Abstract
OBJECTIVE
Animal studies suggest that low serum 25-hydroxyvitamin D (25[OH]D) may impair insulin synthesis and secretion and be involved in the pathogenesis of diabetes. Results in studies in humans have not been consistent, however. Prediabetes is a stage earlier in the hyperglycemia/diabetes continuum where individuals are at increased risk of developing diabetes and where prevention efforts have been shown to be effective in delaying or preventing the onset of diabetes. However, previous studies have not examined the association between low serum 25(OH)D levels and prediabetes.
RESEARCH DESIGN AND METHODS
We examined the 12,719 participants (52.5% women) in the third National Health and Nutrition Examination Survey aged >20 years who were free of diabetes. Serum 25(OH)D levels were categorized into quartiles (≤17.7, 17.8–24.5, 24.6–32.4, >32.4 ng/mL). Prediabetes was defined as a 2-h glucose concentration of 140–199 mg/dL, or a fasting glucose concentration of 110–125 mg/dL, or an A1C value of 5.7–6.4%.
RESULTS
Lower serum 25(OH)D levels were associated with prediabetes after adjusting for age, sex, race/ethnicity, season, geographic region, smoking, alcohol intake, BMI, outdoor physical activity, milk consumption, dietary vitamin D, blood pressure, serum cholesterol, C-reactive protein, and glomerular filtration rate. Compared with quartile 4 of 25(OH)D (referent), the odds ratio of prediabetes associated with quartile 1 was 1.47 (95% CI 1.16–1.85; P = 0.001 for trend). Subgroup analyses examining the relation between 25(OH)D and prediabetes by sex, BMI, and hypertension categories also showed a consistent positive association.
CONCLUSIONS
Lower serum 25(OH)D levels are associated with prediabetes in a representative sample of U.S. adults.
Several studies have shown that lower 25-hydroxyvitamin D (25[OH]D) levels are related to an increased risk of cardiovascular disease (CVD) (1,2). Some evidence also suggests that 25(OH)D insufficiency may be involved in the development of diabetes (3,4). In animal models of diabetes, 25(OH)D deficiency impaired insulin synthesis and secretion, and pharmacologic doses of 1,25-dihydroxyvitamin D3 delayed the onset of diabetes (5,6).
Unlike animal models, the results have not been similarly consistent in human studies (7). Although some epidemiologic studies have reported a positive association (8–10) between low serum 25(OH)D levels and diabetes, others did not find an independent association after multivariable adjustment (11,12) for confounders, or in subgroup analyses in women (13). Scragg et al. (8) reported an association between serum 25(OH)D levels and diabetes in non-Hispanic whites and Mexican Americans in the third National Health and Nutrition Examination Survey (NHANES III), and Liu et al. (9) reported an association between 25(OH)D levels and type 2 diabetes in the Framingham Study. Pittas et al. (11) reported that dietary vitamin D was associated with diabetes in the Nurses’ Health Study. A recent meta-analysis suggested that there is insufficient evidence to conclude an inverse association exists between 25(OH)D levels and diabetes (7). Furthermore, reduced kidney function is a potential confounder in the association between 25(OH)D levels and diabetes because it has been shown to cause low 25(OH)D levels due to a decrease in renal 1-α hydroxylase activity (14) as well as to diabetes (15). Similarly, markers of inflammation have been shown to be associated with diabetes (16) and also with low 25(OH)D levels (17). However, previous studies of serum 25(OH)D levels and diabetes have not accounted for these confounders.
Prediabetes is a stage earlier in the hyperglycemia/diabetes continuum where individuals are at increased risk of developing diabetes and where prevention efforts, including lifestyle modification or pharmacologic intervention, have been shown to be effective in preventing or delaying the onset of diabetes (1,18). To our knowledge, no previous study has examined the association between serum 25(OH)D levels and prediabetes, so we conducted this study in a nationally representative sample of U.S. adults who were free of clinical CVD and diabetes after adjusting for potential confounders, including serum C-reactive protein (CRP) levels for systemic inflammation, and estimated glomerular filtration rate (eGFR) from serum creatinine for kidney function.
