Abstract
Rhythmic activity of cells and cellular networks plays an important role in physiology. In the nervous system oscillations of electrical activity and/or second messenger concentrations are important to synchronize neuronal activity. At the molecular level, rhythmic activity can be initiated by different routes. We have recently shown that an octopamine-activated G-protein-coupled receptor (GPCR; DmOctα1Rb, CG3856) from Drosophila initiates Ca2+ oscillations. Here, we have unraveled the molecular basis of cellular Ca2+ signaling controlled by the DmOctα1Rb receptor using a combination of pharmacological intervention, site-directed mutagenesis, and functional cellular Ca2+ imaging on heterologously expressed receptors. Phosphorylation of a single amino acid residue in the third intracellular loop of the GPCR by PKC is necessary and sufficient to desensitize the receptor. From its desensitized state, DmOctα1Rb is resensitized by dephosphorylation, and a new Ca2+ signal occurs on octopamine stimulation. Our findings show that transient changes of the receptor's surface profile have a strong effect on its physiological signaling properties. We expect that the detailed knowledge of DmOctα1Rb-dependent signal transduction fosters the identification of specific drugs that can be used for GPCR-mediated pest control, since octopamine serves important physiological and behavioral functions in arthropods.—Hoff M., Balfanz, S., Ehling, P., Gensch, T., Baumann, A. A single amino acid residue controls Ca2+ signaling by an octopamine receptor from Drosophila melanogaster.
Keywords: biogenic amine; G-protein-coupled receptor; inositol-1,4,5-trisphosphate; learning and memory; oscillation
Biogenic amines are small organic compounds that function as neurotransmitters, neurohormones, and neuromodulators. In invertebrates, the phenolamine octopamine has a pivotal physiological role. It controls neuromuscular transmission (1) and lipid and carbohydrate metabolism (2), and also contributes to learning and behavior (3–5). Compared to vertebrate biogenic amines, octopamine and its biochemical precursor tyramine may fulfill similar functions in invertebrates, as epinephrine and norepinephrine do in vertebrates (6, 7). Recent experimental evidence suggests that octopamine and tyramine might be functionally active in vertebrates as trace amines as well (8).
Octopamine binds to specific receptors that belong to the superfamily of G-protein-coupled receptor (GPCR) proteins (9). On the basis of pharmacological and cellular signaling criteria, at least 2 groups of octopamine receptors were defined: OA1 receptors cause Ca2+ elevation, whereas OA2 receptors lead to cAMP production (10). Four octopamine receptor genes have been cloned from Drosophila melanogaster: OAMB (new nomenclature: DmOctα1Ra, CG3856; ref. 11), DmOA1A/DmOA1B (DmOctα1Ra/1Rb, CG3856; ref. 12), DmOA2 (DmOctβ1R, CG6919; ref. 12), and DmOctβ1R–3R (CG6919, CG6989, CG31351; ref. 13). The DmOctα1Ra/1Rb proteins are encoded by one gene and originate from alternative splicing. After heterologous expression, both receptors lead to periodic changes of the intracellular Ca2+ concentration ([Ca2+]i) in the presence of ligand. We could show that Ca2+ oscillations were due to Ca2+ release from intracellular stores rather than by influx from the extracellular side (12). In contrast to these receptors, the DmOctβ1R–3R receptors cause an increase of [cAMP]i (12, 13).
At low ligand concentrations (≤50 nM octopamine), DmOctα1Ra/1Rb-induced Ca2+ signals with a frequency of ∼0.02 Hz occurred. The value increased with increasing ligand concentrations (12). For various GPCRs, homologous and/or heterologous mechanisms that cause receptor desensitization, and thus termination of cellular signaling, have been described (14, 15). The initiating step to uncouple an activated receptor from its signaling partners often occurs by phosphorylation of the GPCR, followed by binding to arrestin-type cytosolic proteins (16). Thereby, the signaling cascade is shut off. Reentry of a desensitized GPCR into a signaling status can be achieved by dephosphorylation of the receptor. Alternatively, GPCRs might be internalized, and, depending on the route taken, they might either be reinserted into the plasma membrane from the vesicular pool or become degraded (17). The latter scenario requires de novo protein synthesis to restore the pool of functional receptors in the membrane.
Here we studied the signaling behavior of the DmOctα1Rb receptor at the molecular level. We initiated a combined pharmacological and mutagenesis approach to unravel the molecular basis of DmOctα1Rb signaling characteristics. Inhibition of PKC led to reproducible and drastically changed Ca2+ responses. Blocking phosphorylation of the activated receptor resulted in a continuous Ca2+ signal instead of Ca2+ oscillations. Furthermore, blocking phosphatase activity in DmOctα1Rb-expressing cells completely abolished the Ca2+ oscillations. Using site-directed mutagenesis, we identified a single threonine residue (T352) in the third intracellular loop of DmOctα1Rb as the target site for PKC-dependent phosphorylation and thus homologous desensitization of the receptor. Our results show that Ca2+ signaling initiated by the octopamine receptor DmOctα1Rb is intimately controlled by kinase and phosphatase activities, resulting in dynamic changes of the intracellular second messenger concentration.
