Abstract
OBJECTIVE
To 1) compare associations of diet-quality scores, which were inversely associated with cardiovascular disease, with incident type 2 diabetes and 2) test for differences in absolute-risk reduction across various strata.
RESEARCH DESIGN AND METHODS
Men from the Health Professionals Follow-Up Study, who were initially free of type 2 diabetes, cardiovascular disease, or cancer (n = 41,615), were followed for ≤20 years. The Healthy Eating Index (HEI) 2005, the alternative HEI (aHEI) the Recommended Food Score, the alternative Mediterranean Diet (aMED) Score, and the Dietary Approaches to Stop Hypertension (DASH) Score were calculated from food-frequency questionnaires. Cox proportional hazard models with time-varying covariates were used to assess risk by quintiles and continuous intervals.
RESULTS
There were 2,795 incident cases of type 2 diabetes. After multivariate adjustment, the aHEI, aMED, and DASH scores were significantly associated with reduced risk. A 1-SD increase was associated with 9–13% reduced risk (P < 0.01), and the DASH score was associated with lower risk independent of other scores. These scores were associated with lower absolute risk among those who were overweight or obese compared with normal weight (P for interaction < 0.01).
CONCLUSIONS
Several diet-quality scores were associated with a lower risk of type 2 diabetes and reflect a common dietary pattern characterized by high intakes of plant-based foods such as whole grains; moderate alcohol; and low intakes of red and processed meat, sodium, sugar-sweetened beverages, and trans fat. High-quality diets may yield the greatest reduction in diabetes cases when followed by those with a high BMI.
Type 2 diabetes remains a major cause of morbidity and mortality worldwide. By 2030, nearly 400 million people will suffer from type 2 diabetes (1). Although the major cause of type 2 diabetes is overweight, which is determined by energy imbalance, diet quality plays an important role (2).
“High-quality” or “prudent” diets are rich in fruits and vegetables, and are associated with a reduced risk of cardiovascular disease (CVD) (3). This is attributed to lower blood lipids, blood pressure, and inflammation (3) but may also be due to lower blood glucose and diabetes risk (2). Thus, high quality diets have the potential to substantially reduce the global burden of several important chronic diseases.
Dietary guidelines for large populations are beginning to reflect high-quality diets. In 1994, the Healthy Eating Index (HEI) was developed from the Dietary Guidelines for Americans (4). This 100-point score awards points for dietary diversity; higher intakes of grains, vegetables, fruit, and milk; and lower intakes of meat, total fat, saturated fat, cholesterol, and sodium. In the Health Professionals Follow-Up Study and the Nurses’ Health Study, the HEI was associated with a modest reduction in the risk of CVD (5,6), however its relationship with type 2 diabetes has not been studied. Since the HEI does not award points for carbohydrate quality (e.g., amount of whole grains), it might not be strongly associated with type 2 diabetes. The relationship between other high-quality diet scores, such as the Dietary Approaches to Stop Hypertension (DASH) Score, and type 2 diabetes has also not been studied, despite them being inversely associated with CVD (7).
It is also unclear whether preexisting diabetes risk factors, such as a high BMI, affect the extent to which high-quality diets are associated with lower absolute risk rather than lower relative risk. A deeper understanding of which subgroups benefit from high-quality diets in terms of number of cases could greatly improve the success of public health messages.
For these reasons, we evaluated the relationship between several diet-quality scores designed for use in the U.S. population with risk of type 2 diabetes in a well-characterized cohort of men. We also tested whether age, smoking status, alcohol intake, family history, physical activity, and BMI altered these relationships when diabetes incidence was the outcome.
RESEARCH DESIGN AND METHODS
The Health Professionals Follow-Up Study is a prospective cohort study of 51,529 male health professionals. Questionnaires were mailed to participants every 2–4 years, beginning in 1986, to assess lifestyle and health status. Approximately 94% of participants completed more than one follow-up questionnaire.
