Abstract
OBJECTIVE
To examine whether sleep duration and quality are associated with fasting glucose, fasting insulin, or estimated insulin resistance in a community-based sample of early middle-aged adults.
RESEARCH DESIGN AND METHODS
This was an ancillary study to the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Habitual sleep duration and fragmentation were estimated from 6 days of wrist actigraphy collected in 2003–2005. Insomnia was defined as self-reported difficulty falling asleep or waking up in the night three or more times per week plus average sleep efficiency of <80% based on actigraphy. Fasting blood samples to measure glucose and insulin were collected after the sleep measures during the CARDIA clinical examination in 2005–2006. Insulin resistance was estimated using the homeostatic model assessment (HOMA) method. Analyses were cross-sectional and stratified by the presence of diabetes.
RESULTS
There was no association between sleep measures and fasting glucose, insulin, or HOMA in the 115 subjects without diabetes. Among the 40 subjects with diabetes, after adjustment for covariates, 10% higher sleep fragmentation was associated with a 9% higher fasting glucose level, a 30% higher fasting insulin level, and a 43% higher HOMA level. Insomnia was associated with a 23% higher fasting glucose level, a 48% higher fasting insulin level, and an 82% higher HOMA level.
CONCLUSIONS
The observed association between poor sleep quality and higher glucose, insulin, and estimated insulin resistance among subjects with diabetes warrants further examination of the effect of sleep disturbances on glucose control in type 2 diabetes.
The prevalence of type 2 diabetes nearly doubled in the U.S. between 1980 and 2006, and rates have been increasing rapidly throughout the world (1). Diabetes can be a debilitating disease associated with reduced quality-of-life, severe complications, shorter life expectancy, and increased economic burden (2,3). Much effort has been devoted to identifying factors associated with the increased risk of developing type 2 diabetes and improved prognosis of people with type 2 diabetes to improve the lives of millions of Americans. Disturbed sleep has recently been proposed as a novel risk factor.
Laboratory studies that manipulated bedtimes observed impaired glucose metabolism after sleep restriction compared with sleep extension (4–6). These laboratory studies lasted only 1 to 2 weeks and the results may not reflect the effects of habitual short sleep. Observational studies have examined the association between self-reported habitual sleep and diabetes risk. Many have found cross-sectional associations that indicated a higher prevalence of diabetes among short sleepers (7–9) and among those with poor subjective sleep quality (10,11). Several prospective studies found higher rates of incident diabetes associated with shorter sleep durations. For example, a meta-analysis reported a pooled risk ratio of 1.28 (95% CI 1.03–1.60) associated with sleep duration ≤6 h compared with 7–8 h per night (12). These observational studies all relied on self-reported sleep, which may not be very accurate (13). A small Italian study did use wrist actigraphy to compare the sleep of patients with type 2 diabetes with healthy control subjects and found higher sleep fragmentation in the patients with diabetes (14). Together, these studies suggest that glucose metabolism may be adversely affected by short sleep duration and poor sleep quality.
The goal of the current study was to examine whether sleep duration or quality measured using wrist actigraphy was associated with levels of fasting glucose, fasting insulin, or estimated insulin resistance in a community-based sample of early middle-aged adults.
RESEARCH DESIGN AND METHODS
This study is ancillary to a large, ongoing cohort study, the Coronary Artery Risk Development in Young Adults (CARDIA) Study, which began in 1985–1986. CARDIA recruited black and white adults aged 18–30 years from four sites in the U.S., including Chicago, which is the site involved in our ancillary study. Those who were not pregnant at the 2000–2001 CARDIA examination were eligible to participate in our study, and 670 participants of 814 (82%) consented. Written informed consent was obtained from each participant, and this protocol was approved by the institutional review boards at Northwestern University and the University of Chicago.
In 2003–2005, sleep measures were collected using wrist actigraphy and questionnaires. In the 2005–2006 CARDIA clinical examination fasting blood samples were obtained along with other clinical and demographic variables.
