessation on oxidative DNA modification estimated by 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion. Carcinogenesis 1998;19:347–351 [DOI] [PubMed] [Google Scholar]
- 10.Biesenbach G, Grafinger P, Janko O, Zazgornik J. Influence of cigarette-smoking on the progression of clinical diabetic nephropathy in type 2 diabetic patients. Clin Nephrol 1997;48:146–150 [PubMed] [Google Scholar]
- 11.Ritz E, Ogata H, Orth SR. Smoking: a factor promoting onset and progression of diabetic nephropathy. Diabetes Metab 2000;26(Suppl. 4):54–63 [PubMed] [Google Scholar]
- 12.Gambaro G, Bax G, Fusaro M, et al. Cigarette smoking is a risk factor for nephropathy and its progression in type 2 diabetes mellitus. Diabetes Nutr Metab 2001;14:337–342 [PubMed] [Google Scholar]
- 13.Henriksen T, Hillestrøm PR, Poulsen HE, Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2′-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic Biol Med 2009;47:629–635 [DOI] [PubMed] [Google Scholar]
- 14.Hinokio Y, Suzuki S, Hirai M, Chiba M, Hirai A, Toyota T. Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia 1999;42:995–998 [DOI] [PubMed] [Google Scholar]
- 15.Goodarzi MT, Navidi AA, Rezaei M, Babahmadi-Rezaei H. Oxidative damage to DNA and lipids: correlation with protein glycation in patients with type 1 diabetes. J Clin Lab Anal 2010;24:72–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa T, Sasahara T, Kiritoshi S, et al. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care 2003;26:1507–1512 [DOI] [PubMed] [Google Scholar]
- 17.Xu GW, Yao QH, Weng QF, Su BL, Zhang X, Xiong JH. Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal 2004;36:101–104 [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 2007;9:343–353 [DOI] [PubMed] [Google Scholar]
- 19.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 2005;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viberti G, Wheeldon NM, MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002;106:672–678 [DOI] [PubMed] [Google Scholar]
- 21.Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 2000;49:1399–1408 [DOI] [PubMed] [Google Scholar]
- 22.Karalliedde J, Viberti G. Microalbuminuria and cardiovascular risk. Am J Hypertens 2004;17:986–993 [DOI] [PubMed] [Google Scholar]
- 23.Bruno G, Merletti F, Biggeri A, et al. Progression to overt nephropathy in type 2 diabetes: the Casale Monferrato Study. Diabetes Care 2003;26:2150–2155 [DOI] [PubMed] [Google Scholar]
- 24.Nilsson PM, Gudbjörnsdottir S, Eliasson B, Cederholm J, Steering Committee of the Swedish National Diabetes Register Smoking is associated with increased HbA1c values and microalbuminuria in patients with diabetes—data from the National Diabetes Register in Sweden. Diabetes Metab 2004;30:261–268 [DOI] [PubMed] [Google Scholar]
- 25.Chuahirun T, Simoni J, Hudson C, et al. Cigarette smoking exacerbates and its cessation ameliorates renal injury in type 2 diabetes. Am J Med Sci 2004;327:57–67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.