Abstract
OBJECTIVE
To characterize birth size distribution in infants born to mothers with type 1 diabetes. In particular, the relationship between birth weight (BW) and length (BL) was studied because it may provide information on different causal pathways of fetal macrosomia commonly seen in diabetic pregnancies.
RESEARCH DESIGN AND METHODS
This was a population-based cohort study of 3,705 infants of type 1 diabetic mothers (1,876 boys), with a gestational age of 28–43 weeks, born in Sweden between 1998 and 2007. BW and BL were retrieved from the Medical Birth Registry and expressed as SD scores (SDS). Ponderal index (PI) was calculated as BW in g/length in cm3. A BW >90th and a PI ≤90th percentile was defined as proportionate large-for-gestational age (LGA), whereas if both BW and PI >90th percentile, the infant was categorized as disproportionately large. Values are mean (SD).
RESULTS
The BW distribution for offspring of type 1 diabetic mothers was bell-shaped, significantly broader, and markedly shifted to the right (BWSDS: 1.27 [1.48]) of the reference. Of the infants born to diabetic mothers, 47% were LGA, and among them, 46% were disproportionately large compared with 35% in nondiabetic LGA infants (P < 0.001). Female offspring of type 1 diabetic mothers had significantly higher BWSDS than males (1.34 vs. 1.20, P < 0.01), and preterm infants had higher BWSDS than term infants (1.41 vs. 1.23, P < 0.01)
CONCLUSIONS
Fetal macrosomia in type 1 diabetic pregnancies is due to a right-shift and broadening of the entire BW distribution. The large number of disproportionate LGA infants born to type 1 diabetic mothers suggests an underlying metabolic problem. Fetal macrosomia was more pronounced in preterm and female offspring of type 1 diabetic mothers.
Fetal macrosomia is one of the most frequent complications in the type 1 diabetic pregnancy. We have recently reported a 31% incidence of large-for-gestational age (LGA) infants, defined as birth weight (BW) >2 SD above the mean, adjusted for gestational age (GA) and sex, in a national cohort of more than 5,000 offspring of type 1 diabetic mothers in Sweden between 1991 and 2003 (1). Compared with data from 1982 to 1985 (2), the prevalence of LGA infants born to mothers with type 1 diabetes has increased by 50%. High rates of fetal macrosomia in type 1 diabetic pregnancies have also been reported from other European countries (3).
High BW in offspring of type 1 diabetic mothers is associated with increased risks of obstetric and perinatal complications (4–6) as well as with future risk of overweight, impaired glucose tolerance, cardiovascular disease, and diabetes (7–10). The increasing incidence of fetal macrosomia in type 1 diabetic pregnancies is therefore worrying and warrants an explanation. The high incidence of fetal macrosomia cannot only be attributed to poor metabolic control (1,2). In fact, the rate of fetal macrosomia seems to remain high, despite apparently good metabolic control (11).
Although there are several definitions of fetal macrosomia, a shared limitation with all current definitions of LGA is that they do not distinguish between normal and abnormal body composition. In larger epidemiologic studies, a proxy for body composition that can be used is the relationship between the infant’s BW and birth length (BL), expressed as the ponderal index (PI) and calculated as BW in g/length in cm3. The LGA infant may exhibit a proportionate relationship between BW and BL or disproportionate (i.e., high BW/BL ratio). Some have proposed that proportionate largeness at birth reflects constitutional and genetic factors, whereas disproportionate largeness (i.e., infants with a high BW/BL ratio) reflects an abnormal intrauterine metabolic environment (12). Accordingly, adding analyses of proportionality to BW distributions may provide important additional information on different causal pathways of fetal macrosomia in type 1 diabetic pregnancies. A higher frequency than normal of disproportionately large infants (PI >90th percentile) has so far been reported in two smaller series (n = 144 and 170) of offspring of type 1 diabetic mothers (6,13).
The primary aim of the current study was to characterize the distributions of BW, BL, and head circumferences (HC) in a large, population-based cohort of infants born to mothers with type 1 diabetes. A secondary aim was to determine the proportion of these offspring who were not only large at birth but also overweight, defined as a PI >90th percentile adjusted for GA and sex.