RESEARCH DESIGN AND METHODS
The current study used data from the NHANES III. A detailed description of the NHANES III study design and methods is available elsewhere (19). Briefly, the NHANES survey included a stratified multistage probability sample representative of the civilian noninstitutionalized U.S. population. Selection was based on counties, blocks, households, and individuals within households, and included the oversampling of non-Hispanic blacks and Mexican Americans to provide stable estimates of these groups.
The current study consisted of participants aged >20 years who were randomly assigned to be examined in the morning after an overnight fast. Serum 25(OH)D levels were measured in 18,883 participants who had surplus sera available. We excluded 1,883 subjects with diabetes, 952 with CVD, and 3,329 with missing data for covariates included in the multivariable model. This resulted in 12,719 participants without diabetes, 4,057 of whom had prediabetes.
Main outcome of interest: prediabetes
Plasma glucose was measured by a modified hexokinase enzymatic method (20). Glycosylated hemoglobin (A1C) was measured using an ion-exchange high-performance liquid chromatography method using the Diamat Analyzer System (Hercules, CA). Prediabetes was defined as adults without diabetes who had impaired glucose tolerance (2-h glucose concentration of 140–199 mg/dL [7.8–11.0 mmol/L]), or impaired fasting glucose (fasting glucose concentration 110–25 mg/dL [5.6–6.9 mmol/L]), or an A1C value of 5.7–6.4%, consistent with recent American Diabetes Association recommendations (1).
Exposure measurements
A questionnaire was used to assess age, sex, race/ethnicity, geographic region, smoking, alcohol intake (g/day), history of diabetes, oral hypoglycemic intake or insulin administration, and antihypertensive medication use. Individuals who had not smoked ≥100 cigarettes in their lifetimes were considered never smokers; those who had smoked ≥100 cigarettes in their lifetimes were considered former smokers if they answered negatively to the question “Do you smoke now?”, and current smokers if they answered affirmatively. BMI was calculated as weight (kg)/height (m2). Season of blood collection was assessed from the date of the respondent’s physical examination. Dietary vitamin D intake and milk consumption was assessed from a single 24-h dietary recall. Outdoor physical activity as a surrogate for sunlight exposure was assessed from self-reported physical activities likely to be performed outdoors, including walking, jogging or running, bicycling, swimming, and garden/yard activity.
Rigorous procedures with quality control checks were used in blood collection, as detailed in the NHANES “Laboratory Procedures Manual.” Measurements of serum 25(OH)D were performed at the National Center for Environmental Health, Centers for Disease Control and Prevention (Atlanta, GA) using a radioimmunoassay (RIA) kit (DiaSorin, Stillwater, MN) (19). The interassay coefficient of variation was 15–25% for lower values (20–62 nmol/L) and 14–18% for higher values (86–143 nmol/L) (19). Serum CRP was analyzed using a modification of the Behring Latex-Enhanced CRP assay on the Behring Nephelometer Analyzer System (Somerville, NJ). Serum total cholesterol was measured enzymatically. Serum creatinine was measured using a modified kinetic Jaffe reaction on a Roche Hitachi 737 analyzer (Indianapolis, IN), and the NHANES values in NHANES III were calibrated to the standard creatinine values from the Cleveland Clinic Foundation (CCF) laboratory, as recommended previously (21). The eGFR was calculated from serum creatinine level by using the four-variable Modification of Diet in Renal Disease equation.