Diet-quality scores
Participants completed food-frequency questionnaires (FFQs) every 4 years, which were validated against diet records (8). Correlations for dietary patterns (prudent = 0.5), macronutrients (protein = 0.4, total fat = 0.6, and carbohydrate = 0.7), and minerals (sodium = 0.5, potassium = 0.7) were similar (8,9). Reliability of the FFQ was assessed by repeat annual administration, and correlations were between 0.5 and 0.7 (8,9).
Diet-quality scores were selected from the literature and publications from our research group (Supplementary Table A). These scores included the HEI as revised in 2005 (HEI-2005), which was adapted to our FFQs by T.T.F. and S.E.C. It includes updated recommendations on whole grains, dark green and orange vegetables, and legumes (4). McCullough et al. (10) created the alternative HEI (aHEI) by combining fruit and vegetable categories (e.g., total plus whole fruits), eliminating others (e.g., total grains), and adding foods associated with chronic disease in recent studies (e.g., nuts, cereal fiber). The 51-point Recommended Food Score (RFS) was developed by Kant et al. (11) to measure dietary diversity in the National Health and Nutrition Examination Survey. It awards points for weekly intake of 51 foods (e.g., fruit, vegetables, whole grains, lean meats, and low-fat dairy) and was adapted by M. McCullough and L.d.K. for our FFQs (10). The alternative Mediterranean Diet (aMED) Score was developed by Fung et al. (12) from the 9-point Trichopolou MED Score and awards one point for above-median intakes of vegetables (no potatoes), legumes, whole grains, fruits, nuts, and fish; ratio of monounsaturated to saturated fat; moderate intakes of alcohol; and below-median intakes of red and processed meat. The 40-point DASH score was developed by T.T.F. and S.E.C. and awards points for higher intakes of foods related to a lower risk of hypertension (fruits, vegetables, low-fat dairy, nuts, legumes, and whole grains) and lower intakes of harmful foods (sodium, red and processed meats, and sweetened beverages) (7).
Confirmation of type 2 diabetes
Self-reported cases of type 2 diabetes were confirmed when at least one of the symptoms, positive diagnostic glucose tests, and medication use were reported on a supplementary questionnaire. Glucose criteria were from the National Diabetes Data Group (cases prior to 1998) and the American Diabetes Association (cases after 1998) (13). In a validation study, 97% of cases were confirmed by medical record review (13).
Statistical analysis
Participants with type 2 diabetes, CVD (heart attack, stroke, angina, or coronary artery bypass graft), cancer, or implausible energy intake (<800 or >4200 kcal/day) were excluded at baseline, leaving 41,615 participants.
Person-time was calculated from the return of the 1986 questionnaire until 31 January 2006, death, loss to follow-up, development of type 2 diabetes, or whichever occurred first. Associations between quintiles of diet-quality scores and type 2 diabetes were tested with Cox proportional hazard models with time-varying covariates. Diet-quality scores and dietary covariates (coffee, total energy) were calculated as cumulative averages at each time point and were not updated if participants reported a diagnosis of CVD or cancer. Coffee, which is inversely associated with type 2 diabetes, was adjusted for because it was not captured in the diet-quality scores and could result in some confounding. Other covariates were updated at each time point. A secondary analysis tested whether baseline diet quality was associated with type 2 diabetes risk.
Regression covariates were smoking (never, previous, current [1–14 cigarettes/day or >14 cigarettes/day], or missing), physical activity (quintiles; metabolic equivalents hours/week or missing), coffee intake (quintiles; cups/day), family history of type 2 diabetes, BMI (<23, 23–23.9, 24–24.9, 25–26.9, 27–28.9, 29–30.9, 31–32.9, 33–34.9, or >35 kg/m2 or missing), and total energy intake (quintiles; kcal/day). Missing values for smoking, physical activity, and BMI were imputed from the previous assessment. Linear trends were evaluated using the Wald test of the median diet score in each quintile.
Strength of association was evaluated by comparing risk in the top versus the bottom quintiles and continuous intakes (per 1 SD). Differences were defined as significant when the 95% CIs did not overlap. Model fit was assessed by Akaike information criteria (AIC).