RESEARCH DESIGN AND METHODS
This study used information from the Swedish Medical Birth Registry (MBR). The MBR prospectively collects information on maternal demographics, clinical characteristics, and outcomes for all mothers and their infants discharged from Swedish maternity hospitals and neonatal units. The MBR is regularly evaluated by the Swedish National Board of Health and covers >99% of all pregnancies in Sweden. In 1998, a quality assurance check was preformed in which 581 original case records were compared with the information in the MBR database. The conclusion of this validation was that most fields in the MBR are reliable (14).
In Sweden, there is free access to antenatal care, and almost all women comply with 7 to 10 visits to the midwife. Women with type 1 diabetes are also frequently seen throughout their pregnancy by diabetes teams. Equipment for home monitoring of blood glucose, insulin pens/insulin pumps, and insulin are free of charge.
Dating of pregnancy is based on a routine ultrasound examination in the 16th to the 18th postmenstrual week. At the first antenatal visit, the woman is interviewed about her civil status, alcohol consumption, smoking habits, and medical and obstetric history. Maternal weight and height, delivery, and perinatal data are continuously entered into the MBR. For this purpose, standardized individual obstetric and neonatal forms with prospectively defined variables are used. Maternal disease, complications during pregnancy and delivery, as well as neonatal complications, are classified according to ICD-10. All diagnoses are made by a physician before hospital discharge, and copies of obstetric and infant records are forwarded to the MBR.
Study cohort
We conducted a population-based cohort study of all infants delivered by women with type 1 diabetes, born alive after 28 to 43 weeks of gestation in Sweden between 1998 and 2007. Preterm birth was defined as delivery before 37 completed weeks of gestation. Women with type 1 diabetes’ pregnancies were identified using ICD-10 code O 240. Stillborn infants, multiple pregnancies, infants delivered before 28 or after 43 completed weeks of gestation, those with missing data on BW, BL, sex, or GA, or with a BW, BL, or HC >6 SD above or below the mean, were excluded from the study group. Infants with major malformations (i.e., a malformation considered fatal or potentially life-threatening or that would likely lead to a serious handicap if not surgically corrected) were also excluded, as well as records with extreme values on maternal age (<13 years), maternal weight (<40 or ≥200 kg), and maternal height (<120 or ≥200 cm).
Reference population and collection and categorization of outcome
According to standardized operational procedures, all infant anthropometrics were measured within 12 h after birth. BW was registered to the nearest gram on an electronic scale; BL was measured using a measuring board for length, and tape measure was used for HC.
Reference data for normal fetal growth were based on all singletons born to mothers without type 1 diabetes in our dataset. Exclusion criteria for the reference group were multiple pregnancies, stillborn infants, severe malformations, records with missing data on BW, BL, GA, or sex, or with a BW, BL, or HC >6 SD above or below the mean. Also excluded were records with extreme values on maternal age (<13 years), maternal weight (<40 or ≥200 kg), and maternal height (<120 or ≥200 cm). Using this reference population, including 24 women with type 2 diabetes (0.003%) and 8,929 women with gestational diabetes (1%), we formed age- and sex-adjusted percentiles for BW, BL, HC, and PI. The SDS for BW, BL, and HC were also calculated by subtracting the reference mean from the individual BW, BL, and HC values, respectively, and dividing this difference with the reference SD.
LGA was defined as a BW >90th percentile adjusted for GA and sex. Appropriate-for-gestational age (AGA) was defined as a BW between the 10th and 90th percentile, and small-for-gestational age (SGA) was a BW <10th percentile in the reference population. To discriminate between proportionate and disproportionate fetal growth, PI was calculated and a cutoff value was set at the 90th customized percentile. A PI >90th percentile was defined as neonatal overweight. We defined proportionate LGA as a BW >90th percentile together with a PI ≤90th percentile and disproportionate LGA as both a BW and a PI >90th percentile.
Maternal characteristics
Maternal characteristics included nationality, defined as Nordic (Sweden, Finland, Denmark, Norway, and Island) or non-Nordic, smoking habits during the first trimester of pregnancy, age, parity, prepregnancy weight (kg), height (m), and BMI. BMI was calculated as kg/m2.
Statistical analyses
Data are presented as means (SD) and proportions (%). PI and SDSs were calculated for BW, BL, and HC. The Student t test, Wilcoxon rank sum test, and χ2 test were used for comparisons of group means and proportions. Only infants with full data on BW, BL, GA, and sex were included in the analysis. A value of P < 0.05 was considered statistically significant. All statistical analyses were performed using STATA 10 SE software (StataCorp, College Station, TX).
RESULTS
Study group and reference group
During the study period, there were 947,064 pregnancies, including 4,208 type 1 diabetic pregnancies. The final study cohort comprised 3,705 infants (1,876 boys) of mothers with type 1 diabetes, excluding stillborn infants (0.81%), multiple pregnancies (2.8%), infants with major malformations (4.2%), those delivered before 28 (0.64%) or after 43 (0%) completed weeks of gestation, and infants with missing data on BW, BL, sex, or GA (4.6%). No infants were excluded from the study group because of values for BW, BL, or HC >6 SD above or below the mean.
Records of 883,163 infants (452,809 boys) were used as a reference for singleton fetal growth. Excluded from the reference group were records of infants of mothers with type 1 diabetes (0.44%); were stillborn (0.31%); with major malformations (1.84%); from multiple pregnancies (3.01%); with BW, BL, or HC >6 SD above or below the mean (0.1%); and with missing data on BW, BL, GA, or sex (1.65%).
Mothers with type 1 diabetes were more often of Nordic origin and had a significantly higher mean BMI than mothers in the reference group. There were no significant differences in parity and smoking habits between mothers in the study and reference groups. Despite a significantly lower mean GA in the study group, the mean BW was significantly higher in infants of mothers with type 1 diabetes compared with the reference population. BL and HC were not significantly different between the two groups. Maternal and infant characteristics are presented in Table 1.
Table 1.
Maternal and infant characteristics for singleton pregnancies
| Variable | Type 1 diabetes |
Reference population |
P |
|---|---|---|---|
| n = 3,705 | n = 883,163 | ||
| Maternal characteristics | |||
| Nordic origin | 3,409 (92.0%) | 736,327 (83.4%) | <0.001 |
| Age (years) | 30.4 (5.0) | 30.0 (5.1) | <0.001 |
| Prepregnancy weight (kg) | 72.5 (13.6) | 67.7 (12.8) | <0.001 |
| Height (cm) | 166.6 (6.3) | 166.4 (6.3) | 0.242 |
| BMI (kg/m2) | 26.1 (4.7) | 24.5 (4.4) | <0.001 |
| Primipara | 1,665 (44.9%) | 389,430 (44.1%) | 0.302 |
| Smoking (first trimester) | 373 (10.1%) | 81,502 (9.2%) | 0.078 |
| Infant characteristics | |||
| Gestational age (weeks) | 37.7 (2.0) | 39.4 (1.7) | <0.001 |
| Male | 1,876 (50.6%) | 452,809 (51.3%) | 0.44 |
| BW (g) | 3,775 (711) | 3,570 (547) | <0.001 |
| BW (SDS) | 1.27 (1.5) | 0.01 (1.0) | <0.001 |
| BL (cm) | 50.5 (2.8) | 50.5 (2.4) | 0.88 |
| BL (SDS) | 0.70 (1.2) | 0.00 (1.0) | <0.001 |
| HC (cm) | 35.0 (1.8) | 35.0 (1.7) | 0.29 |
| HC (SDS) | 0.57 (1.0) | 0.06 (1.0) | <0.001 |
| LGA* | 1,753 (47.3%) | 87,283 (9.9%) | <0.001 |
| PI (g/cm3) | 2.90 (0.3) | 2.76 (0.4) | <0.001 |
| PI >90th percentile | 1,111 (30.0%) | 91,173 (10.3%) | <0.001 |
| Proportionately LGA | 943 (25.5%) | 56,634 (6.4%) | <0.001 |
| Disproportionately LGA† | 810 (21.9%) | 30,649 (3.5%) | <0.001 |
Data are mean (SD) or n (%).
*Birth weight >90th percentile.
†BW and PI both >90th percentile.