MATERIALS AND METHODS
Materials
Receptor ligands were purchased from RBI (Munich, Germany) and Sigma (Neustadt, Germany). The Ca2+-sensitive fluorescent dye Fluo-4 AM was from Invitrogen (Leek, Netherlands). Phosphatase inhibitors [inhibitor cocktail set IV containing (−)-p-bromotetramisole oxalate, cantharidine, and calyculin A] and β-glycerophosphate (β-GP) were from Calbiochem (Darmstadt, Germany). Okadaic acid was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The PLC inhibitor (U73122) was from Sigma. The PKA inhibitor [4-cyano-3-methyl-isoquinoline (CMIQ)] was from Merck (Darmstadt, Germany), and bisindolylmaleimide PKC inhibitors (Bis VIII and Bis X) were from Sigma. The PKG inhibitor KT5823 was from Merck. The adenylyl cyclase activator NKH477 was from Biotrend (Cologne, Germany).
Construction of DmOctα1Rb mutants
Phosphorylation sites of DmOctα1Rb (CG3856) were identified using the KinasePhos 2.0 Web page (http://kinasephos2.mbc.nctu.edu.tw) (18). A PCR-based site-directed mutagenesis approach was applied to neutralize PKC phosphorylation sites in DmOctα1Rb. The cDNA was cut into 3 fragments, BamHI/BamHI, BamHI/SalI, and SalI/XbaI, to facilitate a modular cloning and mutagenesis strategy. For each fragment, a pair of specific primers was designed, binding to the 5′ and 3′ ends, respectively. To substitute serine or threonine residues of PKC consensus sites, PCRs with flanking and mutagenesis primers (see Table 1) were performed according to Herlitze and Koenen (19). For amplification, KOD Hot Start DNA Polymerase (Merck) was used. Typical PCR conditions consisted of an initial denaturing step for 150 s at 94°C, followed by 30 cycles with 30 s at 94°C (denaturing), 40 s at 55–60°C (annealing), and 30 s at 72°C (extension). PCR products were cut with restriction enzymes, purified by agarose gel electrophoresis, and subcloned into pBluescript SK− vector by standard cloning techniques (20). Sequencing was performed according to the dideoxy nucleotide sequencing technique (21).
Table 1.
Primers used for PCR amplifications, identified by laboratory number
| Primer | Position | Sequence |
|---|---|---|
| 1407 | P1 B/B | CTCAGGAATTCCACCATGAATGAAACAGAGTGC |
| 4017 | P2 B/B | CGCGGATCCATCGATGGGTGCG |
| 4968 | P1 B/S | ATCAGGATCCGCGGAGATTCGGATTGCG |
| 4969 | P2 B/S | ATCAGTCGACGCAGTCCTGGCAGAACGGC |
| 4970 | P1S/X | ATCAGTCGACCCGCTGCTCTTTTCGGTGC |
| 4971 | P2 S/X | ATCATCTAGAGTTAACTATGCATAATCTGGGACG |
| 4018 | MP2 48 | GTCTTCTGTGCGAATAAGTTG |
| 4019 | MP1 48 | CAACTTATTGGCACAGGAGAC |
| 4020 | MP2 133 | CCAAGCATAATGGCCACGAAG |
| 4021 | MP1 133 | CTTCGTGGCCATTATGCTTGG |
| 4972 | MP2 133/134 | AGCATAATGGCCGCGAAGAAGGCCAAGTCCTTAATCG |
| 4973 | MP1 133/134 | GCCTTCTTCGCGGCCATTATGCTTGGGTAGGTGAC |
| 4974 | MP2 325 | GTGAGAGCGACGAGAGCCATCAACCAGGG |
| 4975 | MP1 325 | GCTCTCGTCGCTCTCACGGCAGCCCGGTAG |
| 4976 | MP2 335 | GCTTCAAGGCCACCAAGGGTTCCAAGGGCATCG |
| 4977 | MP1 335 | CCCTTGGTGGCCTTGAAGCCCTGGTTGATGGC |
| 4978 | MP2 352 | AACGCCTCGCATTGCGCATACATAGAGGACGAG |
| 4979 | MP1 352 | ATGCGCAATGCGAGGCGTTGCTCCTCGAAGC |
| 4980 | MP2 393 | AGTACGCCGCTAGGCGACTGCACCACCACG |
| 4981 | MP1393 | AGTCGCCTAGCGGCGTACTTCGACAACCTCTC |
| 4982 | MP2 598 | AGTCCGTCGCCTTGAAGAGCTCCCGCCGGGG |
| 4983 | MP1598 | CTCTTCAAGGCGACGGACTGGCGGGAGCAG |
| 4984 | MP2 601/602 | CCTTGAAGGCGGCCCGCCGGGGATCTGACATGTC |
| 4985 | MP1 601/602 | CCCCGGCGGGCCGCCTTCAAGGAGACGGACTGGC |
P denotes flanking primer, where P1 represents the primer binding to the very 5′ end, and P2 represents binding to the very 3′ end of the restriction fragment; MP denotes mutagenesis primer, with MP1 primers allowing amplification in 5′–3′ direction, and MP2 primers allowing amplification in 3′–5′ direction. Amplifications were performed on restriction fragments of DmOctα1Rb: BamHI (B)/B, B/SalI (S), and S/XbaI (X). Positions of mutated amino acids are indicated with the respective MPs. Nucleotide sequence of primers is in 5′–3′ direction. Triplets encoding the modified residues are underscored.
Construction of expression constructs
The cDNA encoding the wild-type DmOctα1Rb receptor had been already modified at the 3′ end with a sequence tag coding for the hemagglutinin A (HA) epitope to facilitate immunological detection of expressed protein. All mutant constructs also contained this modification. At the 5′ end, a Kozak consensus sequence (CCACC; ref. 22) was introduced preceding the initiation codon (ATG; see ref. 12). For transient expression in HEK 293 cells, all constructs were cloned into pcDNA1.amp vector (Invitrogen). Prior to transfection, the constructs were controlled by restriction analysis and sequencing.