Statistical analysis
Serum levels of 25(OH)D were analyzed both as a continuous and a categoric variable. We categorized serum 25(OH)D level as quartiles (≤17.7, 17.8–24.5, 24.6–32.4, >32.4 ng/mL). We were interested in the association between low 25(OH)D levels and prediabetes. The odds ratio (OR) and 95% CI of prediabetes for each lower serum 25(OH)D quartile was calculated by taking the highest quartile as the referent, using multivariable logistic regression models. We used two models: age-, sex-, ethnicity-adjusted model and the multivariable model, additionally adjusting for season (January–March, April–June, July–September, October–December), geographic region (Northeast, Midwest, South, West), smoking (never smoker, former smoker, current smoker), alcohol intake (g/day), BMI (kg/m2), outdoor physical activity (yes, no), milk consumption (yes, no), dietary vitamin D (μg), hypertension (yes, no), systolic blood pressure (mmHg), serum total cholesterol (mg/dL), CRP (mg/dL), and eGFR (mL/min/1.73 m2).
Tests for trends were performed by modeling serum 25(OH)D categories as an ordinal variable. To examine the consistency of the association, we performed subgroup analyses by age, sex, geographic region, season of blood draw, BMI, CRP, eGFR, outdoor physical activity, dietary intake of vitamin D, and hypertension. We formally evaluated interactions between serum 25(OH)D levels and other covariates such as age, sex, race/ethnicity, geographic region, month of blood collection, dietary intake of vitamin D, and outdoor physical activity by including cross-product interaction terms in the corresponding multivariable models. Sample weights that account for the unequal probabilities of selection, oversampling, and nonresponse were applied for all analyses using SUDAAN 8.0 (Research Triangle Institute, Research Triangle Park, NC) and SAS 9.2 (SAS Institute, Cary, NC) software; SEs were estimated using the Taylor series linearization method. All values presented are weighted to represent the U.S. civilian population.
To examine the dose-response relationship of the observed association between the serum 25(OH)D level and prediabetes without linearity assumptions, we used flexible nonparametric logistic regression using the generalized additive modeling approach with the R system for statistical computing (Comprehensive R Archive Network, http://www.CRAN.R-project.org) to calculate odds of prediabetes, adjusting for all covariates in the multivariable model. The predicted odds of prediabetes were then plotted against increasing 25(OH)D levels (log scale).
In a supplementary analysis, we repeated the multivariable model additionally adjusting for A1C. Second, to examine whether the association between 25(OH)D and prediabetes is confounded by central obesity, we adjusted for waist circumference, a surrogate for central obesity in the multivariable model. Third, we examined the association between serum 25(OH)D and prediabetes using clinically relevant categories of serum 25(OH)D (<10, 10–20, 21–30, and >30 ng/mL).
RESULTS
Among 12,719 participants included for the current analysis, 4,057 had prediabetes. Those in the lowest quartile of 25(OH)D were more likely to be a current smoker and obese; have higher levels of diastolic blood pressure, CRP, and eGFR; and were less likely to be women, non-Hispanic white, current drinkers, and physically active (data not shown).
As reported in Table 1, compared with those with normal glucose levels, individuals with prediabetes were more likely to be older, former/current smokers, overweight and obese; have higher levels of systolic and diastolic blood pressure, total cholesterol, and CRP; were less likely to be women, non-Hispanic white, current drinkers, and physically active; and have lower levels of eGFR (Table 1).
Table 1.