Scores that were significantly associated with type 2 diabetes were included pairwise in the same model to test for independent associations. This would indicate unique, residual dietary variation related to type 2 diabetes.
To assess the public health impact of high-quality diets according to age (<65 vs. ≥65 years), smoking (ever vs. never smoked), alcohol intake (drinkers vs. abstainers), family history of type 2 diabetes, physical activity (low [quintiles 1–2], medium [quintiles 3–4], or high [quintile 5]), and BMI (<25, 25–30, or ≥30 kg/m2), we stratified our analysis and performed interaction tests over the entire follow-up using absolute risk as the outcome. Interaction significance was evaluated using the Wald test of cross-product terms (e.g., median diet score times median BMI).
Analyses were repeated with continuous covariates to assess residual confounding.
SAS version 9.1 (Cary, NC) was used for analysis, and a P value ≤ 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
The mean of the HEI-2005 was 67.4 (SD 9.8), aHEI score 44.2 (11.2), RFS 17.6 (7.3), aMED score 4.3 (2.0), and DASH score 23.8 (5.4). Higher scores were associated with a significantly higher glycemic load and cereal fiber intake except for the HEI-2005, which was associated with a lower glycemic load (Table 1). Higher scores also were associated with significantly higher intakes of polyunsaturated fat and lower intakes of trans fat and heme iron. All scores were associated with a lower BMI, higher physical activity, and a lower prevalence of current smoking. Alcohol intake was inversely associated with all scores except for the aHEI, which was associated with higher alcohol intake. The HEI-2005 had a weak inverse association with family history.
Table 1.
Age-adjusted characteristics of participants by quintiles of diet-quality scores, at baseline
| HEI-2005 |
aHEI |
RFS |
aMED |
DASH |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | |
| Quintile score range | 24–59 | 77–99 | 8–34 | 54–87 | 0–11 | 24–51 | 0–2 | 7–9 | 8–19 | 29–40 |
| n | 8,659 | 7,714 | 8,557 | 8,015 | 8,778 | 8,780 | 8,118 | 5,951 | 9,419 | 8,551 |
| Glycemic load | 133 (52) | 112 (40) | 101 (39) | 151 (51) | 97.2 (40.2) | 154 (49) | 102 (41) | 152 (49) | 107 (43) | 148 (49) |
| Cereal fiber (g/day) | 4.2 (2.5) | 7.9 (4.9) | 4.5 (2.6) | 7.6 (4.9) | 5.0 (3.8) | 6.5 (3.5) | 4.5 (2.9) | 7.2 (3.9) | 4.2 (2.5) | 7.6 (4.5) |
| Polyunsaturated fat (% E) | 12.7 (3.6) | 13.3 (3.5) | 12.1 (3.1) | 14.1 (4.0) | 13.1 (3.8) | 13.0 (3.1) | 12.1 (3.1) | 14.0 (3.5) | 12.9 (3.3) | 13.2 (3.8) |
| Trans fat (% E) | 3.2 (1.2) | 2.2 (0.9) | 3.7 (1.2) | 1.9 (0.8) | 3.3 (1.3) | 2.3 (0.9) | 3.4 (1.1) | 2.2 (0.9) | 3.4 (1.1) | 2.1 (0.9) |
| Heme iron (g/day) | 1.5 (0.6) | 1.2 (0.5) | 1.6 (0.6) | 1.0 (0.4) | 1.4 (0.6) | 1.2 (0.5) | 1.5 (0.6) | 1.1 (0.4) | 1.6 (0.5) | 1.0 (0.4) |
| Energy (kcal/day) | 2,329 (661) | 1,672 (476) | 1,724 (541) | 2,254 (633) | 1,671 (535) | 2,339 (625) | 1,761 (549) | 2,285 (607) | 1,825 (577) | 2,188 (601) |
| Alcohol (g/day) | 20.8 (22.2) | 6.8 (7.0) | 9.6 (18.1) | 12.6 (12.6) | 12.4 (17.0) | 10.4 (13.4) | 12.3 (18.5) | 11.5 (11.7) | 12.6 (16.9) | 9.7 (13.4) |
| BMI (kg/m2) | 25.7 (3.4) | 24.9 (3.1) | 25.9 (3.5) | 24.8 (3.2) | 25.6 (3.3) | 25.3 (3.4) | 25.8 (3.3) | 24.9 (3.2) | 25.8 (3.5) | 24.8 (3.1) |
| Physical activity (METS/day) | 16.8 (24.8) | 27.0 (34.9) | 14.9 (22.9) | 31.0 (36.1) | 16.7 (27.9) | 27.4 (33.0) | 15.6 (23.4) | 29.8 (34.7) | 15.1 (22.8) | 30.4 (35.9) |
| Current smoking (%) | 19 | 4 | 16 | 5 | 15 | 6 | 16 | 5 | 17 | 4 |
| Family history of type 2 diabetes (%) | 11 | 11 | 12 | 11NS | 12 | 12NS | 12 | 11NS | 11 | 12NS |
Data are means (SD) for continuous variables or percentages for dichotomous variables. All trends are statistically significant (P < 0.05) except when labeled NS (not significant). METS, metabolic equivalents.