Diabetes in pregnancy and size at birth
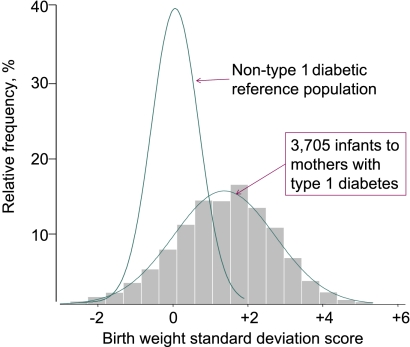
Infants of mothers with type 1 diabetes showed a normal (bell-shaped) BW distribution that was, however, significantly wider and markedly shifted to the right of the normal reference (Fig. 1).
Figure 1.
Distribution of BWSDS in 3,705 infants born to type 1 diabetic mothers and in a nondiabetic reference population (n = 883,163).
The mean BWSDS of offspring of type 1 diabetic mothers was 1.27 (SD 1.48; P < 0.001) compared with the reference population. The BL distribution among infants of mothers with type 1 diabetes was also normal, broadened, and significantly shifted to the right of the normal reference, with a mean BLSDS of 0.70 (1.22); that is, with a smaller right shift than for BW, indicating disproportionate fetal growth. The HCSDS distribution was also right-shifted in the study cohort (mean 0.57 [1.03]; P < 0.001) versus the reference population, but not to the same degree as for BW and BL (Table 1).
The proportion of LGA infants born to mothers with type 1 diabetes was 47%. In addition, 27% of offspring of type 1 diabetic mothers had a BW >97.5th percentile in the reference population; and in 14%, the BW >4500 g. Three percent of infants in the study group were SGA (BW <10th percentile) compared with 9.1% in the reference population.
The mean (SD) PI was significantly higher in offspring of type 1 diabetic mothers than in the reference population (2.90 [0.33] vs. 2.76 [0.35] g/cm3; P < 0.001). In 30% of all infants of type 1 diabetic mothers, PI exceeded the 90th percentile, and in diabetic LGA infants, 46% were disproportionately large at birth, with a PI >90th percentile. The corresponding proportions of neonatal overweight (PI >90th percentile) were 10% in the overall reference population and 35% among nondiabetic LGA infants (Table 1).
Birth size in relation to GA
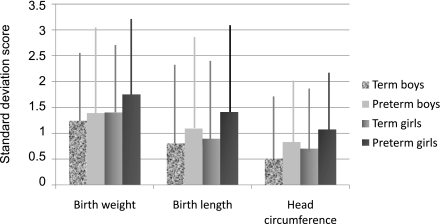
Preterm infants of mothers with type 1 diabetes had a significantly higher mean BWSDS (1.41 vs. 1.23) and BLSDS (0.80 vs. 0.68) than infants born at term (P < 0.05 for both comparisons; Fig. 2). The proportion of LGA infants of mothers with type 1 diabetes was also significantly larger in preterm versus term infants (55.2 vs. 45.3%; P < 0.001). The average PI was significantly higher in term compared with preterm infants of type 1 diabetic mothers (2.93 vs. 2.78 g/cm3; P < 0.001). However, the proportion of infants of type 1 diabetic mothers with neonatal overweight (PI >90th percentile) was significantly larger in the preterm versus the term group (36.5 vs. 28.4%; P < 0.001). Among LGA infants there was no significant difference in the proportion of disproportionate fetal growth in preterm compared with term offspring of type 1 diabetic mothers.
Figure 2.
SDSs for BW, BL, and HC in offspring of type 1 diabetic mothers, stratified by sex and gestational age. The bars show the mean values and the vertical lines indicate the SDs for the SDSs.
Birth size in relation to sex
Girls born to mothers with type 1 diabetes had significantly higher mean BWSDS (1.34 vs. 1.20), BLSDS (0.76 vs. 0.65), and HCSDS (0.62 vs. 0.53) compared with boys (P < 0.01 for all comparisons; Fig. 2). LGA was noted in 50% of girls born to type 1 diabetic mothers compared with 45% of boys (P = 0.003). The mean PI was also significantly higher in girls born to type 1 diabetic mothers than in boys (2.93 vs. 2.88 g/cm3; P < 0.001), but the number of disproportionate LGA infants was similar in both sexes.