Characteristics of the study population by prediabetes status*
| Characteristic | Prediabetes absent | Prediabetes present | P† |
|---|---|---|---|
| Unweighted sample size | 8,662 | 4,057 | |
| Age (years) | 39.33 ± 0.36 | 51.13 ± 0.61 | <0.0001 |
| Female (%) | 55.67 ± 0.69 | 42.96 ± 1.29 | <0.0001 |
| Smoking categories (%) | <0.0001 | ||
| Never smoker | 50.29 ± 1.07 | 42.02 ± 1.02 | |
| Former smoker | 21.03 ± 0.83 | 28.42 ± 0.92 | |
| Current smoker | 28.68 ± 0.99 | 29.55 ± 1.07 | |
| Current drinker (%) | 59.18 ± 1.28 | 49.74 ± 2.00 | <0.0001 |
| BMI (kg/m2) | <0.0001 | ||
| <25 | 51.86 ± 0.93 | 33.10 ± 1.27 | |
| 25–29.9 | 31.16 ± 0.71 | 37.61 ± 1.01 | |
| ≥30 | 16.98 ± 0.80 | 29.29 ± 0.90 | |
| Race/ethnicity (%) | <0.0001 | ||
| Non-Hispanic white | 79.08 ± 1.28 | 71.40 ± 1.74 | |
| Non-Hispanic black | 8.64 ± 0.54 | 14.27 ± 0.89 | |
| Mexican American | 4.99 ± 0.42 | 5.49 ± 0.55 | |
| Others | 7.30 ± 0.88 | 8.84 ± 1.28 | |
| Outdoor physical activity (%) | 54.28 ± 1.14 | 48.07 ± 1.37 | <0.0001 |
| Hypertension (%) | 22.02 ± 0.84 | 41.14 ± 1.28 | <0.0001 |
| Blood pressure (mmHg) | |||
| Systolic | 117.89 ± 0.31 | 128.46 ± 0.58 | <0.0001 |
| Diastolic | 73.13 ± 0.22 | 76.80 ± 0.27 | <0.0001 |
| Total cholesterol (mg/dL) | 197.19 ± 0.80 | 215.76 ± 1.13 | <0.0001 |
| HDL cholesterol (mg/dL) | 58.10 ± 3.27 | 56.06 ± 2.89 | 0.08 |
| Serum CRP (mg/dL) | 0.35 ± 0.01 | 0.47 ± 0.02 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 96.27 ± 0.53 | 87.78 ± 0.58 | <0.0001 |
Data are means ± SD unless otherwise indicated.
*All values presented are weighted to represent the U.S. civilian population, 1988–1994.
†P value represents differences in means (SD) or proportions, using ANOVA or χ2 test.
As reported in Table 2, lower 25(OH)D levels were associated with prediabetes in both the age-, sex-, ethnicity-adjusted as well as the multivariable-adjusted model that adjusted for various confounders, including CRP levels and eGFR. Models evaluating trend in this association were also statistically significant (P < 0.001).
Table 2.
Association between serum 25(OH)D levels and prediabetes
| Serum 25(OH)D level | N at risk (prediabetes cases) | Age-, sex-, ethnicity-adjusted OR (95% CI)* | Multivariable OR (95% CI)*† |
|---|---|---|---|
| Quartile IV (>32.4 ng/mL) | 3,185 (892) | 1.00 (ref) | 1.00 (ref) |
| Quartile III (24.6–32.4 ng/mL) | 3,171 (1,008) | 1.15 (0.99–1.33) | 1.10 (0.94–1.30) |
| Quartile II (17.8–24.5 ng/mL) | 3,215 (1,083) | 1.43 (1.19–1.71) | 1.24 (1.03–1.51) |
| Quartile I (≤17.7 ng/mL) | 3,148 (1,074) | 1.73 (1.41–2.13) | 1.47 (1.16–1.85) |
| P (trend) | <0.0001 | 0.001 | |
| OR for 10-unit decrease in 25(OH)D | 12,719 (4,057) | 1.21 (1.13–1.28) | 1.13 (1.06–1.21) |
*OR values presented are weighted to represent the U.S. civilian population, 1988–1994.
†Adjusted for age (years), sex (men, women), race/ethnicity (non-Hispanic whites, non-Hispanic blacks, Mexican Americans, others), geographic region (Northeast, Midwest, South, West), season of blood drawn (January–March, April–June, July–September, October–December), smoking categories (never, former, current), current drinker (yes, no), milk consumption (yes, no), BMI (kg/m2), dietary vitamin D (μg), outdoor physical activity (yes, no), hypertension (yes, no), systolic blood pressure (mmHg), total cholesterol (mg/dL), high-sensitivity CRP level (mg/dL), eGFR (mL/min/1.73 m2).
