Abstract
OBJECTIVE
The prognostic significance of diabetic retinopathy (DR) for death and cardiovascular (CV) outcomes is debated. We investigated the association of DR with all-cause mortality and CV events in patients with diabetes by a systematic review and meta-analysis.
RESEARCH DESIGN AND METHODS
The electronic databases Medline and Embase were searched for cohort studies that evaluated DR in type 2 or type 1 diabetic patients and reported total mortality and/or fatal and nonfatal CV events, including myocardial infarction, angina pectoris, coronary artery bypass graft, ischemic changes on a conventional 12-lead electrocardiogram, transient ischemic attack, nonfatal stroke, or lower leg amputation. Data extraction was performed by two reviewers independently. Pooled effect estimates were obtained by using random-effects meta-analysis.
RESULTS
The analysis included 20 studies that fulfilled the inclusion criteria, providing data from 19,234 patients. In patients with type 2 diabetes (n = 14,896), the presence of any degree of DR increased the chance for all-cause mortality and/or CV events by 2.34 (95% CI 1.96–2.80) compared with patients without DR. In patients with type 1 diabetes (n = 4,438), the corresponding odds ratio was 4.10 (1.50–11.18). These associations remained after adjusting for traditional CV risk factors. DR was also predictive of all-cause mortality in type 2 diabetes (odds ratio 2.41 [1.87–3.10]) and type 1 diabetes (3.65 [1.05–12.66]).
CONCLUSIONS
The presence of DR was associated with an increased risk of all-cause mortality and CV events in both type 2 and type 1 diabetic patients.
Diabetic retinopathy (DR) is a common chronic microvascular diabetes complication. Approximately 29% of U.S. adults with type 2 diabetes have DR (1), whereas DR will develop in 95% of type 1 diabetic individuals during their lifetime (2). DR has been associated with increased all-cause and cardiovascular (CV) mortality risk in both type 2 and type 1 diabetes (3–7). Associations with DR have been more extensively evaluated in type 2 diabetes, whereas studies in type 1 diabetes are scarce.
Considering these data, identification of DR could possibly add to the diabetic patient’s CV risk stratification. Furthermore, the fundus examination is inexpensive and is routinely performed for the screening of chronic diabetes complications. Therefore, it is worthwhile to comprehensively review data on the predictive role of DR. The aim of the current study was to investigate the association of DR with all-cause mortality and CV events (fatal and nonfatal) in type 2 and type 1 diabetic patients by a systematic review and meta-analysis of cohort studies.
RESEARCH DESIGN AND METHODS
Data sources and searches
The electronic databases (beginning in 1950 until July 2010) Medline and Embase were searched for the medical subject headings (MeSH) “Diabetic Retinopathy” and “mortality” or “cardiovascular disease” to identify observational studies that report the incidence of all-cause mortality, and fatal and nonfatal CV events in diabetic patients whose DR was evaluated (regardless of the language).
Study selection
Studies were considered eligible for inclusion if they fulfilled the following inclusion criteria:
presented original data of prospective, observational studies;
evaluated the presence of DR, defined as any degree and/or severity according to well-validated scales, such as the Early Treatment Diabetic Retinopathy Study severity scale (8), in type 2 or type 1 diabetic patients; and
reported all-cause mortality and/or fatal or nonfatal CV events.
When studies reported more than one outcome separately, only all-cause mortality data were included. Fatal and nonfatal CV events were defined as a positive medical history of a CV event, including death due to CV disease and/or any of the following: myocardial infarction, angina pectoris, coronary artery bypass graft, ischemic changes on a conventional 12-lead electrocardiogram, transient ischemic attack, nonfatal stroke, or lower leg amputation.
Data extraction and quality assessment
Data were extracted independently by two investigators with an agreement value of κ = 96%. Disagreements were resolved by a third author.
Extracted data included the clinical characteristics of participants, study design, and follow-up, assessment of DR, and the number of participants who had the outcome according to DR status. Numeric data reported in the articles were used. In the few studies not reporting these data, risk estimates were calculated from the survival curves.
The Newcastle-Ottawa Scale for assessing quality of nonrandomized studies in meta-analysis was used (9).
Data synthesis and analysis
An overall odds ratio (OR) was calculated to assess the predictive value of DR for all-cause mortality and/or CV events (composite outcome). DR was evaluated either as “any degree” or “advanced DR” compared with the group without DR in analyses stratified by type of diabetes (type 1 and type 2). Advanced DR was defined as the most severe category of DR described: proliferative DR, severe nonproliferative DR, sight-threatening DR, or any combination of these categories.
The Cochran χ2 and the I2 tests were used to evaluate heterogeneity among studies and a threshold value of P = 0.10 was considered significant (10). The risk estimates were obtained with random-effects meta-analysis because a significant heterogeneity was found among studies in preliminary models.
Meta-regression analyses were used to investigate potential sources of heterogeneity. The factors investigated were age, male sex, proportion of smokers, A1C test, and follow-up period (previously chosen based on their biologic relevance). In a meta-regression analysis of type 1 diabetes, only follow-up and A1C tests were included because the number of studies limited a broader evaluation of potential confounders. A sensitivity analysis including only all-cause mortality as an end point was performed to minimize the influence of CV risk factors as potential confounders.
Receiver operating characteristic (ROC) curves were constructed to obtain the pooled estimates of sensitivity and specificity of DR for the development of outcomes. The average likelihood ratio of the positive and negative test result was calculated. For practical clinical purposes, the post-test probability of the outcome was estimated using the Bayes normogram, considering the pretest probability as the mean proportion of the event in the included population (11).
The possibility of publication bias was evaluated using a funnel plot of a trial’s effect size against the SE. Funnel plot asymmetry was evaluated by the Begg and Egger tests. The trim-and-fill computation was used to estimate the effect of publication bias (12).
All statistical analyses were performed using Stata 11.0 software (StataCorp, College Station, TX).
RESULTS
Literature search results and study characteristics
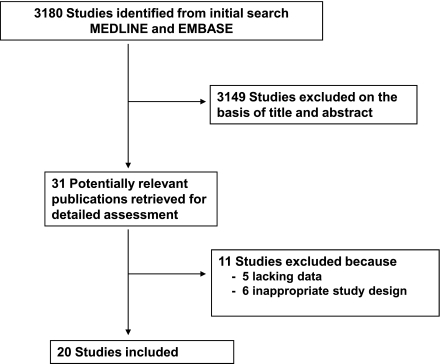
We identified 3,180 studies in the database searches (Fig. 1). Among these studies, 3,149 were excluded based on title and abstract, leaving 31 studies for further evaluation. Of these, 11 studies were excluded after full-text evaluation (Supplementary Table 1). A total of 20 studies, comprising 19,234 patients, fulfilled our inclusion criteria and were included.
Figure 1.
Flow diagram shows the literature search to identify cohort studies that evaluated DR and all-cause mortality and/or CV events (fatal and nonfatal) of diabetic patients.
Table 1 summarizes the characteristics of the included studies. A total of 17 studies with a mean follow-up of 8.95 years evaluated type 2 diabetic patients (n = 14,896; mean age, 58.4 years) and four studies with a mean follow-up of 12.37 years evaluated type 1 diabetic patients (n = 4,438; mean age, 32.7 years).
Table 1.