Birth size in relation to maternal BMI
Mothers of disproportionate LGA infants born at term had a significantly higher mean BMI than mothers who delivered proportionate LGA infants at term. This was true for the study group (BMI 26.6 vs. 25.8 kg/m2; P = 0.003) and the reference population (BMI 26.7 vs. 26.0 kg/m2; P < 0.001). The prevalence of maternal overweight (BMI >25 kg/m2) was also significantly higher in disproportionate LGA versus proportionate LGA infants born at term, in both the study (60.3 vs. 54.3%; P = 0.03) and the reference group (60.9 vs. 56.6%; P < 0.001). Among preterm infants, no relation was found between maternal BMI or the incidence of maternal overweight and the proportionality of their infants.
CONCLUSIONS
This study presents several important findings. First, the distribution of BW, BL, and HC was normal in newborn infants of mothers with type 1 diabetes. Second, all distributions were significantly shifted to the right of the normal reference, with the greatest deviation for BW, resulting in 47% incidence of fetal macrosomia (BW >90th percentile). Third, 46% of the macrosomic infants born to type 1 diabetic mothers were disproportionately large (PI >90th percentile) and could thus be considered overweight at birth. However, the corresponding proportion of disproportionate growth in LGA infants of the reference population without type 1 diabetes was also high (35%), implying that factors besides maternal diabetes may be important to explain fetal macrosomia in both diabetic and normal pregnancies. Finally, the degree of fetal macrosomia was more pronounced in female than in male offspring of type 1 diabetic mothers, and was greater in preterm compared with term infants.
The strength of the current study is the population-based cohort, including a large number of pregnancies complicated by type 1 diabetes. This analysis offers objective estimates of anthropometric data in offspring of type 1 diabetic mothers over a wide range of gestational ages. The care of pregnant women with type 1 diabetes in Sweden is uniform. We know from our previous national study of type 1 diabetic pregnancies that major outcomes do not vary with geographic area and hospital size (1).
One limitation of the current study is that the MBR does not contain data on duration of diabetes, prevalence of pre-existing microangiopathy, or glycemic control during pregnancy. However, outcomes of type 1 diabetic pregnancies in Sweden have improved during the last 2 decades: the stillbirth rate decreased from 1.5% (1991 to 2003) to 0.81% (1998 to 2007, present study) (1). It is reasonable to assume that this favorable outcome over time can at least partly be attributed to improved glycemic control.
We are aware of two previous studies depicting the distribution of BWSDS in offspring of type 1 diabetic mothers (15,16). The finding in the current study of a bell-shaped BWSDS distribution with a mean of 1.27 is in line with earlier findings. The distribution of BW was more shifted to the right than the corresponding BL distribution, indicating disproportionate fetal growth. This finding is supported by the higher mean PI in offspring of type 1 diabetic mothers (2.90 g/cm3) versus in the reference population (2.76 g/cm3), with 30% of infants in the study group having a PI >90th percentile. Disproportionate fetal macrosomia was exhibited in 22% of all offspring of type 1 diabetic mothers. This is in accordance with earlier findings in smaller series of offspring of type 1 diabetic mothers (6,13).
Fourty-six percent of LGA infants had a disproportionate body constitution with a high PI. Disproportionate macrosomia is proposed to be mainly the result of fetal hyperinsulinemia (12). Maternal hyperglycemia is recognized to lead to fetal hyperglycemia and hyperinsulinemia. Experimental and clinical studies have shown that fetal hyperinsulinemia is strongly associated with fetal macrosomia and increased adipose tissue mass (17,18).
Maternal glucose values in type 1 diabetic pregnancies can only explain a minor proportion of the variance in BW (16,19). Furthermore, reported rates of fetal macrosomia remain high, despite tight glycemic control during pregnancy (11,19). This, and the present finding of a high incidence of disproportionate macrosomia also in the reference group, implies that other factors besides maternal hyperglycemia are important to explain disproportionate fetal growth in type 1 diabetic and normal pregnancies.
In the recently published Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study of nondiabetic pregnancies, maternal BMI was strongly associated with birth weight >90th percentile, hyperinsulinemia, and fetal adiposity. These associations were independent of fasting and postload glucose values from a 75-g oral glucose tolerance test early in the third trimester (20). In the current study, the mean maternal BMI and incidence of maternal overweight were significantly higher in disproportionate LGA compared with proportionate LGA infants, born at term, in both the study and reference group. The average BMI of pregnant women has increased over time in Sweden and has been demonstrated to be an important contributing factor to the increasing incidence of fetal macrosomia in nondiabetic pregnancies (21). Taken together, these findings support the effect of BMI on fetal growth both in type 1 diabetic and nondiabetic pregnancies.
A high PI may reflect an increased lean mass, fat mass, or both. The high mean PI in our study cohort is most likely a reflection of primarily increased fat mass (17). This hypothesis is supported by significantly higher cord blood concentrations of insulin, C-peptide, and leptin in the offspring of type 1 diabetic mothers with a PI >90th percentile compared with those with a PI <90th percentile (22).
An unexpected finding in the current study was the significantly higher values of BWSDS in term and preterm girls born to diabetic mothers. Studies of newborn infants of nondiabetic mothers have shown that term and preterm girls have significantly higher levels of insulin and proinsulin in the cord blood and proportionally greater amounts of body fat than boys, despite a significantly lower mean absolute BW (23). The present finding of significantly higher PI values in female offspring of type 1 diabetic mothers can most likely be attributed to a greater accumulation of fat in the female than in the male fetus. Cord blood concentrations of C-peptide/insulin are much elevated in the offspring of type 1 diabetic mothers, but whether there is a sex difference is unclear. One could, however, speculate that hyperinsulinemia is more pronounced in female than in male infants of type 1 diabetic mothers.
In line with previous findings, 55% of the preterm infants were LGA (24). The high BWSDS in preterm infants suggests an early onset of enhanced fetal growth. This is in accordance with ultrasound measurements of fetal abdominal circumference, showing growth deviation already in the second trimester of type 1 diabetic pregnancies (25). Poor glycemic control in early pregnancy has been associated with an increased risk of preterm delivery (24). Furthermore, concentrations of C-peptide in the cord blood are significantly higher in preterm than in term offspring of type 1 diabetic mothers (18). The higher mean BWSDS in preterm compared with term infants could thus be an effect of maternal hyperglycemia in early pregnancy that is more pronounced than in women giving birth at term.
In conclusion, this study shows that all anthropometric measures in the offspring of type 1 diabetic mothers are significantly shifted to the right of the normal reference, which implies an early onset of enhanced fetal growth. The incidence of fetal macrosomia was very high, and 46% of LGA infants were overweight at birth. The proportion of LGA infants with a PI >90th percentile was also high in the reference population. The degree of fetal macrosomia was more pronounced in preterm and female offspring of type 1 diabetic mothers. Other factors besides maternal overweight and maternal glycemic control must be considered to explain the persistently high rate of fetal macrosomia in type 1 diabetic pregnancies.
Acknowledgments
This study was supported by unrestricted research grants from The Samariten Foundation, Sällskapet Barnavård, The Swedish Order of Freemasons, and the Stockholm County Council. D.P. was funded by the Tommy's Baby Charity during this study.
No potential conflicts of interest relevant to this article were reported.
M.P. conceived and designed the research, drafted the manuscript, acquired and analyzed data, and interpreted results. D.P. conceived and designed the research, analyzed data, interpreted results, and critically revised the manuscript. U.H. conceived and designed the research, acquired data, interpreted results, and critically revised the manuscript. M.N. conceived and designed the research, interpreted results, drafted the manuscript, handled funding, and supervised.
The authors appreciate the help from the Swedish MBR, which provided them with data.