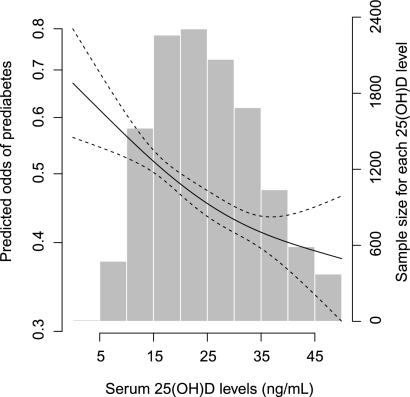
As reported in Table 3, we observed an overall statistically significant positive association between lower 25(OH)D levels and prediabetes within subgroups of age, sex, race/ethnicity, geographic region, season of blood draw, BMI, CRP, eGFR levels, outdoor physical activity, dietary intake of vitamin D, and hypertension, and the ORs ranged from 0.96 to 1.24 (all P > 0.1 for interaction). Although no statistically significant interaction was found between serum 25(OH)D and the odds of prediabetes for race/ethnicity, the CIs in Table 3 show that the ORs among non-Hispanic whites were significant, whereas the ORs among non-Hispanic blacks, Mexican Americans, and others were not significant. In nonparametric graphic models, we observed a continuous inverse association between serum 25(OH)D level and prediabetes, without any threshold effect (Fig. 1).
Table 3.
Association between serum 25(OH)D levels and prediabetes, within selected subgroups
| Subgroup of interest | N at risk (cases) | Multivariable OR (95% CI) for 10-unit decrease in serum 25(OH)D*† | P (interaction)‡ |
|---|---|---|---|
| Age | |||
| <60 years | 9,558 (2,383) | 1.15 (1.07–1.24) | 0.91 |
| ≥60 years | 3,161 (1,674) | 1.04 (0.93–1.17) | |
| Sex | |||
| Women | 6,850 (1,860) | 1.15 (1.05–1.25) | 0.83 |
| Men | 5,869 (2,197) | 1.13 (1.04–1.24) | |
| Race/ethnicity | |||
| Non-Hispanic white | 5,275 (1,567) | 1.09 (1.02–1.17) | 0.93 |
| Non-Hispanic black | 3,435 (1,282) | 1.00 (0.89–1.12) | |
| Mexican American | 3,491 (1,033) | 1.00 (0.88–1.15) | |
| Others | 518 (175) | 1.13 (0.85–1.50) | |
| Geographic region | |||
| Northeast | 1,720 (525) | 1.23 (1.08–1.40) | 0.48 |
| Midwest | 2,513 (788) | 1.12 (1.01–1.25) | |
| South | 5,440 (1,855) | 1.16 (1.03–1.30) | |
| West | 3,046 (889) | 1.01 (0.91–1.11) | |
| Season | |||
| January–March | 3,325 (1,097) | 1.04 (0.93–1.17) | 0.18 |
| April–June | 3,332 (1,023) | 0.96 (0.89–1.04) | |
| July–September | 3,134 (960) | 1.18 (1.11–1.26) | |
| October–December | 2,928 (977) | 1.24 (1.08–1.41) | |
| BMI (kg/m2) | |||
| <25 | 5,365 (1,226) | 1.08 (0.97–1.21) | 0.88 |
| 25–29.9 | 4,408 (1,561) | 1.17 (1.05–1.30) | |
| ≥30 | 2,946 (1,270) | 1.15 (1.00–1.33) | |
| Hypertension | |||
| Yes | 4,002 (1,831) | 1.13 (1.02–1.25) | 0.65 |
| No | 8,717 (2,226) | 1.13 (1.04–1.23) | |
| CRP (mg/dL) | |||
| <0.3 | 8,797 (2,477) | 1.11 (1.02–1.19) | 0.24 |
| ≥0.3 | 3,922 (1,580) | 1.19 (1.06–1.34) | |
| eGFR (mL/min/1.73 m2) | |||
| <95 | 6,239 (2,483) | 1.13 (1.04–1.22) | 0.74 |
| ≥95 | 6,480 (1,574) | 1.13 (1.01–1.27) | |
| Outdoor physical activity | |||
| Yes | 9,116 (2,847) | 1.13 (1.06–1.21) | 0.99 |
| No | 3,603 (1,210) | 1.11 (0.96–1.30) | |
| Dietary vitamin D (μg) | |||
| <4.6 | 7,742 (2,436) | 1.07 (0.96–1.20) | 0.28 |
| ≥4.6 | 4,977 (1,621) | 1.20 (1.10–1.30) |
*OR values presented are weighted to represent the U.S. civilian population, 1988–1994.
†Adjusted for age (years), sex (men, women), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, others), geographic region (Northeast, Midwest, South, West), season blood drawn (January–March, April–June, July–September, October–December), smoking categories (never, former, current), current drinker (yes, no), milk consumption (yes, no), BMI (kg/m2), dietary vitamin D (μg), outdoor physical activity (yes, no), hypertension (yes, no), systolic blood pressure (mmHg), total cholesterol (mg/dL), CRP level (mg/dL), eGFR (mL/min/1.73 m2).
‡P value associated with the cross-product interaction term between the corresponding stratification variable and the 25(OH)D variable in the multivariable model.
Figure 1.
Multivariable-adjusted odds of prediabetes according to serum 25(OH)D level (ng/mL). The predicted odds of prediabetes from nonparametric logistic regression (solid line) are shown with 95% CI (dashed lines) for the nonparametric logistic regression estimates. The nonparametric logistic regression was adjusted for age (years), sex (men, women), race/ethnicity (non-Hispanic whites, non-Hispanic blacks, Mexican Americans, others), geographic region (Northeast, Midwest, South, West), season of blood drawn (January–March, April–June, July–September, October–December), smoking categories (never, former, current), current drinker (yes, no), milk consumption (yes, no), BMI (kg/m2), dietary vitamin D (μg), outdoor physical activity (yes, no), hypertension (yes, no), systolic blood pressure (mmHg), total cholesterol (mg/dL), high-sensitivity CRP level (mg/dL), eGFR (mL/min/1.73 m2). x axis: Serum 25(OH)D level (ng/mL). y1 axis: Predicted odds of prediabetes plotted in log scale. y2 axis: Participant number for each serum 25(OH)D level.
In a supplementary analysis, we examined the association between lower serum 25(OH)D levels and prediabetes additionally adjusting for A1C levels to the multivariable-adjusted model in Table 2; the association was slightly attenuated but still present. The multivariable OR (95% CI) of prediabetes per 10-unit decrease in serum 25(OH)D levels was 1.08 (1.00–1.18).
Second, when we added waist circumference as a covariate, instead of BMI in the multivariable model in Table 2, the results were slightly accentuated. Compared with quartile 4, the multivariable OR (95% CI) of prediabetes was 1.16 (0.99–1.36) in quartile 3, 1.38 (1.31–2.05) in quartile 2, and 1.64 (1.31–2.05) in quartile 1 of serum 25(OH)D.
Third, when we repeated the multivariable analysis in Table 2 using clinically relevant categories of 25(OH)D compared with those with 25(OH)D levels of >30 ng/mL, the multivariable OR (95% CI) of prediabetes was 1.22 (1.06–1.42), 1.35 (1.11–1.64), and 1.58 (1.04–2.40) among those with 25(OH)D levels of 21–30, 10–20, and <10 ng/mL, respectively (P = 0.002 for trend).
CONCLUSIONS
Lower serum 25(OH)D levels were positively associated with prediabetes in this representative sample of U.S. adults. The association between 25(OH)D and prediabetes was independent of confounding factors, including age, sex, race/ethnicity, smoking, alcohol intake, BMI, physical activity, hypertension, systolic blood pressure, serum total cholesterol, CRP levels, and eGFR. The association between serum 25(OH)D and prediabetes was also consistently present in subgroup analyses by sex, BMI, and hypertension. Our results contribute to the existing literature on the effect of low serum 25(OH)D on diabetes by demonstrating for the first time that 25(OH)D levels are independently associated with prediabetes, a stage in the disease continuum where diabetes prevention efforts have been shown to be effective in preventing the onset of diabetes (1,18).
Previous reports have shown that lower serum 25(OH)D levels are related to CVD and CVD mortality (2,22). Several lines of recent evidence also suggest that 25(OH)D insufficiency may be involved in the risk of developing diabetes (3,4), a risk factor for CVD. In animal models, low levels of 25(OH)D have been shown to impair insulin synthesis and secretion, and treatment with 25(OH)D has, in turn, been shown to delay the onset of diabetes (5,6).
However, the results from human studies have not been similarly consistent (7). First, although some epidemiologic studies have reported a positive association (8–10) between low serum 25(OH)D levels and diabetes, others did not find an association after multivariable adjustment (11,12) or in an analysis among women (13). Pittas et al. (7), in a recent meta-analysis, concluded that evidence was insufficient to support an inverse association between 25(OH)D levels and diabetes. Also, to our knowledge, the association between serum 25(OH)D levels and prediabetes has not been previously examined.
The few intervention studies that have evaluated the effect of 25(OH)D supplementation on glucose regulation have also shown mixed results. In a small, short-term clinical trial, treatment with 25(OH)D3 improved insulin sensitivity and first-phase insulin secretion in the intervention group (23). In a placebo-controlled trial of nondiabetic subjects with impaired fasting glucose at baseline, treatment with 25(OH)D3 and calcium attenuated a rise in fasting glucose and progression of insulin resistance (24). In contrast, treatment with 25(OH)D3 and calcium in the Women’s Health Initiative trial involving postmenopausal women did not reduce the risk of developing diabetes (25). However, this trial defined diabetes by self-report and not by blood glucose measurements, and the 25(OH)D3 doses were lower than in other studies. In the face of our finding of a positive association between low levels of serum 25(OH)D and prediabetes, results from these 25(OH)D intervention studies indirectly suggest that 25(OH)D3 supplementation may be potentially beneficial in preventing or delaying diabetes development in individuals with prediabetes.
In the current study, low serum 25(OH)D levels were positively associated with prediabetes. The magnitude of the association observed between low 25(OH)D and prediabetes, its persistence even after multivariable adjustment, and the consistency of these findings in subgroup analyses by sex, BMI categories, and hypertension, suggest that these findings are less likely to be due to chance.
Reduced GFR is a potential confounder in the association between serum 25(OH)D levels and diabetes because it has been shown to cause low 25(OH)D levels (14), as well as to cause diabetes (15). Similarly, CRP levels were associated with diabetes (16) and with low 25(OH)D levels (17). However, previous studies of serum 25(OH)D levels and diabetes did not account for the confounding effect of reduced kidney function or systemic inflammation. The observed association between low serum 25(OH)D levels and prediabetes was attenuated in the current study but still persisted after adjustment for eGFR and CRP levels in the multivariable model, suggesting the association is independent of these processes.
In the current study, the positive association between serum 25(OH)D and prediabetes was significant only in non-Hispanic whites but not in non-Hispanic blacks, Mexican Americans, and others. Ethnic variations in 25(OH)D levels have been recognized in the U.S.: non-Hispanic whites have higher levels of 25(OH)D than Mexican Americans and non-Hispanic blacks (19). Our results are also consistent with the previous analyses of NHANES III data by Scragg et al. (8), who reported an inverse association between 25(OH)D and undiagnosed diabetes among non-Hispanic whites and Mexican Americans but no association among non-Hispanic blacks. The association between serum 25(OH)D and prediabetes among non-Hispanic whites only suggests that non-Hispanic whites may be more sensitive to 25(OH)D deficiency compared with other races and ethnicities.
The main strengths of our study include its population-based nature, inclusion of a representative multiethnic sample, adequate sample size, and the availability of data on confounders for multivariable adjustment.
The main limitation of our study is the cross-sectional nature of NHANES. It is possible that our results could be biased by residual confounding by variables, including the use of vitamin D supplements and parathyroid hormone levels. Further, the high coefficient of variation at lower values of 25(OH)D suggests that there may be measurement error and therefore exposure misclassification of 25(OH)D.
In summary, in a representative sample of U.S. adults, low serum levels of 25(OH)D were positively associated with prediabetes among subjects without diabetes. This association was independent of confounding factors, including BMI, blood pressure, serum cholesterol, CRP levels, and eGFR. Because prediabetes is a stage in the disease continuum where diabetes prevention has been shown to be effective (1,18), it would be of public health relevance to see if vitamin D supplementation in this stage can help to prevent or delay the onset of diabetes.
Acknowledgments
This study was funded by an American Heart Association National Clinical Research Program grant (to A.S.).
No potential conflicts of interest relevant to this article were reported.
A.S. originated the study, wrote the initial draft, supervised data analysis, and contributed to the intellectual development of the manuscript. C.S. and S.K. performed the statistical analyses, were involved in critical corrections of the manuscript, and contributed to the intellectual development of the manuscript.
References
- 1.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia 2005;48:1247–1257 [DOI] [PubMed] [Google Scholar]
- 4.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu C, Laureys J, Sobis H, Vandeputte M, Waer M, Bouillon R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 1992;41:1491–1495 [DOI] [PubMed] [Google Scholar]
- 6.Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 1994;37:552–558 [DOI] [PubMed] [Google Scholar]
- 7.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 2010;152:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scragg R, Sowers M, Bell C, Third National Health and Nutrition Examination Survey Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–2818 [DOI] [PubMed] [Google Scholar]
- 9.Liu E, Meigs JB, Pittas AG, et al. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr 2010;91:1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattila C, Knekt P, Männistö S, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care 2007;30:2569–2570 [DOI] [PubMed] [Google Scholar]
- 11.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006;29:650–656 [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005;28:2926–2932 [DOI] [PubMed] [Google Scholar]
- 13.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008;19:666–671 [DOI] [PubMed] [Google Scholar]
- 14.Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab 1988;67:876–881 [DOI] [PubMed] [Google Scholar]
- 15.Donahue RP, Stranges S, Rejman K, Rafalson LB, Dmochowski J, Trevisan M. Elevated cystatin C concentration and progression to pre-diabetes: the Western New York study. Diabetes Care 2007;30:1724–1729 [DOI] [PubMed] [Google Scholar]
- 16.Shankar A, Li J. Positive association between high-sensitivity C-reactive protein level and diabetes mellitus among US non-Hispanic black adults. Exp Clin Endocrinol Diabetes 2008;116:455–460 [DOI] [PubMed] [Google Scholar]
- 17.Manolagas SC, Provvedini DM, Tsoukas CD. Interactions of 1,25-dihydroxyvitamin D3 and the immune system. Mol Cell Endocrinol 1985;43:113–122 [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 2008;88:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunter EW, Lewis BG, Koncikowski SM. Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994 [Internet]. National Center for Health Statistics, Centers for Disease Control and Prevention. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf Accessed 27 May 2010
- 21.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 2007;50:918–926 [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromsø study. Eur J Endocrinol 2010;162:935–942 [DOI] [PubMed] [Google Scholar]
- 23.Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract 2003;57:258–261 [PubMed] [Google Scholar]
- 24.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30:980–986 [DOI] [PubMed] [Google Scholar]
- 25.de Boer IH, Tinker LF, Connelly S, et al. Women’s Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008;31:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]