Glucose and insulin were both assayed in the fasting blood sample. Each participant was asked to fast for 12 h and refrain from smoking for 2 h before the examination. Participants were asked to report the time of their last meal, and the length of the fasting period was calculated. Glucose was measured on a Cobas Mira Plus chemistry analyzer (Roche Diagnostic Systems, Indianapolis, IN) using the hexokinase ultraviolet method. Insulin was measured by radioimmunoassay using an overnight equilibrium incubation format. As part of quality control, reassays of stored samples were conducted and recalibration equations were developed for the glucose and insulin values from the 2005–2006 CARDIA examination. The current report uses the calibrated values of glucose and insulin.
Insulin resistance was estimated from the homeostatic model assessment (HOMA) index, which was calculated as: [fasting plasma insulin (mU/L) × fasting plasma glucose (mmol/L)]/22.5 (15). Higher values indicate greater estimated insulin resistance.
A person was considered to have diabetes if one of three criteria was met: 1) the participant had a fasting plasma glucose level of ≥6.99 mmol/L, 2) he or she reported having been diagnosed with diabetes, or 3) he or she reported taking medications identified as a treatment for diabetes (e.g., insulin, Amaryl, Avandia, Glucophage, glipizide, Glucotrol, metformin).
Participants wore an Actiwatch-16 wrist activity monitor (Mini-Mitter Inc, Bend, OR) for 3 days on two occasions approximately 1 year apart between 2003 and 2005. These monitors use highly sensitive omnidirectional accelerometers to count the number of wrist movements in 30-s epochs. The software scores each 30-s epoch as sleep or wake based on a threshold of activity counts that is estimated using activity within the epoch being scored as well as the epochs 2 min before and after that epoch. Bedtime and wake time are set by the researcher using the event markers, a sleep log, and data. Wrist actigraphy has been validated against polysomnography, demonstrating a correlation for sleep duration between 0.82 in people with insomnia and 0.97 in healthy subjects (16).
Sleep duration and fragmentation were calculated using Actiware 3.4 software, supplied by the manufacturer. Sleep duration is the amount of actual sleep obtained at night. Sleep fragmentation is an index of restlessness during the sleep period expressed as a percentage. It is calculated by summing two percentages: 1) the percentage of the sleep period spent moving (an epoch with >2 activity counts is considered moving) and 2) the percentage of the number of immobile phases (consecutive epochs with no movement) that are only ≤1 min long. For each participant, the mean across all available nights was used. For 92% of participants in these analyses, 6 days of actigraphy recording were available and the remaining 8% had 3 to 5 days of actigraphy recording.
We also created a dichotomous variable to identify insomnia based on subjective and objective assessments of sleep. A person was considered to have insomnia if both of the following were true: 1) on the Pittsburgh Sleep Quality Index questionnaire (17), he or she reported not falling asleep within 30 min three or more times per week or waking up in the middle of the night three or more times per week, 2) his or her average sleep efficiency (sleep duration divided by time in bed) from the actigraphy data was <80%.
Participants also completed the Berlin Sleep Apnea questionnaire (18). A participant was considered to have frequent snoring symptoms if he or she indicated two of three of the following conditions on this questionnaire: 1) snored three or more times per week, 2) snoring was louder than talking or very loud, or 3) experienced breathing pauses three or more times per week.
Covariates included race, sex, and age. BMI (kg/m2) was measured in 2005–2006. Finally, income (seven-level ordinal variable ranging from <$16,000 to ≥$100,000) and education (a five-level ordinal variable: less than high school, high school graduate, some college, college graduate, more than college degree) in 2005–2006 were included in the fully adjusted models.
Statistical analysis
Because the mechanisms of glucose regulation are markedly different between people with and without diabetes, all analyses were stratified by diabetes status. Means (SD) of all continuous variables and proportions of categorical variables were calculated. Differences in means between those with and without diabetes were tested using unpaired Student t tests, and differences in proportions were tested using χ2 tests. Linear regression models were used to examine the cross-sectional association between the sleep measures and fasting glucose, fasting insulin, and HOMA of insulin resistance (HOMA-IR) at the 20-year examination. Each of the four sleep measures—sleep duration, sleep fragmentation, insomnia, and frequent snoring—was entered into a separate regression model. Fasting glucose, fasting insulin, and HOMA-IR were all transformed using the natural log in the analyses because they were skewed to the right. The first regression model was unadjusted, and the second model was adjusted for age, race, sex, BMI, education, and income. The robust variance estimator was used in linear regression models to calculate confidence intervals and P values. All statistical analyses were conducted using StataSE 10 software (StataCorp, College Station, TX).
We excluded 9 participants with <3 days of wrist actigraphy data, 87 who reported a fasting period of <8 h before the clinical examination, 2 who were missing a value for fasting glucose or fasting insulin at 20 years, and one whose diabetes status could not be ascertained, which resulted in a final sample size of 571 participants.
RESULTS
Table 1 presents the means (SD), and proportions of key variables in the analysis stratified by diabetes status. BMI, fasting glucose, fasting insulin, HOMA-IR, and prevalence of insomnia were all significantly higher in the diabetes group.
Table 1.
Description of sample by diabetes status
| Variable | No diabetes (n = 531) |
Type 2 diabetes (n = 40) |
P |
||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | t test | |
| Age (years) | 45.2 (3.6) | 37–52 | 45.6 (3.8) | 38–51 | 0.51 |
| BMI (kg/m2) | 28.4 (6.5) | 17.2–61.0 | 37.9 (9.2) | 24.3–60.3 | <0.0001 |
| Glucose (mmol/L) | 5.3 (0.5) | 3.5–6.8 | 8.2 (2.7) | 4.8–19.4 | <0.0001 |
| Insulin (pmol/L) | 79.1 (50.7) | 4.5–504.8 | 151.2 (88.3) | 46.2–493.1 | <0.0001 |
| HOMA | 3.1 (2.2) | 0.2–21.9 | 9.1 (5.8) | 2.1–30.2 | <0.0001 |
| Actual sleep (hours) | 6.1 (1.0) | 2.6–8.8 | 5.9 (1.3) | 2.3–7.7 | 0.18 |
| Sleep fragmentation (%) | 19.0 (7.8) | 4.4–63.9 | 20.3 (8.7) | 6.5–41.8 | 0.29 |
| Sample (%) | Sample (%) | χ2 test | |||
|---|---|---|---|---|---|
| Women | 57 | 70 | 0.11 | ||
| African Americans | 41 | 60 | 0.018 | ||
| Insomnia | 17 | 35 | 0.005 | ||
| Frequent snorers | 12 | 23 | 0.06 |
The results of the cross-sectional analyses examining the association between the sleep measures and fasting glucose, fasting insulin, and HOMA-IR are presented in Table 2. In subjects without diabetes in the unadjusted models, frequent snoring was associated with higher fasting glucose, higher insulin, and higher HOMA values, and shorter sleep duration was associated with higher insulin levels and higher HOMA values, but these associations were no longer statistically significant in the fully adjusted models. Among subjects without diabetes, the fully adjusted models showed insomnia was associated with lower insulin levels and, as a direct result of lower insulin levels, lower HOMA. The regression coefficients indicated that having insomnia was associated with a 14% lower fasting insulin level and a 14% lower HOMA level.
Table 2.