References
- 1.Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care 2009;32:2005–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanson U, Persson B. Outcome of pregnancies complicated by type 1 insulin-dependent diabetes in Sweden: acute pregnancy complications, neonatal mortality and morbidity. Am J Perinatol 1993;10:330–333 [DOI] [PubMed] [Google Scholar]
- 3.Jensen DM, Damm P, Moelsted-Pedersen L, et al. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care 2004;27:2819–2823 [DOI] [PubMed] [Google Scholar]
- 4.Lewis DF, Edwards MS, Asrat T, Adair CD, Brooks G, London SJ. Can shoulder dystocia be predicted? Preconceptive and prenatal factors. J Reprod Med 1998;43:654–658 [PubMed] [Google Scholar]
- 5.Berk MA, Mimouni F, Miodovnik M, Hertzberg V, Valuck J. Macrosomia in infants of insulin-dependent diabetic mothers. Pediatrics 1989;83:1029–1034 [PubMed] [Google Scholar]
- 6.Ballard JL, Rosenn B, Khoury JC, Miodovnik M. Diabetic fetal macrosomia: significance of disproportionate growth. J Pediatr 1993;122:115–119 [DOI] [PubMed] [Google Scholar]
- 7.Rijpert M, Evers IM, de Vroede MA, de Valk HW, Heijnen CJ, Visser GH. Risk factors for childhood overweight in offspring of type 1 diabetic women with adequate glycemic control during pregnancy: nationwide follow-up study in the Netherlands. Diabetes Care 2009;32:2099–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buinauskiene J, Baliutaviciene D, Zalinkevicius R. Glucose tolerance of 2- to 5-yr-old offspring of diabetic mothers. Pediatr Diabetes 2004;5:143–146 [DOI] [PubMed] [Google Scholar]
- 9.Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia 2002;45:991–996 [DOI] [PubMed] [Google Scholar]
- 10.Cardwell CR, Stene LC, Joner G, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia 2010;53:641–651 [DOI] [PubMed] [Google Scholar]
- 11.Evers IM, de Valk HW, Mol BWJ, ter Braak EWMT, Visser GHA. Macrosomia despite good glycaemic control in type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia 2002;45:1484–1489 [DOI] [PubMed] [Google Scholar]
- 12.Van Assche FA. Symmetric and asymmetric fetal macrosomia in relation to long-term consequences. Am J Obstet Gynecol 1997;177:1563–1564 [DOI] [PubMed] [Google Scholar]
- 13.Djelmis J, Buković D, Pfeifer D, Ivanisević M. Ponderal index and disproportionate fetal growth in IDDM pregnancies. Coll Antropol 1998;22:491–495 [PubMed] [Google Scholar]
- 14.The National Board of Health and Welfare (Socialstyrelsen) [Internet]. Available from http://www.sos.se/epc/fodelse/mfr.htm Accessed 12 December 2010
- 15.Bradley RJ, Nicolaides KH, Brudenell JM. Are all infants of diabetic mothers “macrosomic”? BMJ 1988;297:1583–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnstone FD, Mao JH, Steel JM, Prescott RJ, Hume R. Factors affecting fetal weight distribution in women with type I diabetes. BJOG 2000;107:1001–1006 [DOI] [PubMed] [Google Scholar]
- 17.Susa JB, McCormick KL, Widness JA, et al. Chronic hyperinsulinemia in the fetal rhesus monkey: effects on fetal growth and composition. Diabetes 1979;28:1058–1063 [DOI] [PubMed] [Google Scholar]
- 18.Sosenko IR, Kitzmiller JL, Loo SW, Blix P, Rubenstein AH, Gabbay KH. The infant of the diabetic mother: correlation of increased cord C-peptide levels with macrosomia and hypoglycemia. N Engl J Med 1979;301:859–862 [DOI] [PubMed] [Google Scholar]
- 19.Persson B, Hanson U. Fetal size at birth in relation to quality of blood glucose control in pregnancies complicated by pregestational diabetes mellitus. Br J Obstet Gynaecol 1997;104:120–121 [DOI] [PubMed] [Google Scholar]
- 20.HAPO Study Cooperative Research Group Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 2010;117:575–584 [DOI] [PubMed] [Google Scholar]
- 21.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol 2004;104:720–726 [DOI] [PubMed] [Google Scholar]
- 22.Leperq J, Taupin P, Dubois-Laforgue D, et al. Heterogeneity of fetal growth in type 1 diabetic pregnancy. Diabet Metab 2001;27:339–344 [PubMed] [Google Scholar]
- 23.Shields BM, Knight B, Hopper H, et al. Measurement of cord insulin and insulin-related peptides suggests that girls are more insulin resistant than boys at birth. Diabetes Care 2007;30:2661–2666 [DOI] [PubMed] [Google Scholar]
- 24.Jensen DM, Damm P, Ovesen P, et al. Microalbuminuria, preeclampsia, and preterm delivery in pregnant women with type 1 diabetes: results from a nationwide Danish study. Diabetes Care 2010;33:90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong SF, Chan FY, Oats JJ, McIntyre DH. Fetal growth spurt and pregestational diabetic pregnancy. Diabetes Care 2002;25:1681–1684 [DOI] [PubMed] [Google Scholar]