Heterologous expression of DmOctα1Rb variants
For expression of receptor constructs, 8–15 μg of DmOctα1Rb recombinants were introduced into exponentially growing (∼2×105 cells/50 mm dish) HEK 293 cells by a modified calcium phosphate method (23). At 36 to 48 h post-transfection, octopamine-induced cellular signaling was analyzed by Ca2+ fluorimetry (see below). For generation of a stably transfected cell line, the DmOctα1RbT352A mutant was subcloned into pcDNA3.1(+) vector (Invitrogen) and transfected into HEK 293 cells. Transformed cell clones were selected in the presence of 1.0 mg/ml of the antibiotic G418. Isolated foci were propagated and analyzed individually for receptor-mediated changes of [Ca2+]i using Ca2+ fluorimetry, as described previously for the stable cell line expressing the wild-type receptor DmOctα1Rb (12).
Ca2+ fluorimetry
The ability of DmOctα1Rb variants to induce changes in [Ca2+]i was monitored with the Ca2+-sensitive fluorescence dye Fluo-4. Experiments were done on transiently and stably transfected HEK 293 cells. Cells were incubated at 37°C in extracellular solution (ES; in mM: 150 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 30 glucose at pH 7.4) containing 2 μM Fluo-4AM (Invitrogen) and 0.02% Pluronic F-127 (Sigma). After 45 min, cells were washed with dye-free ES. For receptor activation, cells were superfused with ES containing octopamine. The effect of kinase, phosphatase, and PLC inhibitors was examined by coapplication of the respective substances and octopamine via the superfusion system. To measure [Ca2+]i-dependent changes of Fluo-4 fluorescence, a single-cell photon-counting system (PhoCal; Life Science Resources, Cambridge, UK) was used. Excitation wavelength was 480 nm (Xe lamp, 100 W; Nikon, Tokyo, Japan). Fluorescence emission was detected at 520–560 nm. The sampling rate of the photon-counting system was adjusted to 100 ms. Alternatively, an upright laser-scanning fluorescence microscope (BX51; Olympus, Hamburg, Germany) with 2-photon excitation (80-fs pulse length, 75-MHz repetition frequency; Newport Spectra-Physics, Darmstadt, Germany) was used. Excitation was at 750 nm, where Fluo-4 has a large 2-photon absorption coefficient. Fluorescence emission was detected with a 500-nm long-pass filter. Because of different geometries of the incubation chambers used for the two Ca2+ imaging setups, initiation of Ca2+ signals was delayed at the 2-photon-excitation microscope (see Figs. 3 and 4).
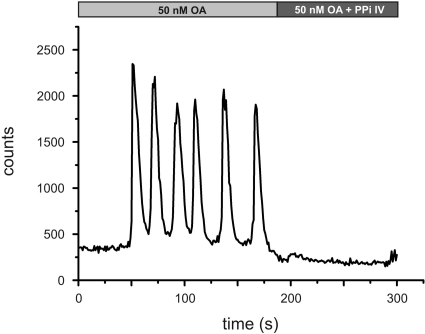
Figure 3.
Ca2+ fluorimetry of DmOctα1Rb-expressing cells in the absence or presence of phosphatase inhibitors. HEK 293 cells stably transfected with DmOctα1Rb were loaded with Fluo-4. As shown in Figs. 1 and 2, application of 50 nM octopamine led to Ca2+ oscillations. Simultaneous application of octopamine and a phosphatase inhibitor cocktail (PPi IV) completely abolished Ca2+ signals.
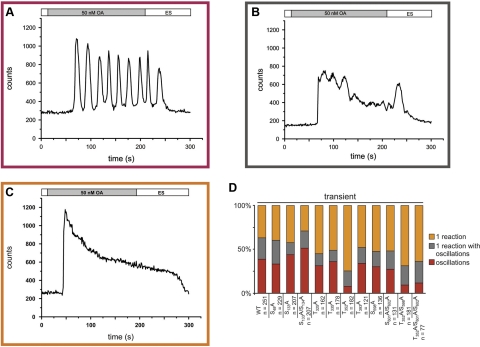
Figure 4.
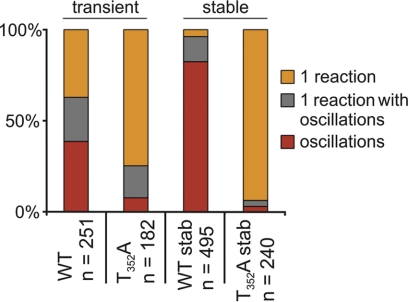
Octopamine-induced Ca2+ signals in HEK 293 cells transiently transfected with DmOctα1Rb mutants. HEK 293 cells were transiently transfected with DmOctα1Rbwt (WT) or mutant constructs. For Ca2+ imaging, cells were loaded with Fluo-4. Ca2+ responses of individual cells were examined in the presence of 50 nM octopamine. Three types of Ca2+ responses were observed. A) Ca2+ oscillations as shown for the stable cell line. B) Ca2+ oscillations or Ca2+ peaks on top of an elevated Ca2+ level. C) Initial transient increase followed by sustained and elevated [Ca2+]i. D) Percentage of cells showing any of the 3 signals, quantified and summarized. Number of cells (n) analyzed for each construct is given. Color code: red, [Ca2+]i oscillation; gray, oscillation on top of elevated [Ca2+]I; orange, transient rise followed by sustained elevated [Ca2+]i.