Summary of cohort studies evaluating the association between DR and all-cause mortality and/or CV events (nonfatal and fatal) in patients with type 2 and type 1 diabetes
| Author, year | Follow-up (years) | Population* (N) | DR evaluation | Age (years) | Men (%) | A1C test (%) | Smoking (%) | TC (mg/dL) | SBP (mmHg) | DBP (mmHg) | Outcome(s) (n events) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 2 diabetes | |||||||||||
| Sasaki, 1989 (19) | 9.4 | 1,939 Japanese | NA | 53.0 | 62 | NA | NA | NA | NA | NA | All-cause mortality (503) |
| Hanis, 1993 (16) | 8.0 | 321 Mexican Americans | Direct and indirect ophthalmoscopy and fundus photography | 55.0 | 37 | 10.8 | 20.3 | 208 | 143 | 80 | All-cause mortality (67) |
| Miettinen, 1996 (7) | 7.0 | 1,059 Finnish | Ophthalmoscopy after mydriasis | 58.0 | 54 | 9.7 | 18.0 | 258 | NA | NA | CVD death or nonfatal myocardial infarction (255) |
| Forsblom, 1998 (20) | 9.0 | 131 Europeans | Direct ophthalmoscopy and angiography | 57.5 | 51 | 9.9 | 23.5 | 243 | 154 | 86 | All-cause mortality (38) |
| Klein, 1999 (4) | 16.0 | 1,370 Americans | Indirect ophthalmoscopy and fundus photography | 66.6 | 46.4 | 9.6 | 14.2 | NA | 147 | 79 | All-cause mortality (1,052) |
| Kim, 2002 (21) | 2.3 | 365 Koreans after PCI | Direct and indirect ophthalmoscopy | 60.5 | 64 | NA | 34.0 | NA | NA | NA | CVD death (5) |
| Ono, 2002 (22) | 11.6 | 223 Japanese after CABG | Indirect ophthalmoscopy, fundus photography, and angiography | 60.4 | 75 | NA | 50.0 | 192 | NA | NA | All-cause mortality (75) |
| Rius Riu, 2003 (23) | 6.3 | 176 Spanish | NA | 54.0 | 36.4 | 7.2 | 21.9 | 226 | 144 | 77 | Fatal and nonfatal CVD (28) |
| Gimeno-Orna, 2006 (24) | 8.0 | 458 Spanish | Direct and indirect ophthalmoscopy | 65.0 | 40 | 7.8 | 11.7 | 217 | 145 | 79 | All-cause mortality (93) |
| Cheung, 2007 (25) | 7.8 | 1,617 Americans | Fundus photography | 60.0 | 47 | NA | 16.0 | 207 | NA | NA | Stroke (75) |
| Cheung, 2007 (26) | 7.8 | 1,524 Americans | Fundus photography | 60.0 | 48 | NA | 17.0 | 210 | NA | NA | Fatal and nonfatal CVD (209) |
| Lövestam-Adrian, 2007 (27) | 10.0 | 363 Swedish | Fundus photography and indirect ophthalmoscopy | 54.0 | 64 | 8.1 | NA | NA | 146 | 85 | All-cause mortality (99) |
| Juutilainen, 2007 (28) | 18.0 | 824 Finnish | Indirect ophthalmoscopy | 58.0 | 48 | 10.0 | 16.0 | 253 | 151 | 86 | CVD death (558) |
| Tong, 2007 (29) | 3.4 | 4,416 Chinese | Direct and indirect ophthalmoscopy | 57.6 | 42.5 | 7.7 | 13.0 | 208 | 134 | 77 | All-cause mortality (110) |
| Cheung, 2008 (30) | 9.0 | 1,021 Americans | Fundus photography | 60.0 | 47 | 6.6 | 44.0 | 208 | NA | NA | Heart failure (106) |
| Liew, 2009 (31) | 12.0 | 199 Australians | Fundus photography | >49 | NA | NA | NA | NA | NA | NA | CVD deaths (32) |
| Gimeno-Orna, 2009 (32) | 6.7 | 458 Spanish | Direct and indirect ophthalmoscopy | 65.0 | 40 | 7.8 | NA | 220 | 145 | 79 | Fatal and nonfatal CVD (176) |
| Type 1 diabetes | |||||||||||
| Klein, 1999 (4) | 16.0 | 966 Americans | Indirect ophthalmoscopy and fundus photography | 29.3 | 51.4 | 10.8 | 24.6 | NA | 125 | 79 | All-cause mortality (136) |
| Weis, 2001 (33) | 14.0 | 147 Australians | Direct and indirect ophthalmoscopy | 32.3 | 56 | 10.8 | 19.0 | 205 | 128.3 | 77 | All-cause mortality (28) |
| Torffvit, 2005 (34) | 12.0 | 462 Swedish | Fundus photography and ophthalmoscopy | 37.0 | 56.7 | 8.5 | NA | NA | 141 | 80 | Nonfatal CVD (70) |
| Soedamah-Muthu, 2008 (5) | 7.0 | 2,787 Europeans | Fundus photography | 32.16 | 50 | 8.4 | NA | 200 | 122 | 76 | All-cause mortality (102) |
CABG, coronary artery bypass grafting; CVD, CV disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; NA, not available; PCI, percutaneous coronary intervention; TC, total cholesterol.
*Included all patients who completed the follow-up.
In accordance with the Newcastle-Ottawa quality assessment scale for cohort studies, all studies achieved at least six stars, indicating an overall good quality (Table 2). Funnel plots and the Egger regression test suggested a borderline significant asymmetry in the analysis of type 2 diabetes (P = 0.10). However, the trim-and-fill computation revealed that there were no missing trials, indicating that the publication bias did not interfere with the interpretation of the results. There were no publication biases in analysis of type 1 diabetes in both tests (P = 0.21).
Table 2.
Newcastle-Ottawa quality assessment scale for cohort studies
| Study reference (author, year) | Selection | Comparability | Outcome |
|---|---|---|---|
| Type 2 diabetes | |||
| Sasaki, 1989 (19) | **** | * | *** |
| Hanis, 1993 (16) | **** | * | *** |
| Miettinen, 1996 (7) | *** | * | *** |
| Forsblom, 1998 (20) | *** | * | *** |
| Klein, 1999 (4) | **** | * | ** |
| Kim, 2002 (21) | *** | * | *** |
| Ono, 2002 (22) | *** | * | *** |
| Rius Riu, 2003 (23) | **** | * | *** |
| Gimeno-Orna, 2006 (24) | *** | * | *** |
| Cheung, 2007 (25) | **** | * | *** |
| Cheung, 2007 (26) | **** | * | *** |
| Lövestam-Adrian, 2007 (27) | *** | * | ** |
| Juutilainen, 2007 (28) | **** | * | *** |
| Tong, 2007 (29) | *** | * | *** |
| Cheung, 2008 (30) | **** | * | *** |
| Liew, 2009 (31) | **** | * | *** |
| Gimeno-Orna, 2009 (32) | *** | * | *** |
| Type 1 diabetes | |||
| Klein, 1999 (4) | **** | * | ** |
| Weis, 2001 (33) | *** | * | *** |
| Torffvit, 2005 (34) | **** | * | *** |
| Soedamah-Muthu, 2008 (5) | **** | * | *** |
DR and all-cause mortality and/or CV events
Type 2 diabetes.
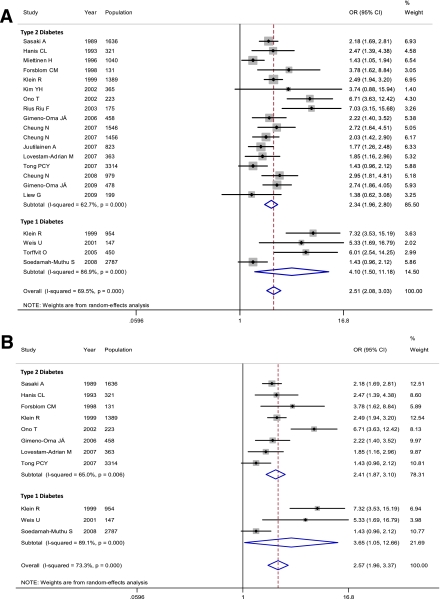
In the pooled analysis of the 17 included studies, the OR for all-cause mortality and/or CV events of the presence of DR was 2.34 (95% CI 1.96–2.80) compared with patients without DR (Fig. 2A). All but three studies reported an increased risk for events. However, a significant heterogeneity among the individual estimates was evident when the magnitude of the association was evaluated (I2 = 62.7%, P < 0.001).
Figure 2.
Meta-analyses of the association between presence of DR and (A) all-cause mortality and/or CV events (fatal and nonfatal) and (B) all-cause mortality in both type 2 and type 1 diabetic patients. (A high-quality color representation of this figure is available in the online issue.)
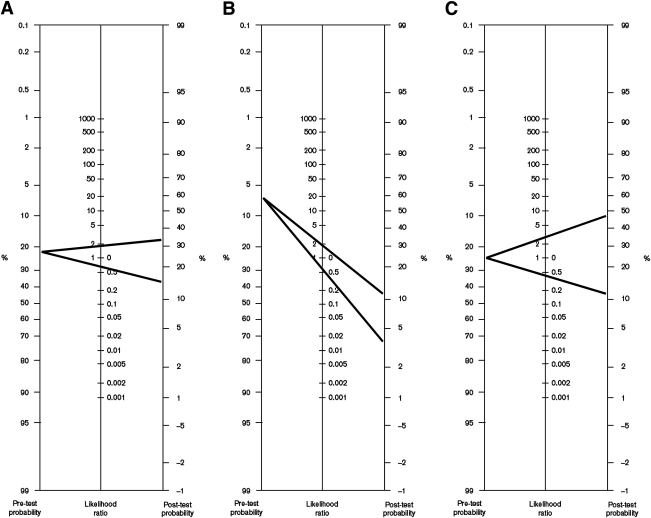
The overall sensitivity of any DR for the composite outcome was 43% (95% CI 36–50), and the specificity was 75% (71–80). The positive likelihood ratio was 1.78 (1.57–2.00) and the negative likelihood ratio was 0.75 (0.68–0.82). The post-test probability of the composite outcome for patients who had any degree of DR was 34% considering a pretest probability of 23% (Fig. 3A).
Figure 3.
Bayes normogram for DR shows post-test probability of all-cause mortality and/or CV event in type 2 (A) and in type 1 (B) diabetic patients with DR (upper line) and without DR (lower line). C: Nomogram is shown for advanced DR in type 2 diabetic patients.
In an exploratory attempt to identify the sources of heterogeneity among trials, we undertook a meta-regression analysis considering as covariates baseline age, proportion of men, proportion of smokers, A1C test, and follow-up. None of them explained the among-studies variance (overall P = 0.80).
The ORs for the composite outcome were also adjusted for CV risk factors (age, sex, diabetes duration, blood pressure, smoking status, albuminuria, and lipid profile) in 12 of the included studies. A meta-analysis was performed taking these adjusted ORs into account, even though these studies did not include the same covariates. The presence of DR still predicted events in this adjusted meta-analysis (OR 1.61 [95% CI 1.32–1.90]).
When advanced DR was evaluated (10 reports), the OR for all-cause mortality and/or CV events was 4.22 (95% CI 2.81–6.33) compared with patients without retinopathy (I2 = 63.0%, P = 0.004). The overall sensitivity of advanced DR for the combined outcome was 19% (13–28), and the specificity was 94% (91–96). The positive likelihood ratio was 3.64 (2.52–5.26) and the negative likelihood ratio was 0.84 (0.77–0.92). The post-test probability of an event occurring in an individual with advanced DR was 48% by using the Bayes normogram and considering a pretest probability of 23% (Fig. 3C).
Type 1 diabetes.
In the pooled analysis of four included studies, the OR for all-cause mortality and/or CV events of any DR was 4.10 (95% CI 1.50–11.18) compared with individuals without retinopathy (Fig. 2). A significant heterogeneity among trials was present (I2 = 86.9%, P < 0.001).