Cross-sectional analysis predicting the natural log of glucose, insulin, and HOMA from each sleep variable separately stratified by diabetes*
| Variable | Model 1 (unadjusted) |
Model 2 (adjusted)† |
||
|---|---|---|---|---|
| No diabetes | Diabetes | No diabetes | Diabetes | |
| Outcome: ln(glucose) | ||||
| Sleep duration (h) | ||||
| β | –0.003 | –0.022 | 0.006 | 0.033 |
| 95% CI | –0.011 to 0.005 | –0.084 to 0.040 | –0.003 to 0.015 | –0.032 to 0.097 |
| P | 0.46 | 0.48 | 0.20 | 0.31 |
| Sleep fragmentation (per 10%) | ||||
| β | 0.008 | 0.133 | 0.001 | 0.089 |
| 95% CI | –0.004 to 0.019 | 0.042–0.223 | –0.010 to 0.012 | 0.006–0.172 |
| P | 0.199 | 0.005 | 0.89 | 0.036 |
| Insomnia | ||||
| β | 0.001 | 0.285 | –0.001 | 0.204 |
| 95% CI | –0.022 to 0.024 | 0.069–0.501 | –0.025 to 0.023 | –0.006 to 0.414 |
| P | 0.94 | 0.011 | 0.93 | 0.056 |
| Frequent snoring | ||||
| β | 0.031 | –0.088 | 0.014 | –0.148 |
| 95% CI | 0.008–0.055 | –0.290 to 0.113 | –0.009 to 0.037 | –0.323 to 0.026 |
| P | 0.008 | 0.38 | 0.23 | 0.092 |
| Outcome: ln(insulin) | ||||
| Sleep duration (h) | ||||
| β | –0.059 | –0.042 | 0.011 | –0.051 |
| 95% CI | –0.111 to –0.007 | –0.209 to 0.123 | –0.038 to 0.060 | –0.181 to 0.078 |
| P | 0.025 | 0.61 | 0.66 | 0.42 |
| Sleep fragmentation (per 10%) | ||||
| β | 0.056 | 0.224 | –0.015 | 0.264 |
| 95% CI | –0.009 to 0.121 | 0.010–0.438 | –0.073 to 0.042 | 0.086–0.441 |
| P | 0.093 | 0.040 | 0.60 | 0.005 |
| Insomnia | ||||
| β | –0.059 | 0.216 | -0.145 | 0.389 |
| 95% CI | –0.203 to 0.085 | –0.133 to 0.565 | –0.275 to −0.014 | –0.068 to 0.710 |
| P | 0.42 | 0.22 | 0.030 | 0.019 |
| Frequent snoring | ||||
| β | 0.235 | 0.208 | 0.010 | 0.209 |
| 95% CI | 0.073–0.397 | –0.215 to 0.631 | –0.133 to 0.153 | –0.117 to 0.535 |
| P | 0.004 | 0.33 | 0.89 | 0.20 |
| Outcome: ln(HOMA) | ||||
| Sleep duration (h) | ||||
| β | –0.062 | –0.065 | 0.017 | –0.019 |
| 95% CI | –0.118 to −0.007 | –0.228 to 0.097 | –0.036 to 0.070 | –0.164 to 0.126 |
| P | 0.028 | 0.43 | 0.53 | 0.79 |
| Sleep fragmentation (per 10%) | ||||
| β | 0.064 | 0.356 | –0.015 | 0.353 |
| 95% CI | –0.007 to 0.134 | 0.172–0.541 | –0.075 to 0.046 | 0.197–0.508 |
| P | 0.077 | <0.001 | 0.64 | <0.001 |
| Insomnia | ||||
| β | –0.058 | 0.501 | –0.146 | 0.593 |
| 95% CI | –0.212 to 0.096 | 0.123–0.880 | –0.284 to −0.008 | 0.284 to 0.902 |
| P | 0.46 | 0.011 | 0.038 | <0.001 |
| Frequent snoring | ||||
| β | 0.266 | 0.120 | 0.024 | 0.061 |
| 95% CI | 0.096−0.437 | –0.378 to 0.618 | –0.128 to 0.176 | –0.307 to 0.428 |
| P | 0.002 | 0.63 | 0.76 | 0.74 |
Boldface type indicates statistical significance.
*Each sleep-related predictor was included in a separate regression model.
†Model 2 is adjusted for age, race, sex, BMI, education, and income.
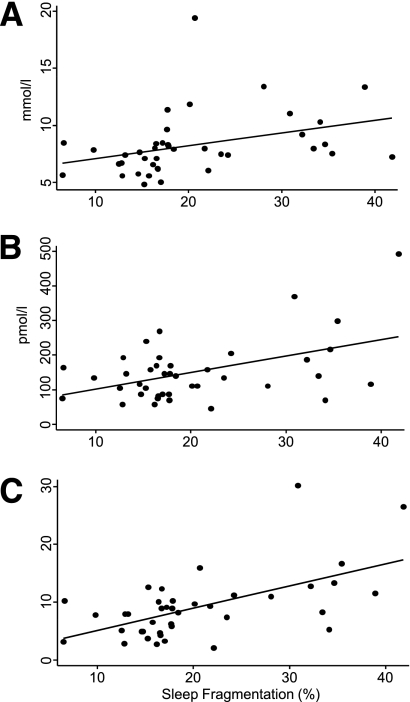
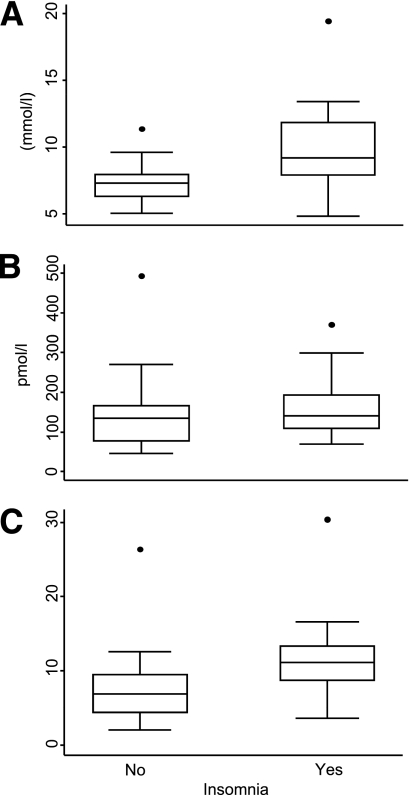
Among subjects with diabetes, greater sleep fragmentation and insomnia, but not sleep duration, were associated with higher fasting glucose, higher fasting insulin, and higher HOMA even in the fully adjusted models. Figure 1 presents the unadjusted associations between sleep fragmentation and each of the metabolic variables. Figure 2 presents the association between insomnia and each of the metabolic variables. The regression coefficients from the fully adjusted regression models (Table 2) indicated that among subjects with diabetes, a 10% higher sleep fragmentation was associated with a 9% higher fasting glucose level, a 30% higher fasting insulin level, and a 43% higher HOMA value.
Figure 1.
Scatterplots present the unadjusted association between sleep fragmentation and fasting glucose (A), insulin (B), and HOMA (C) among subjects with diabetes. Unadjusted regression is represented by the line.
Figure 2.
Box and whisker plots present the unadjusted association between insomnia and fasting glucose (A), insulin (B), and HOMA (C) among subjects with diabetes. The median is marked by the line inside the box, the ends of the box are the 25th and 75th percentiles, the whiskers represent the largest and smallest observed values that are not outliers, and the circles represent an outlier defined as 1.5 × interquartile range.
To translate these statistical associations, let us consider a diabetic patient who has good sleep quality with sleep fragmentation of only 5% and who has a fasting glucose level of 7.8 mmol/L. Let us consider a second diabetic patient with identical demographics, but a sleep fragmentation of 15%, which is the clinical threshold for insomnia. His or her average fasting glucose level is estimated to be 0.7 mmol/L higher than the first patient. Finally, insomnia among subjects with diabetes was associated with a 23% higher fasting glucose level, a 48% higher fasting insulin level, and an 82% higher level of HOMA. Again as an example, if two patients differ only in the presence of insomnia and the one without insomnia had a fasting glucose level of 7.8 mmol/L, the average fasting glucose level in the patient with insomnia would be 1.6 mmol/L higher.
CONCLUSIONS
Our analyses suggest that habitual sleep duration objectively assessed by multiple days of wrist actigraphy is not associated with markers of glucose metabolism assessed after an overnight fast in middle-aged adults with and without diabetes. Sleep fragmentation and insomnia, however, were both associated with higher fasting glucose, insulin, and estimated insulin resistance, but in subjects with diabetes only. Frequent snoring, which is a possible indicator of sleep-disordered breathing, was associated with higher fasting glucose, insulin, and insulin resistance in subjects without diabetes in unadjusted models only, which suggests that this association may be mediated by one of the covariates, such as BMI. Frequent snoring was not significantly associated with any of the metabolic measures in subjects with diabetes.
The lack of association between sleep duration or fragmentation and fasting glucose among subjects without diabetes in our sample is consistent with laboratory studies of sleep restriction in both young and middle-aged healthy subjects (4–6,19). These studies observed a significant deleterious impact of experimentally reduced sleep duration or quality on glucose metabolism in response to a glucose challenge, but did not observe a significant change in fasting levels of glucose or insulin. Two epidemiologic studies in adults also reported no association between self-reported sleep duration and fasting glucose, insulin, or HOMA in subjects without diabetes (9,20). Thus, the effects of short sleep duration or poor sleep quality on glucose metabolism in healthy individuals may be detectable only after a glucose challenge.
It is possible that the effects of sleep disturbances are more pronounced in subjects with diabetes because the mechanisms responsible for glucose homeostasis are already deficient and therefore more vulnerable. Laboratory studies in healthy subjects have indicated that lower cerebral glucose utilization, elevated sympathetic activity, higher evening cortisol levels, alterations in growth hormone release, and elevated markers of inflammation are on the pathways that mediate the adverse effects of reduced sleep duration or quality on glucose metabolism, or both. Each of these pathways is already dysregulated in the diabetic state and is likely to be more vulnerable to an additional perturbation, such as sleep disturbance.
Our data suggest that the effect of impaired sleep on glucose control in patients with type 2 diabetes may be clinically relevant. Among subjects with diabetes, fragmented sleep and insomnia were associated with fasting glucose levels that were 0.7–1.8 mmol/L higher than in those who had well-consolidated sleep or no insomnia. The strength of these associations suggests that the diagnosis and therapeutic treatment of type 2 diabetes should take into account the possible existence of sleep disturbances.
Sleep disturbances may be more common in people with diabetes (21). A study in Italy found significantly higher sleep fragmentation using wrist actigraphy in patients with type 2 diabetes compared with healthy controls (14). We did not observe significant differences in sleep fragmentation between subjects with and without type 2 diabetes, but the prevalence of insomnia symptoms in subjects with diabetes was double that in those without diabetes. A variety of factors may be responsible for the higher insomnia prevalence among those with diabetes, including increased pain associated with neuropathy, nocturia, poor glucose control, and sleep-disordered breathing. Our dataset does not provide indications on the cause of insomnia in the participants with type 2 diabetes.
Effects of insomnia on glucose metabolism have not been studied extensively. Insomnia often involves sleep loss but can also include impaired sleep quality, including increased sleep fragmentation. Insomnia may be associated with increased activity in the hypothalamic-pituitary-adrenal axis (22), which could impair glucose metabolism, particularly in those whose metabolism is already compromised. The link between the presence of insomnia and slightly, but significantly, lower fasting insulin values without significant elevation of glucose levels in nondiabetic individuals with insomnia in our fully adjusted model is intriguing. An exploratory analysis showed that this association became significant after adjustment for BMI, and reflected the contribution of the nondiabetic individuals with insomnia who were not overweight (BMI <25 kg/m2). The unadjusted mean (SD) fasting insulin level was 43.3 (20.7) pmol/L in the 22 subjects with insomnia and 54.9 (22.4) pmol/L in the 145 individuals without insomnia. This finding will need replication in a larger sample.
This study has some limitations that need to be considered. First, our study design does not allow for determination of causal direction. Although available evidence from epidemiologic and laboratory studies supports the concept that disturbed sleep impairs glucose metabolism, poorly controlled glucose levels may also impair sleep (21).
Second, we do not have polysomnographic measures of sleep in this cohort, so we cannot accurately detect sleep disorders like obstructive sleep apnea, which appears to be highly prevalent in patients with type 2 diabetes (23) and is associated with poorer glycemic control (24). We did use a validated screening questionnaire and examined the frequent snoring component of this questionnaire as a surrogate marker of apnea risk, but frequent snoring was not associated with any of the outcome measures in the fully adjusted models.
A third limitation is that participants reported having fasted for at least 8 h, but there was no way to objectively verify whether they were indeed fasting. Thus, some of the glucose and insulin measures may not have been representative of a fasting state. Another important limitation is that the sample only contained 40 individuals with diabetes, which is consistent with the mean age of the participants.
Finally, sleep was not assessed immediately before the clinical examination, but previous analysis of the actigraphy data demonstrated little year-to-year variability (25). Future research should enroll larger numbers of diabetic patients and measure glucose metabolism in a controlled setting.
Patients with type 2 diabetes are at a higher risk of diabetes complications if their glucose levels are poorly controlled. Our analyses indicated an association between poor sleep quality and higher glucose, insulin, and estimated insulin resistance that is likely to be of clinical significance. Additional research to examine the effect of impaired sleep on glycemic control in people with type 2 diabetes is important. If improving sleep can help improve glucose control in people with diabetes, then a potentially new therapeutic intervention may be identified.
Acknowledgments
Research for this study was supported by Grant AG 11412 from the National Institute on Aging. CARDIA is supported by U.S. Public Health Service contracts NO1-HC-48047, NO1-HC-48048, NO1-HC-48049, NO1-HC-48050, and NO1-HC-95095 from the National Heart, Lung, and Blood Institute.
No potential conflicts of interest relevant to this article were reported.
K.L.K. wrote the manuscript and researched data. E.V.C. reviewed data analysis and interpretation, and reviewed and edited the manuscript. P.Z. and K.L. reviewed and edited the manuscript. D.S.L. researched data and reviewed and edited the manuscript.
References
- 1.Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. J Diabetes Complications 2007;21:363–370 [DOI] [PubMed] [Google Scholar]
- 2.Ettaro L, Songer TJ, Zhang P, Engelgau MM. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics 2004;22:149–164 [DOI] [PubMed] [Google Scholar]
- 3.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med 2007;167:1145–1151 [DOI] [PubMed] [Google Scholar]
- 4.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–1439 [DOI] [PubMed] [Google Scholar]
- 5.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009;94:3242–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165:863–867 [DOI] [PubMed] [Google Scholar]
- 8.Chaput JP, Després JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia 2007;50:2298–2304 [DOI] [PubMed] [Google Scholar]
- 9.Chao CY, Wu JS, Yang YC, et al. Sleep duration is a potential risk factor for newly diagnosed type 2 diabetes mellitus. Metabolism. 14 September 2010 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep 2007;30:219–223 [DOI] [PubMed] [Google Scholar]
- 11.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun 2008;22:960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2009;33:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology 2008;19:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trento M, Broglio F, Riganti F, et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol 2008;45:225–229 [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 16.Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills 1997;85:207–216 [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 18.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–491 [DOI] [PubMed] [Google Scholar]
- 19.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 2008;105:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafalson L, Donahue RP, Stranges S, et al. Short sleep duration is associated with the development of impaired fasting glucose: the Western New York Health Study. Ann Epidemiol 2010;20:883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins RC, Andersen ML, Tufik S. The reciprocal interaction between sleep and type 2 diabetes mellitus: facts and perspectives. Braz J Med Biol Res 2008;41:180–187 [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am 2002;31:15–36 [DOI] [PubMed] [Google Scholar]
- 23.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 2010;181:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521–530 [DOI] [PubMed] [Google Scholar]
- 25.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep 2007;30:793–796 [DOI] [PMC free article] [PubMed] [Google Scholar]