For graphical display of experimentally registered Fluo-4 fluorescence, the programs OriginPro6.1 (OriginLab Co., Northampton, MA, USA) and Image J 1.42q (Wayne Rasband, U.S. National Institutes of Health, Bethesda, MD, USA) were used.
RESULTS
Inhibition of PLC blocks DmOctα1Rb receptor-mediated signaling
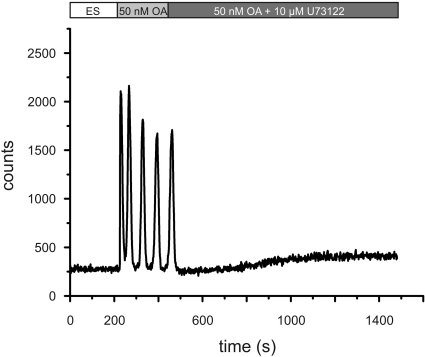
Application of octopamine to DmOctα1Rb (CG3856)-expressing cells induces intracellular Ca2+ oscillations (12). To identify the downstream effectors participating in this signaling pathway, cells were incubated with 50 nM octopamine either in the absence or presence of 10 μM U73122, a specific inhibitor of PLC. Octopamine-induced Ca2+ oscillations were completely abolished in the presence of U73122 (Fig. 1). This result suggests that DmOctα1Rb activation leads to PLC-mediated IP3 production, followed by IP3-receptor activation in the endoplasmic reticulum (ER), and subsequently to an elevation of intracellular Ca2+. Measurements performed in nominally Ca2+-free extracellular solutions did not elicit changes in DmOctα1Rb signaling (12).
Figure 1.
Ca2+ fluorimetry of DmOctα1Rb-expressing cells in the absence or presence of the PLC inhibitor U73122. HEK 293 cells stably transfected with DmOctα1Rb were loaded with Fluo-4. ES applied before octopamine stimulation did not result in changes of [Ca2+]i. Incubation with 50 nM octopamine in ES (light shaded bar) led to Ca2+ oscillations: Simultaneous application of the PLC inhibitor U73122 in the presence of 50 nM octopamine (dark shaded bar) completely abolished Ca2+ oscillations in DmOctα1Rb-expressing cells.
Inhibition of PKC leads to noninactivating Ca2+ responses
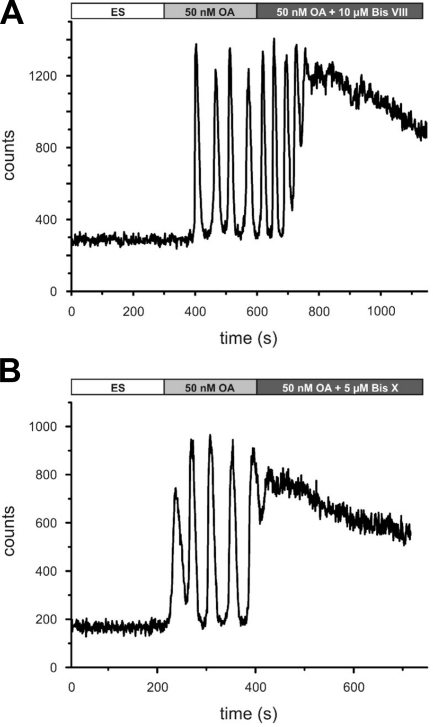
Whether the Ca2+ oscillations evoked by DmOctα1Rb during continuous stimulation with octopamine were caused by homologous or heterologous desensitization, e.g., phosphorylation of the receptor, was investigated by a series of experiments. To test for effects of PKA, cells expressing DmOctα1Rb were stimulated with octopamine in the absence or presence of NKH477, a specific agonist of membrane-bound adenylyl cyclases. The increase of the intracellular cAMP concentration did not change DmOctα1Rb-mediated signaling (Supplemental Fig. S1A). The simultaneous application of octopamine and 10 μM CMIQ, a PKA inhibitor (Supplemental Fig. S1B) also did not result in a change of Ca2+ signals. The same observation was made when a PKG inhibitor was applied (KT5823; 10 μM; 1 μM; Supplemental Fig. S1C). In contrast, application of octopamine and PKC inhibitors (Bis VIII or Bis X) had a strong effect on DmOctα1Rb-induced Ca2+ signals. Both inhibitors completely abolished the Ca2+ oscillations and resulted in elevated, slowly declining Ca2+ signals (Fig. 2). We did not determine dose-response curves for both substances, but concentrations >0.25 μM of externally applied Bis VIII or Bis X inhibitor were sufficient to block the Ca2+ oscillations. These results favor an interpretation that DmOctα1Rb receptors are desensitized homologously by PKC. Full activation of the kinase requires both diacylglycerol (DAG), originating from PIP2 breakdown catalyzed by PLC, and elevated [Ca2+]i, which is provided by opening of IP3 receptors. Both second messengers are produced once octopamine is bound to DmOctα1Rb, and the receptor activates its associated G protein. But how does a receptor recover from its desensitized state? One possibility might be that phosphatases cleave off the phosphate groups added by PKC. Therefore, we examined whether inhibition of cellular phosphatases interferes with DmOctα1Rb signaling. Application of a phosphatase-inhibitor set containing (−)-p-bromotetramisole oxalate, cantharidine, and calyculin A in the presence of octopamine abolished the Ca2+ signals (Fig. 3). The effect was reversible once the phosphatase inhibitors were washed out. The inhibitors block alkaline phosphatases, PP2A and both PP1 and PP2A, respectively. β-Glycerophosphate, a rather nonselective phosphatase inhibitor, also blocked DmOctα1Rb-mediated Ca2+ signals, and at high concentrations (≥50 mM, Supplemental Fig. S1D). In contrast to these substances, okadaic acid (150 nM), an inhibitor of PP1 and PP2A, did not alter Ca2+ signals induced by DmOctα1Rb. The results demonstrate that DmOctα1Rb-mediated signaling strongly depends on the activity of PKC and, most likely, alkaline phosphatases, which cause desensitization and resensitization of the receptor, respectively.
Figure 2.
Ca2+ fluorimetry of DmOctα1Rb-expressing cells in the absence or presence of PKC bisindolylmaleimide inhibitors. HEK 293 cells stably transfected with DmOctα1Rb were loaded with Fluo-4. Application of 50 nM octopamine led to Ca2+ oscillations. Simultaneous application of octopamine and 10 μM bisindolylmaleimide VIII (A; Bis VIII) or octopamine and 5 μM Bis X (B) led to sustained and elevated Ca2+ signals.
Identification of the residue phosphorylated by PKC
To identify the residues that are prone to PKC phosphorylation, we initiated a site-directed mutagenesis approach. Supplemental Fig. S2 displays the amino acid residues of DmOctα1Rb that can be phosphorylated by PKC. The serine- or threonine-encoding triplets harbored in these PKC consensus motifs were substituted for alanine-encoding triplets by recombinant PCR. In total, 7 mutants containing single amino acid exchanges, 3 mutants containing double substitutions, and 1 construct containing a triple exchange were generated (Table 2). All constructs were transiently transfected into HEK 293 cells, and octopamine-induced Ca2+ signals were compared to cells that had been transiently transfected with wild-type DmOctα1Rb. The overall transfection efficacy was similar for the different constructs with ∼50% of transfectants, as determined by immunocytochemical staining. We observed 3 different signal patterns (Fig. 4) in cells transfected with the wild-type construct: cells showing the previously described Ca2+ oscillations, cells showing Ca2+ oscillations and/or peaks on top of an elevated Ca2+ concentration, and cells showing a single transient Ca2+ peak followed by an elevated and sustained Ca2+ level (Fig. 4A–C). For all DmOctα1Rb constructs, we determined the percentage of cells showing either signal. The results are depicted in Fig. 4D. Approximately 40% of cells that had been transfected with the wild-type receptor showed Ca2+ oscillations. Also, ∼40% of the cells showed a single transient Ca2+ increase, and 20% of cells showed oscillations on top of increased intracellular Ca2+. When examining the results for the receptor mutants, most of the single amino acid substitutions led to signaling patterns that were similar to wild-type DmOctα1Rb (Fig. 4D). The T352A mutant, however, behaved differently. Here, ∼75% of transfected cells showed an elevated and sustained level of [Ca2+]i and only 8% of cells showed Ca2+ oscillations. In the double and triple mutants harboring the T532A substitution (i.e., T352A/S598A and T532A/S601A/S602A), the pattern of Ca2+ signals was very similar to the single T352A mutant (Fig. 4D). These results let us hypothesize that phosphorylation of T532, a position located in the third intracellular loop of the receptor (Supplemental Fig. S2) dominates DmOctα1Rb signaling. However, we were surprised by the rather heterogeneous Ca2+ signals that were registered from cells expressing either wild-type DmOctα1Rb or the T352A mutant. An explanation could be that transient transfection may lead to variable levels of receptor protein in different cells due to unequal amounts of plasmid transfected per cell. Therefore, we decided to generate a stable cell line expressing the T352A mutant and to compare its signaling with the stable cell line expressing the wild-type construct (12).
Table 2.
Overview of DmOctα1Rb mutants
| Position in DmOctα1Rb | Mutated residues |
|---|---|
| IL1 | S48A |
| IL2 | S133A |
| IL3 | S325A |
| IL3 | T335A |
| IL3 | T352A |
| IL3 | T393A |
| C terminus | S598A |
| IL2 | S133A/T134A |
| C terminus | S601A/S602A |
| IL3/C terminus | T352A/S598A |
| IL3/C terminus | T352A/S601A/S602A |
Position of the substituted residue and its location in the intracellular domains of DmOctα1Rb is given for each mutant.
T352 of DmOctα1Rb controls the receptors' signaling behavior
Expression of the mutant protein was monitored by immunocytochemistry and Western blot analysis (Supplemental Fig. S3). Calcium imaging was performed as described previously. In Fig. 5, the percentage of the different Ca2+ signals is displayed as introduced for Fig. 4. The cellular responses were much more homogeneous in the stable cell lines (Fig. 5, right bars) than in transiently transfected cells (Fig. 5, left bars). Furthermore, our hypothesis that T352 plays a key role for receptor regulation is corroborated by the fact that ∼95% of cells expressing the mutant receptor now show sustained elevated Ca2+ levels, whereas in wild-type receptor-expressing cells, ∼92% of cells show Ca2+ oscillations and ≤ 4% of cells show sustained elevated Ca2+ levels. Hence, phosphorylation of a single amino acid residue in the third cytoplasmic loop of DmOctα1Rb by PKC is sufficient to desensitize this GPCR.
Figure 5.
Octopamine-induced Ca2+ signals in HEK 293 cells stably transfected with DmOctα1Rbwt or DmOctα1RbT352A. HEK 293 cells stably transfected either with DmOctα1Rbwt (WTstab) or DmOctα1RbT352A (T352Astab) were loaded with Fluo-4. Ca2+ responses of individual cells were examined in the presence of 50 nM octopamine. Classification and color code are same as in Fig. 4. Number of cells (n) analyzed for each construct is given. For comparison, results from transiently transfected cells are included (taken from Fig. 4).
DISCUSSION
GPCRs constitute a large family of membrane receptors and functionally regulate and modulate a variety of cellular reactions. Among the signaling pathways controlled by GPCRs, modulation of [cAMP]i, [cGMP]i, and [Ca2+]i are the most prominent. Here, we have examined the effect of receptor activity on transient changes of [Ca2+]i. Our results demonstrate that phosphorylation of DmOctα1Rb at a single residue is sufficient to desensitize the signaling pathway and to shut off Ca2+ influx from intracellular stores. Reactivation of Ca2+ signals occurs repetitiously by dephosphorylation of the receptor, even in the continuous presence of the ligand.
Signal transduction in nonexcitable, as well as excitable cells, relies on the complex interplay between extracellular ligands that activate specific receptor proteins. The latter evoke metabotropic or electrical signals in the target cell. Changes of the intracellular concentration of second messengers like cAMP, cGMP, IP3, and Ca2+ are mostly achieved by the activity of GPCRs. With >1000 members, GPCR-encoding genes constitute the largest gene family in eukaryotes. Ligands binding to these receptors include volatile odorants (24), water-soluble nutrients (25), quanta of light (26), and small organic compounds, such as neurotransmitters or neuropeptides (27). Once the ligand is bound to its receptor, a specific cascade of intracellular signaling events is induced. Cells, however, possess efficient mechanisms to block long-lasting receptor activities. To reduce binding of the ligand-bound receptor to its cognate G protein, phosphorylation of intracellular loops has been shown to be a rather effective strategy (15, 28). The addition of phosphate groups modifies the receptors' surface such that the affinity to binding to the G protein is strongly reduced (17, 29). The receptor desensitizes, and subsequently the intracellular reaction cascade is turned off.
While phosphorylation of the receptor is a primary event to stop GPCR-mediated signaling, several subsequent reactions are necessary to eventually reset the full signaling capability of the receptor. One such process involves binding of arrestin proteins to the phosphorylated receptor. The affinity of arrestins for phosphorylated receptors is greater than that of G proteins (17). Evidence has accumulated that arrestin-bound receptors are likely to be endocytosed in a clathrin-dependent way (16). Thereby, the total number of active receptors on the cell surface is reduced. Afterward, two pathways exist for these receptors. They can either be reinserted into the plasma membrane (15) or be targeted to endosomal degradation (30). The last process would require de novo protein synthesis to reestablish the surface concentration of receptors. Which route of internalization is taken seems to depend on the amount of phosphate groups that has been added to the receptor. Experimental evidence suggests that GPCRs that have been phosphorylated by receptor-specific kinases (GRKs) are more likely to enter the degradative pathway than those that were phosphorylated by PKA or PKC (17). Because the former receptors are lost for further signaling, this phenomenon was dubbed down-regulation of receptors (31). In terms of signaling efficacy, it would be more favorable to save and reinsert receptors into the plasma membrane from a submembraneous pool of vesicles. This keeps a cell more flexible to respond to changing extracellular conditions.
Activation of DmOctα1Rb receptors in HEK 293 cells with octopamine led to sustained Ca2+ oscillations at a frequency of ∼0.02 Hz. This time frame would fit neither a scenario with internalization and recycling of the receptor nor degradation and de novo protein synthesis. Therefore, we assumed that transient modifications, e.g., phosphorylation of the receptor, might be the molecular explanation for the cessation of DmOctα1Rb signaling. Both our pharmacological treatments with PKC inhibitors and the mutagenesis approach, strongly support this hypothesis. A single threonine residue in the third intracellular loop, T352, is phosphorylated in a receptor activity-dependent way and is sufficient to inhibit further Ca2+ signaling of DmOctα1Rb. In contrast to PKC, neither PKA nor PKG caused desensitization of DmOctα1Rb.
A similar signaling behavior as observed for DmOctα1Rb has been described for a mammalian glutamate receptor (mGluR5a). Activation of this GPCR causes Ca2+ oscillations both in heterologous cell systems (32) and in cultured astrocytes (33). The reactions could be blocked by PKC inhibitors. A homologous receptor (mGluR1α), on the contrary, led to a singular and sustained Ca2+ increase in cells (32). Site-directed mutagenesis and domain shuffling between both receptors identified a threonine residue in the C-terminal loop of mGluR5a that is phosphorylated by PKC. This threonine residue is substituted for an aspartate in mGluR1α, which does not allow PKC modification (32). Thus, timely consecutive phosphorylation and dephosphorylation events on receptors can lead to recurring transient changes of intracellular second-messenger concentrations. The position of the modified residues, however, is not strictly conserved, with T352 of DmOctα1Rb being located in the third IL and T840 of mGluR5a being located in the C-terminal tail of the receptor, respectively.
Receptor-dependent Ca2+ signals in cells can occur also along other routes. The downstream targets of DmOctα1Rb are IP3 receptors in the ER. These universal ligand-gated Ca2+ channels display a fascinating complexity of regulation. Whereas IP3 is pivotal to activate the channel, the [Ca2+]i has a great effect on channel activity, too. A biphasic dependence to [Ca2+]i exists for IP3-evoked Ca2+ release, with free [Ca2+] below or above 300 nM substantially inhibiting IP3-receptor activity (34). From this characteristic IP3-receptor behavior, one could assume that the Ca2+ oscillations observed in DmOctα1Rb-expressing cells might be due to IP3-receptor signaling rather than DmOctα1Rb signaling. On the basis of our pharmacological and mutagenesis data, we do not favor this interpretation. To our knowledge, dephosphorylation of IP3 receptors has not been addressed as a possible means of its regulation. However, when phosphatase activity in DmOctα1Rb-expressing cells was blocked, Ca2+ signals were completely abolished. Furthermore, activation of the T352A mutant, which cannot be phosphorylated, no longer caused Ca2+ oscillations but led to sustained elevated levels of [Ca2+]i that are unlikely to occur by IP3-receptor activity.
Another way to generate and maintain Ca2+ signals in cells may occur via the crosstalk of STIM proteins in the ER and Orai proteins in the plasma membrane (35). STIM proteins register the efflux of Ca2+ ions from the ER by means of a low-affinity Ca2+ binding site. A reduction of [Ca2+]ER causes assembly of STIM proteins and movement to cellular domains where intracellular and plasma membranes come in close contact. It is here that STIM and Orai proteins physically interact. The protein-protein contact activates the Orai Ca2+ channel, giving way to Ca2+ influx from the extracellular side. This leads to the replenishment of intracellular Ca2+ stores (36). Once the [Ca2+]ER rises, STIM and Orai dissociate, because Ca2+ ions bind to STIM in the ER lumen and thereby initiate disassembly of STIM oligomers (35). Although it has been reported that membrane channels are likely to contribute to the signaling behavior of the mGluR1α receptor, we do not suppose such a mechanism for DmOctα1Rb. We have previously shown that DmOctα1Rb signals can be induced with 1 μM octopamine in extracellular solution (ES) containing nominally no Ca2+ (12). In the present study, we performed additional measurements with 10–50 nM octopamine in Ca2+-free ES. Under these conditions, we observed Ca2+ oscillations as in regular ES (Supplemental Fig. S4).
From all these experiments, we conclude that Ca2+ oscillations induced by octopamine-activated DmOctα1Rb rely on the consecutive interplay of PKC and phosphatase activities (Fig. 6). Phosphorylation by PKC leads to homologous desensitization from which DmOctα1Rb is relieved by dephosphorylation. The dynamics of such receptor-induced cellular Ca2+ signaling events are also physiologically relevant. Recently, it has been reported that intracellular Ca2+ dynamics can regulate the free-running circadian clock oscillation in Drosophila (37). Most likely, this effect is mediated by calmodulin and/or CaM kinase II pathways. A contribution of [Ca2+]i dynamics has been also reported for pheromone signaling in moths (38), in which pheromone perception causes phasic PLC signal transduction cascades and thus Ca2+ signals in the respective olfactory neurons. Female Drosophila mutants lacking a splice variant of DmOctα1Rb, i.e., DmOctα1Ra (11, 12), are severely impaired in oviposition (39). Since both receptors share Ca2+ signaling properties (12), it will be interesting to examine in a forthcoming study how these octopamine-induced signals control the functional properties of the reproductive tract in wild-type flies.
Figure 6.
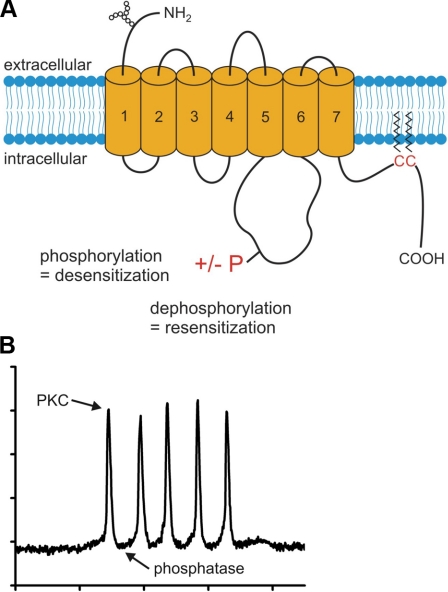
Summary of DmOctα1Rb signaling. A) Cartoon of a GPCR. Phosphorylation and dephosphorylation of T352 in the third intracellular loop of DmOctα1Rb cause desensitization and resensitization of receptor activity, respectively. B) Respective enzymatic activity of PKC and cellular phosphatases is depicted in a representative Ca2+ imaging trace of a DmOctα1Rb-expressing cell activated with 50 nM octopamine.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the assistance of Joachim Schmitz in the cell culture and Dr. M. Krähling during the initial phases of pharmacological tests on DmOctα1Rb. The authors also thank Dr. D. Kaschuba and A. Meisenberg for comments on the manuscript.
The work was supported in part by a Deutsche Forschungsgemeinschaft grant to A.B. (Ba 1541/3-1).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Malamud J. G., Mizisin A. P., Josephson R. K. (1988) The effects of octopamine on contraction kinetics and power output of a locust flight muscle. J. Comp. Physiol. A 162, 827–835 [Google Scholar]
- 2. Orchard I., Ramirez J. M., Lange A. B. (1993) A multifunctional role for octopamine in locust flight. Annu. Rev. Entomol. 38, 227–249 [Google Scholar]
- 3. Livingstone M., Harris-Warrick R. M., Kravitz E. A. (1980) Serotonin and octopamine produce opposite postures in lobsters. Science 208, 76–79 [DOI] [PubMed] [Google Scholar]
- 4. Menzel R., Leboulle G., Eisenhardt D. (2006) Small brains, bright minds. Cell 124, 237–239 [DOI] [PubMed] [Google Scholar]
- 5. Verlinden H., Vleugels R., Marchal E., Badisco L., Pflüger H. J., Blenau W., Broeck J. V. (2010) The role of octopamine in locusts and other arthropods. J. Insect Physiol. 56, 854–867 [DOI] [PubMed] [Google Scholar]
- 6. Roeder T., Seifert M., Kahler C., Gewecke M. (2003) Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch. Insect Biochem. Physiol. 54, 1–13 [DOI] [PubMed] [Google Scholar]
- 7. Scheiner R., Baumann A., Blenau W. (2006) Aminergic control and modulation of honeybee behaviour. Curr. Neuropharmacol. 4, 259–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burchett S. A., Hicks T. P. (2006) The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Progr. Neurobiol. 79, 223–246 [DOI] [PubMed] [Google Scholar]
- 9. Hauser F., Cazzamali G., Williamson M., Park Y., Li B., Tanaka Y., Predel R., Neupert S., Schachtner J., Verleyen P., Grimmelikhuijzen C. J. (2008) A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum. Front. Neuroendocrinol. 29, 142–165 [DOI] [PubMed] [Google Scholar]
- 10. Farooqui T. (2007) Octopamine-mediated neuromodulation of insect senses. Neurochem. Res. 32, 1511–1529 [DOI] [PubMed] [Google Scholar]
- 11. Han K.-A., Millar N. S., Davis R. L. (1998) A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J. Neurosci. 18, 3650–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balfanz S., Strünker T., Frings S., Baumann A. (2005) A family of octopamine receptors that specifically induce cAMP production or Ca2+ release are expressed in Drosophila melanogaster. J. Neurochem. 93, 440–451 [DOI] [PubMed] [Google Scholar]
- 13. Maqueira B., Chatwin H., Evans P. D. (2005) Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 94, 547–560 [DOI] [PubMed] [Google Scholar]
- 14. Tobin A. B., Butcher A. J., Kong K. C. (2008) Location, location, location.site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol. Sci. 29, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly E., Bailey C. P., Henderson G. (2008) Agonist-selective mechanisms of GPCR desensitization. Brit. J. Pharmacol. 153, S379–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lefkowitz R. J., Shenoy S. K. (2005) Transduction of receptor signals by β–arrestins. Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 17. Hanyaloglu A. C., Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 18. Wong Y. H., Lee T. Y., Liang H. K., Huang C. M., Yang Y. H., Chu C. H., Huang H. D., Ko M. T., Hwang J. K. (2007) KinasePhos 2.0: a web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 35, W588–W594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herlitze S., Koenen M. (1990) A general and rapid mutagenesis method using polymerase chain reaction. Gene 91, 143–147 [DOI] [PubMed] [Google Scholar]
- 20. Sambrook J., Russell R.W. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA [Google Scholar]
- 21. Sanger F., Nicklen S., Coulson A. R. (1977) DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74, 5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kozak M. (1984) Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 12, 857–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen C., Okayama H. (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7, 2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Touhara K., Vosshall L. B. (2009) Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 71, 307–332 [DOI] [PubMed] [Google Scholar]
- 25. Roper S. D. (2007) Signal transduction and information processing in mammalian taste buds. Pflügers Arch. 454, 759–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fain G. L., Hardie R., Laughlin S. B. (2010) Phototransduction and the evolution of photoreceptors. Curr. Biol. 20, R114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenbaum D. M., Rasmussen S. G. F., Kobilka B. K. (2009) The structure and function of G-protein-coupled receptors. Nature 459, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gehret A. U., Hinkle P. M. (2010) Importance of regions outside the cytoplasmic tail of G-protein-coupled receptors for phosphorylation and dephosphorylation. Biochem. J. 428, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lohse M. J., Andexinger S., Pitcher J., Trukawinski S., Codina J., Faure J. P., Caron M. G., Lefkowitz R. J. (1992) Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J. Biol. Chem. 267, 8558–8564 [PubMed] [Google Scholar]
- 30. Wolfe B. L., Trejo J. (2007) Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 8, 462–470 [DOI] [PubMed] [Google Scholar]
- 31. Prossnitz E. R. (2004) Novel roles for arrestins in the post-endocytic trafficking of G protein-coupled receptors. Life Sci. 75, 893–899 [DOI] [PubMed] [Google Scholar]
- 32. Kawabata S., Tsutsumi R., Kohara A., Yamaguchi T., Nakanishi S., Okada M. (1996) Control of caclium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 383, 89–92 [DOI] [PubMed] [Google Scholar]
- 33. Nakahara K., Okada M., Nakanishi S. (1997) The metabotropic glutamate receptor mGluR5 induces calcium oscillations in cultured astrocytes via protein kinase C phosphorylation. J. Neurochem. 69, 1467–1475 [DOI] [PubMed] [Google Scholar]
- 34. Taylor C. W., Laude A.-J. (2002) IP3 receptors and their regulation by calmodulin and cytosolic Ca2+. