Abstract
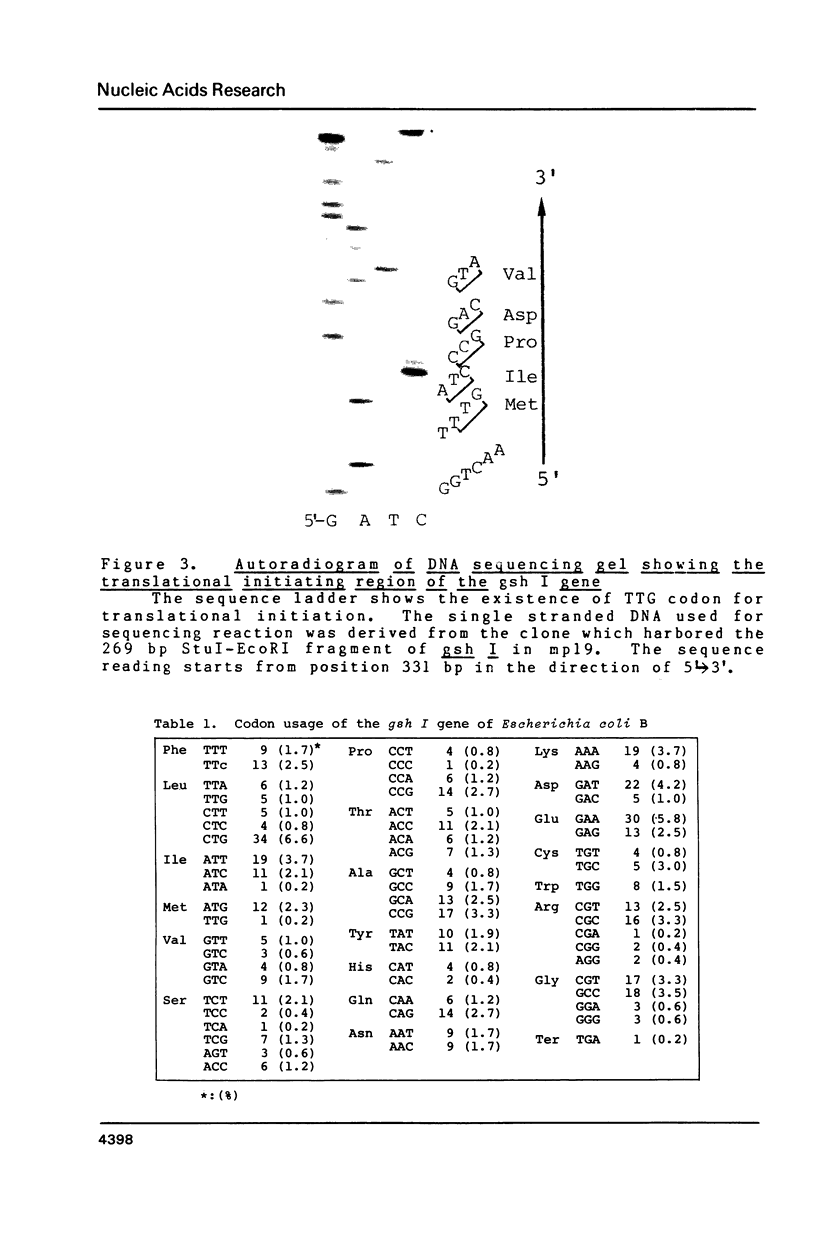
The nucleotide sequence of the gsh I gene for gamma-glutamylcysteine synthetase(GSH I) of Escherichia coli B has been determined. The gsh I structural gene contains 1557 bases in length and predicted a ploypeptide of 518 amino acids with a calculated molecular weight of 58,251. The value is in good agreement with that obtained from gel filtration and SDS/PAGE of GSH I. The initiation codon 5 bp downstream of putative Shine-Dalgarno sequence was an unusual TTG, which encoded methionine. For transcription, two sets of consensus promoter signals(-10 and -35 regions) overlapping each other were identified. The terminator signal shows the favored stem-loop structure with an adequate free energy delta G = -22.80 kcal/mol.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Rudland P. S., Clark B. F., Marcker K. A. A structural requirement for codon-anticodon interaction on the ribosome. Cold Spring Harb Symp Quant Biol. 1969;34:161–166. doi: 10.1101/sqb.1969.034.01.023. [DOI] [PubMed] [Google Scholar]
- Files J. G., Weber K., Coulondre C., Miller J. H. Identification of the UUG codon as a translational initiation codon in vivo. J Mol Biol. 1975 Jun 25;95(2):327–330. doi: 10.1016/0022-2836(75)90398-8. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Marliere P., Kofoid E. C., Louis B. G. Initiator tRNA may recognize more than the initiation codon in mRNA: a model for translational initiation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4587–4591. doi: 10.1073/pnas.82.14.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushima H., Miya T., Murata K., Kimura A. Construction of glutathione-producing strains of Escherichia coli B by recombinant DNA techniques. J Appl Biochem. 1983 Feb-Apr;5(1-2):43–52. [PubMed] [Google Scholar]
- Ikemura T., Ozeki H. Codon usage and transfer RNA contents: organism-specific codon-choice patterns in reference to the isoacceptor contents. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1087–1097. doi: 10.1101/sqb.1983.047.01.123. [DOI] [PubMed] [Google Scholar]
- Lee N., Cozzitorto J., Wainwright N., Testa D. Cloning with tandem gene systems for high level gene expression. Nucleic Acids Res. 1984 Sep 11;12(17):6797–6812. doi: 10.1093/nar/12.17.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A. Nucleotide sequence of the gene for ribosomal protein S20 and its flanking regions. J Biol Chem. 1981 Aug 10;256(15):8177–8182. [PubMed] [Google Scholar]
- Murata K., Kimura A. Some properties of glutathione biosynthesis-deficient mutants of Escherichia coli B. J Gen Microbiol. 1982 May;128(5):1047–1052. doi: 10.1099/00221287-128-5-1047. [DOI] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Roy A., Haziza C., Danchin A. Regulation of adenylate cyclase synthesis in Escherichia coli: nucleotide sequence of the control region. EMBO J. 1983;2(5):791–797. doi: 10.1002/j.1460-2075.1983.tb01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Hammer-Jespersen K., Boetius F., Svendsen I. Structure and function of the intercistronic regulatory deoC-deoA element of Escherichia coli K-12. EMBO J. 1984 Jan;3(1):179–183. doi: 10.1002/j.1460-2075.1984.tb01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyens G., Rose K., Falmagne P., Glansdorff N., Piérard A. Synthesis of Escherichia coli carbamoylphosphate synthetase initiates at a UUG codon. Eur J Biochem. 1985 Jul 1;150(1):111–115. doi: 10.1111/j.1432-1033.1985.tb08995.x. [DOI] [PubMed] [Google Scholar]
- Young I. G., Rogers B. L., Campbell H. D., Jaworowski A., Shaw D. C. Nucleotide sequence coding for the respiratory NADH dehydrogenase of Escherichia coli. UUG initiation codon. Eur J Biochem. 1981 May;116(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05314.x. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]