The overall sensitivity of any DR for the composite outcome was 70% (95% CI 30–93), and the specificity was 60% (29–85). The corresponding positive likelihood ratio was 1.80 (1.20–2.70) and the negative likelihood ratio was 0.47 (0.22–1.04; Fig. 3B). The post-test probability of an event occurring in a patient with DR was 12% by using the Bayes normogram and considering a pretest probability of 7.5%.
In a meta-regression analysis including the follow-up period and baseline A1C test as possible sources of heterogeneity, the follow-up of the studies explained almost 100% of the differences among the studies. Therefore, a sensitive analysis including only the three studies with follow-up >10 years was performed, demonstrating an OR of DR for the composite outcome of 6.45 (95% CI 3.91–10.85) with heterogeneity of 0% (P = 0.90).
Three studies also presented an adjusted OR of the risk conferred by DR. The presence of any DR still predicted all-cause mortality and CV events when a meta-analysis using these adjusted data were performed (OR 1.58 [95% CI 1.33–1.89]).
Three reports also included evaluation of advanced DR. The OR for all-cause mortality and/or CV events of advanced DR was 7.00 (95% CI 2.22–20.0) compared with patients without DR (I2 = 85.0%, P < 0.001). Because only three studies were included in this analysis, the ROC curve could not be constructed.
DR and all-cause mortality in type 2 and type 1 diabetes
In the sensitivity analyses evaluating only the eight studies that reported data on all-cause mortality, the presence of DR increased more than twofold the risk for death (OR 2.41 [95% CI 1.87–3.10]) in patients with type 2 diabetes. The risk for death was also greater in patients with type 1 diabetes and DR compared with those without DR, considering three included studies (OR 3.65 [1.05–12.66]; Fig. 2B).
CONCLUSIONS
These meta-analyses of cohort studies showed that the presence of any degree of DR or advanced DR was associated with an increased risk for all-cause mortality and CV events (fatal and nonfatal) in both type 2 and type 1 diabetic patients. Data from the current study show that funduscopy can be a practical tool to identify patients who are at increased risk for adverse outcomes, as demonstrated by the influence of DR in the post-test probability of having an event.
Our literature search was extensive; we tested for and found no evidence of publication bias. The quality of original studies was checked according to the Newcastle scale statement and most of the studies fulfilled all components. We also are aware that publication bias and the quality issues of individual studies may still exist despite our best efforts to conduct a comprehensive search and the lack of statistical evidence of existence of bias. Another possible limitation is that our adjusted meta-analyses undertaken were not ideal because the authors of the original studies used different statistical models. However, we must take into account that the potential confounders (covariates) were chosen in each original study according to sample characteristics.
The development of DR has been associated with known risk factors for CV disease such as hypertension, hyperglycemia, and albuminuria (2). Therefore, the association of DR with CV events would be expected, and one might think that DR is just a marker of a worse clinical status. However, in the meta-regression analyses, CV confounders did not explain the between-studies variance, suggesting that these risk factors do not fully account for the observed association. In parallel to that, our results did not change when additional meta-analyses were performed, including only the studies where the risk determined by DR was adjusted for possible confounders in both type 2 and type 1 diabetes.
An important aspect that should be considered is that the therapeutic goals in diabetes care have changed over time. The intensification of glycemic control in patients with type 1 diabetes became widespread after the publication of the Diabetes Control and Complications Trial study in 1993 (13). In patients with type 2 diabetes, a significant reduction in LDL cholesterol levels and blood pressure were observed since 1990 (14). These issues might have influenced mortality rates as well as the effects of risk factors in our meta-analysis because the timeline of included studies ranged from the 1960s to 2008. However, despite changes in goals to be achieved in patients with diabetes, the mortality rate was comparable during the periods of 1971 through 1986 and 1988 through 2000 (15). In parallel to that, the timelines of included studies all overlapped.
Highlighting the importance of DR as a possible novel marker for events in patients with diabetes is that DR was strongly associated with all-cause mortality, beyond its association with CV events. In fact, CV disease was the cause of death in less than 35% of individuals in most of the included studies (4,16), suggesting an additional mechanism by which the presence of DR increases death. In this sense, the presence of autonomic neuropathy could be a possible link between DR and CV events. Indeed, it was recently demonstrated that autonomous deregulation could lead to alterations in blood pressure and cardiac rhythm, which were associated with DR (17,18).
In conclusion, the presence of DR was associated with an increased risk for all-cause mortality and CV events in both type 2 and type 1 diabetes. Further studies are needed to understand the role of the inclusion of DR in mortality prediction scores as well as to understand the link between death/CV events and DR, especially the all-cause mortality DR association.
Supplementary Material
Acknowledgments
This study was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico grant 576627/2008-9, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior PNPD 03021/09-2, and Fundo de Incentivo a Pesquisa–Hospital de Clínicas de Porto Alegre, Brazil.
No potential conflicts of interest relevant to this article were reported.
C.K.K. originated and designed the study, acquired data, analyzed statistics, analyzed and interpreted data, drafted the manuscript, and critically revised the manuscript for important intellectual content. T.C.R. acquired data, analyzed and interpreted data, and critically revised the manuscript for important intellectual content. L.H.C. and J.L.G. analyzed and interpreted data and critically revised the manuscript for important intellectual content. M.J.A. originated and designed the study, analyzed and interpreted data, and critically revised the manuscript for important intellectual content.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0079/-/DC1.
References
- 1.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010;304:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong DS, Aiello LP, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care 2004;27:2540–2553 [DOI] [PubMed] [Google Scholar]
- 3.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med 1997;157:1413–1418 [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol 1999;117:1487–1495 [DOI] [PubMed] [Google Scholar]
- 5.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH, EURODIAB Prospective Complications Study Group Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008;31:1360–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hecke MV, Dekker JM, Stehouwer CD, et al. EURODIAB prospective complications study Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care 2005;28:1383–1389 [DOI] [PubMed] [Google Scholar]
- 7.Miettinen H, Haffner SM, Lehto S, Rönnemaa T, Pyörälà K, Laakso M. Retinopathy predicts coronary heart disease events in NIDDM patients. Diabetes Care 1996;19:1445–1448 [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682 [DOI] [PubMed] [Google Scholar]
- 9.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa Hospital Research Institute. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed 2 August 2010
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 11.Fagan TJ. Letter: Nomogram for Bayes theorem. N Engl J Med 1975;293:257. [DOI] [PubMed] [Google Scholar]
- 12.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463 [DOI] [PubMed] [Google Scholar]
- 13.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 14.Preis SR, Pencina MJ, Hwang SJ, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation 2009;120:212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 2007;147:149–155 [DOI] [PubMed] [Google Scholar]
- 16.Hanis CL, Chu HH, Lawson K, et al. Mortality of Mexican Americans with NIDDM. Retinopathy and other predictors in Starr County, Texas. Diabetes Care 1993;16:82–89 [DOI] [PubMed] [Google Scholar]
- 17.Ayad F, Belhadj M, Pariés J, Attali JR, Valensi P. Association between cardiac autonomic neuropathy and hypertension and its potential influence on diabetic complications. Diabet Med 2010;27:804–811 [DOI] [PubMed] [Google Scholar]
- 18.Kramer CK, Leitão CB, Canani LH, et al. Late afternoon blood pressure increase is associated with diabetic retinopathy in normotensive type 2 diabetes mellitus patients. Diabetes Res Clin Pract 2009;84:e12–e14 [DOI] [PubMed] [Google Scholar]
- 19.Sasaki A, Horiuchi N, Hasegawa K, Uehara M. Mortality and causes of death in type 2 diabetic patients. A long-term follow-up study in Osaka District, Japan. Diabetes Res Clin Pract 1989;7:33–40 [DOI] [PubMed] [Google Scholar]
- 20.Forsblom CM, Sane T, Groop PH, et al. Risk factors for mortality in type II (non-insulin-dependent) diabetes: evidence of a role for neuropathy and a protective effect of HLA-DR4. Diabetologia 1998;41:1253–1262 [DOI] [PubMed] [Google Scholar]
- 21.Kim YH, Hong MK, Song JM, et al. Diabetic retinopathy as a predictor of late clinical events following percutaneous coronary intervention. J Invasive Cardiol 2002;14:599–602 [PubMed] [Google Scholar]
- 22.Ono T, Kobayashi J, Sasako Y, et al. The impact of diabetic retinopathy on long-term outcome following coronary artery bypass graft surgery. J Am Coll Cardiol 2002;40:428–436 [DOI] [PubMed] [Google Scholar]
- 23.Rius Riu F, Salinas Vert I, Lucas Martín A, Romero González R, Sanmartí Sala A. A prospective study of cardiovascular disease in patients with type 2 diabetes: 6.3 years of follow-up. J Diabetes Complications 2003;17:235–242 [DOI] [PubMed] [Google Scholar]
- 24.Gimeno Orna JA, Castro Alonso FJ, Sánchez Vañó R, Latre Rebled B, Lou Arnal LM, Molinero Herguedas E. Diabetic retinopathy and mortality in type 2 diabetic patients. Med Clin (Barc) 2006;126:686–689 [in Spanish] [DOI] [PubMed] [Google Scholar]
- 25.Cheung N, Rogers S, Couper DJ, Klein R, Sharrett AR, Wong TY. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007;38:398–401 [DOI] [PubMed] [Google Scholar]
- 26.Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care 2007;30:1742–1746 [DOI] [PubMed] [Google Scholar]
- 27.Lövestam-Adrian M, Hansson-Lundblad C, Torffvit O. Sight-threatening retinopathy is associated with lower mortality in type 2 diabetic subjects: a 10-year observation study. Diabetes Res Clin Pract 2007;77:141–147 [DOI] [PubMed] [Google Scholar]
- 28.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care 2007;30:292–299 [DOI] [PubMed] [Google Scholar]
- 29.Tong PC, Kong AP, So WY, et al. Interactive effect of retinopathy and macroalbuminuria on all-cause mortality, cardiovascular and renal end points in Chinese patients with type 2 diabetes mellitus. Diabet Med 2007;24:741–746 [DOI] [PubMed] [Google Scholar]
- 30.Cheung N, Wang JJ, Rogers SL, et al. ARIC (Atherosclerosis Risk In Communities) Study Investigators Diabetic retinopathy and risk of heart failure. J Am Coll Cardiol 2008;51:1573–1578 [DOI] [PubMed] [Google Scholar]
- 31.Liew G, Wong TY, Mitchell P, Cheung N, Wang JJ. Retinopathy predicts coronary heart disease mortality. Heart 2009;95:391–394 [DOI] [PubMed] [Google Scholar]
- 32.Gimeno-Orna JA, Faure-Nogueras E, Castro-Alonso FJ, Boned-Juliani B. Ability of retinopathy to predict cardiovascular disease in patients with type 2 diabetes mellitus. Am J Cardiol 2009;103:1364–1367 [DOI] [PubMed] [Google Scholar]
- 33.Weis U, Turner B, Gibney J, et al. Long-term predictors of coronary artery disease and mortality in type 1 diabetes. QJM 2001;94:623–630 [DOI] [PubMed] [Google Scholar]
- 34.Torffvit O, Lövestam-Adrian M, Agardh E, Agardh CD. Nephropathy, but not retinopathy, is associated with the development of heart disease in type 1 diabetes: a 12-year observation study of 462 patients. Diabet Med 2005;22:723–729 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.