Diet scores were significantly correlated (Supplementary Table B), ranging from 0.80 (aHEI vs. aMED) to 0.33 (RFS vs. HEI-2005).
Cox regression
There were 2,795 cases of type 2 diabetes over 20 years of follow-up (733,291 person-years). Diet-quality scores were significantly associated with decreased risk in age-adjusted models (Table 2), but after multivariate adjustment the RFS and HEI-2005 were not. For example, participants in the top quintile of the DASH score had a 25% lower risk than those in the bottom quintile (P < 0.01). This reduction was not significantly different compared with the aMED (25%) or aHEI (23%) scores, although the DASH score fit the data best according to the AIC. Using a baseline measure of the dietary scores did not principally alter these results or modify the order of associations (data not shown).
Table 2.
Risk of type 2 diabetes according to quintiles of cumulatively averaged diet-quality scores
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend | |
|---|---|---|---|---|---|---|
| HEI-2005 | ||||||
| Quintile range | 24–59 | 60–65 | 66–70 | 71–76 | 77–99 | |
| Cases/person-years | 623/144,032 | 629/149,600 | 553/151,510 | 533/149,752 | 457/138,397 | |
| Age-adjusted HR | 1.00 | 0.97 (0.87–1.08) | 0.82 (0.73–0.92) | 0.79 (0.70–0.89) | 0.72 (0.64–0.82) | <0.01 |
| Multivariate HR | 1.00 | 1.02 (0.91–1.15) | 0.91 (0.80–1.02) | 0.94 (0.83–1.07) | 0.96 (0.84–1.10) | 0.31 |
| aHEI | ||||||
| Quintile range | 8–34 | 35–40 | 41–46 | 47–53 | 54–87 | |
| Cases/person-years | 671/144,148 | 645/148,099 | 598/148,983 | 494/148,951 | 387/143,110 | |
| Age-adjusted HR | 1.00 | 0.92 (0.82–1.02) | 0.82 (0.73–0.92) | 0.68 (0.60–0.76) | 0.54 (0.47–0.61) | <0.01 |
| Multivariate HR | 1.00 | 0.97 (0.86–1.08) | 0.95 (0.83–1.05) | 0.84 (0.74–0.95) | 0.77 (0.67–0.88) | <0.01 |
| RFS | ||||||
| Quintile range | 0–11 | 12–15 | 16–19 | 20–23 | 24–51 | |
| Cases/person-years | 604/145,982 | 560/146,678 | 591/153,985 | 529/141,478 | 511/145,168 | |
| Age-adjusted HR | 1.00 | 0.92 (0.82–1.03) | 0.91 (0.81–1.02) | 0.86 (0.76–0.97) | 0.84 (0.75–0.95) | <0.01 |
| Multivariate HR | 1.00 | 0.95 (0.84–1.07) | 0.98 (0.87–1.10) | 0.97 (0.86–1.10) | 0.96 (0.84–1.10) | 0.71 |
| aMED | ||||||
| Quintile range | 0–2 | 3 | 4 | 5–6 | 7–9 | |
| Cases/person-years | 705/151,824 | 572/139,328 | 575/145,260 | 538/155,632 | 405/141,248 | |
| Age-adjusted HR | 1.00 | 0.85 (0.76–0.95) | 0.81 (0.72–0.90) | 0.75 (0.67–0.84) | 0.57 (0.50–0.64) | <0.01 |
| Multivariate HR | 1.00 | 0.92 (0.82–1.03) | 0.91 (0.81–1.02) | 0.89 (0.79–1.00) | 0.75 (0.66–0.86) | <0.01 |
| DASH | ||||||
| Quintile range | 8–19 | 20–22 | 23–25 | 26–28 | 29–40 | |
| Cases/person-years | 725/156,191 | 650/147,910 | 506/144,880 | 529/147,425 | 385/136,885 | |
| Age-adjusted HR | 1.00 | 0.89 (0.80–0.99) | 0.70 (0.63–0.79) | 0.71 (0.63–0.79) | 0.55 (0.48–0.62) | <0.01 |
| Multivariate HR | 1.00 | 0.93 (0.83–1.04) | 0.77 (0.69–0.87) | 0.83 (0.74–0.94) | 0.75 (0.65–0.85) | <0.01 |
Data are HR (95% CI). Multivariate models are adjusted for smoking, physical activity, coffee intake, family history of type 2 diabetes, BMI, and total energy. AIC values for the multivariate models are HEI-2005: 21,876; aHEI: 21,861; RFS: 21,880; aMED: 21,863; and DASH: 21,852 (a smaller number indicates a better fit of the model to the data).
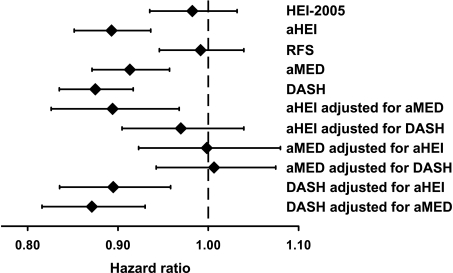
Similar results were obtained using continuous scores. A 1-SD increase in the aHEI, aMED, or DASH scores was associated with 9–13% decreased risk, which was not significantly different from each other (Fig. 1). The DASH score had the lowest risk estimate (hazard ratio [HR] 0.87 [95% CI 0.83–0.92], P < 0.01) and fit the data best according to the AIC.
Figure 1.
Standardized and mutually adjusted associations of diet-quality scores with risk of type 2 diabetes. HRs and their 95% CI are shown for an increase of 1 SD (HEI-2005: 9.8; aHEI: 11.2; RFS: 7.3; aMED: 2.0; and DASH: 5.5). Models are adjusted as in Table 2. The AIC values are as follows: HEI-2005: 21,875; aHEI: 21,853; RFS: 21,874; aMED: 21,861; and DASH: 21,844 (a smaller number indicates a better fit of the model to the data).
After mutually adjusting scores that were significantly related to type 2 diabetes, the DASH score remained significantly and inversely associated with risk (Fig. 1).
There were significant interactions between BMI and the aHEI, aMED, and DASH scores, with absolute risk as the outcome (Table 3). Greater reductions in absolute risk of type 2 diabetes were observed among those who were overweight or obese compared with normal weight.
Table 3.
Stratified analysis of DASH score and absolute risk of type 2 diabetes risk
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend | |
|---|---|---|---|---|---|---|
| Quintile range | 8–19 | 20–22 | 23–25 | 26–28 | 29–40 | |
| Age | ||||||
| <65 years | Reference category | +5 (−44 to +54) | −55 (−104 to −6) | −50 (−99 to −1) | −50 (−99 to −1) | 0.02 |
| ≥65 years | Reference category | −35 (−133 to +63) | −120 (−208 to −32) | −55 (−143 to +33) | −125 (−213 to −37) | 0.01 |
| P for interaction | 0.56 | |||||
| Current or previous smoker | ||||||
| Yes | Reference category | −20 (−89 to +49) | −100 (−169 to −31) | −75 (−144 to −6) | −135 (−204 to −66) | <0.01 |
| No | Reference category | −15 (−74 to +44) | −70 (−129 to −11) | −45 (−104 to +14) | −55 (−114 to −4) | 0.05 |
| P for interaction | 0.06 | |||||
| Consumes alcohol | ||||||
| Yes | Reference category | −20 (−69 to +29) | −80 (−129 to −31) | −75 (−124 to −26) | −95 (−144 to −46) | <0.01 |
| No | Reference category | +10 (−98 to +118) | −100 (−198 to −2) | +25 (−83 to +133) | −75 (−183 to +33) | 0.27 |
| P for interaction | 0.26 | |||||
| Family history of type 2 diabetes | ||||||
| Yes | Reference category | +5 (−191 to +201) | −105 (−291 to +81) | −110 (−296 to +76) | −120 (−316 to +76) | 0.12 |
| No | Reference category | −20 (−69 to +29) | −90 (−129 to −51) | −60 (−99 to −21) | −95 (−144 to −46) | <0.01 |
| P for interaction | 0.28 | |||||
| Physical activity | ||||||
| Low (quintile 1 + 2) | Reference category | +30 (−48 to +108) | −100 (−178 to −22) | −55 (−133 to +23) | −95 (−183 to −7) | 0.01 |
| Medium (quintile 3 + 4) | Reference category | −65 (−134 to −4) | −65 (−134 to +4) | −80 (−149 to −11) | −110 (−179 to −41) | <0.01 |
| High (quintile 5) | Reference category | +10 (−108 to +88) | −115 (−203 to −27) | −50 (−138 to +38) | −95 (−183 to −7) | 0.03 |
| P for interaction | 0.20 | |||||
| BMI | ||||||
| Normal (<25 kg/m2) | Reference category | +15 (−34 to +64) | −40 (−79 to −1) | +10 (−29 to +49) | −30 (−69 to +9) | 0.16 |
| Overweight (25–29.9 k/m2) | Reference category | −40 (−109 to +29) | −135 (−194 to −76) | −115 (−184 to −46) | −155 (−224 to −86) | <0.01 |
| Obese (≥ 30 kg/m2) | Reference category | −25 (−250 to +200) | −80 (−315 to +155) | −165 (−410 to +80) | −205 (−479 to +69) | 0.09 |
| P for interaction | <0.01 | |||||
Data are changes in absolute risk per 100,000 person-years (95% CI). Models are adjusted as in Table 2, except for the stratifying factor. Stratified analysis using the aHEI and aMED scores yielded similar results. Participants can contribute person-time to multiple strata.
Repeating the analysis using continuous covariates did not materially alter the results (data not shown).
CONCLUSIONS
In this analysis, several diet-quality scores were associated with similar reductions in type 2 diabetes risk, which points to a common underlying dietary pattern. High-quality diets were associated with greater reductions in the number of type 2 diabetes cases among individuals with a high BMI.
The effects of high-quality diets on type 2 diabetes may be mediated by many factors. A low glycemic load minimizes postprandial glucose spiking, whereas fiber from whole grains, legumes, and nuts reduces glucose absorption (14,15). Both may improve insulin demand and β-cell function. Magnesium from nuts and whole grains is also a cofactor for cellular glucose uptake and oxidation (16). Polyunsaturated fats from vegetable oils and nuts reduce postprandial triglycerides and increase skeletal muscle cell membrane fluidity and glucose uptake compared with saturated fats (17). Low-fat dairy is included in high-quality diets to reduce the intake of saturated fat but may provide additional benefits because dairy proteins stimulate the secretion of insulinotropic peptides (18). Mediterranean-type diets include alcohol, which, in moderation, increases insulin sensitivity by an unknown mechanism (19). Most high-quality diets restrict the intake of red and processed meat because they are major sources of saturated fat and other potentially harmful components. For example, heme iron can accumulate in tissues and potentially damage β-cells through oxidative stress (20). Nitrates in processed meats are converted into nitrosamines in the intestines and promote insulin resistance in rodents (20). Moreover, advanced glycation end products are formed when meat is cooked at high temperatures and induce insulin resistance in mice (20).
In support of these mechanisms, whole grains (14), alcohol (19), low-fat dairy (18), polyunsaturated fat (17), and magnesium (16) are associated with lower risk of type 2 diabetes, whereas glycemic load (15), red and processed meat (20), sugar-sweetened beverages (21), and trans fat (17) are associated with higher risk in meta-analyses of prospective cohort studies. In a meta-analysis of controlled trials, legumes improved glycemic control in people with or without type 2 diabetes (22), whereas fish oil had no impact on glycemic control among patients with diabetes (23). Interestingly, fruits and vegetables were not associated with type 2 diabetes in a meta-analysis (24), which may be because potatoes, sometimes classified as a vegetable, have a high glycemic index and would bias associations toward the null (15). These findings are consistent with publications from the Health Professionals Follow-Up Study and the Nurses’ Health Study.
Taken together, high-quality diets should have the greatest impact on type 2 diabetes if they include whole grains, nuts, legumes, moderate amounts of alcohol, and low-fat dairy, at the expense of glycemic load, red and processed meat, sugar-sweetened beverages, and trans fat. Fruits and vegetables also should be included because they can replace harmful foods but may not be as important as other components for diabetes prevention. Fish should be included for the same reason and because of its inverse association with CVD mortality (3). Finally, sodium should be minimized because of its positive association with hypertension and CVD (7).
Among the scores tested, the DASH and aHEI reflected this evidence most strongly, whereas the HEI-2005 and RFS reflect this evidence most weakly. Not surprisingly, the HEI-2005 and RFS were not significantly associated with type 2 diabetes after multivariate adjustment. In the Health Professionals Follow-Up Study, participants in the top quintile of the original HEI or RFS had a 28% (6) and a 23% (10) lower CVD risk compared with participants in the bottom quintile. A high HEI (top vs. bottom quintile) also was significantly associated with a 14% lower CVD risk in the Nurses’ Health Study (5). This suggests that these scores are associated with blood lipids and blood pressure but not insulin resistance.
To improve the predictive power of the original HEI on CVD outcomes, the aHEI was developed. In this study, a high aHEI was associated with 23% lower risk of type 2 diabetes and in the Nurses’ Health Study was associated with 36% lower risk (25). A high aHEI also was associated with 39% lower risk of CVD in the Health Professionals Follow-Up Study and 29% lower risk in the Nurses’ Health Study (10). In the Nurses’ Health Study, the aMED and DASH scores had similar associations with CVD (7,12). Combined with their high correlations (r > 0.71), this points to a common underlying dietary pattern. However, the DASH score provided better fit to the data and captured unique dietary variation related to diabetes risk. This could be because it includes sugar-sweetened beverages, which are associated with an increased risk of type 2 diabetes (21).
However, despite these differences, the aHEI, aMED, and DASH scores were associated with nearly identical risk reductions. This is because even though some scores were not optimal, they awarded points to a sufficient number of beneficial components. This suggests that public health messages need not be overly strict, as adequate risk reductions can be achieved even if not all dietary recommendations are followed. But because these scores were associated with greater reductions in absolute risk of type 2 diabetes among the overweight and obese, public health messages should focus on improving diet quality in these groups to prevent the greatest number of cases.
Our study has several strengths. First, it is prospective, which minimizes reverse causality. Second, participants were relatively similar, which reduces residual confounding common to studies of diverse populations. Third, cumulative averages of diet scores were used, which accounts for previous dietary information and controls measurement error. Fourth, recall bias was reduced by not updating dietary data after the diagnosis of a chronic disease. Fifth, time-dependent confounding was adjusted for by using updated covariates. Sixth, a large sample size allowed for modest but potentially meaningful changes in risk to be detected.
Our study also has limitations. The first is that because of its ethnic homogeneity (most were white males), its findings may not be generalizable to other populations. The second limitation is the potential for confounding. Dietary patterns could simply be markers for factors such as health awareness. However, we controlled for many possible confounders of the relationship between diet and type 2 diabetes and used continuous diet scores and covariates to assess residual confounding.
In conclusion, several diet-quality scores were inversely associated with type 2 diabetes. These scores reflect a common dietary pattern characterized by high intake of fruits, vegetables, whole grains, nuts, legumes, and unsaturated fats; moderate intake of alcohol; and lowintake of red and processed meat, sodium, sugar-sweetened beverages, and trans fat. High-quality diets may yield the greatest reduction in diabetes cases when followed by those with a high BMI.
Supplementary Material
Acknowledgments
This analysis was supported by fellowships from the Canadian Institutes of Health Research and the Canadian Diabetes Association (to L.d.K.). The Health Professionals Follow-Up Study is supported by grants from the National Institutes of Health (CA-55075, HL-35464, HL-60712, and DK-58845).
No potential conflicts of interest relevant to this article were reported.
L.d.K. contributed to design and analysis and wrote the manuscript. S.E.C. and T.T.F. contributed to programming and edited the manuscript. W.C.W. and E.B.R. contributed to funding and study management and edited the manuscript. F.B.H. contributed to study design, funding, data interpretation, manuscript editing, and supervision of the study.
The authors thank M. McCullough (American Cancer Society) for programming several diet scores.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2352/-/DC1.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Kastorini CM, Panagiotakos DB. Dietary patterns and prevention of type 2 diabetes: from research to clinical practice; a systematic review. Curr Diabetes Rev 2009;5:221–227 [DOI] [PubMed] [Google Scholar]
- 3.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009;169:659–669 [DOI] [PubMed] [Google Scholar]
- 4.U.S. Health and Human Services and U.S. Department of Agriculture Dietary Guidelines for Americans 2005. Washington, DC, U.S. Government Printing Office, 2005 [Google Scholar]
- 5.McCullough ML, Feskanich D, Stampfer MJ, et al. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in women. Am J Clin Nutr 2000;72:1214–1222 [DOI] [PubMed] [Google Scholar]
- 6.McCullough ML, Feskanich D, Rimm EB, et al. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr 2000;72:1223–1231 [DOI] [PubMed] [Google Scholar]
- 7.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–720 [DOI] [PubMed] [Google Scholar]
- 8.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126; discussion 1127–1136 [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–249 [DOI] [PubMed] [Google Scholar]
- 10.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–1271 [DOI] [PubMed] [Google Scholar]
- 11.Kant AK, Schatzkin A, Ziegler RG. Dietary diversity and subsequent cause-specific mortality in the NHANES I epidemiologic follow-up study. J Am Coll Nutr 1995;14:233–238 [DOI] [PubMed] [Google Scholar]
- 12.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 14.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med 2007;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barclay AW, Petocz P, McMillan-Price J, et al. Glycemic index, glycemic load, and chronic disease risk: a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–637 [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Wolk A. Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med 2007;262:208–214 [DOI] [PubMed] [Google Scholar]
- 17.Murakami K, Okubo H, Sasaki S. Effect of dietary factors on incidence of type 2 diabetes: a systematic review of cohort studies. J Nutr Sci Vitaminol (Tokyo) 2005;51:292–310 [DOI] [PubMed] [Google Scholar]
- 18.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids 2010;45:925–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2009;32:2123–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aune D, Ursin G, Veierød MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 2009;52:2277–2287 [DOI] [PubMed] [Google Scholar]
- 21.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009;52:1479–1495 [DOI] [PubMed] [Google Scholar]
- 23.Farmer A, Montori V, Dinneen S, Clar C. Fish oil in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2001;(3):CD003205. [DOI] [PubMed] [Google Scholar]
- 24.Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysis. J Hypertens 2007;25:2361–2369 [DOI] [PubMed] [Google Scholar]
- 25.Fung TT, McCullough M, van Dam RM, Hu FB. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care 2007;30:1753–1757 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.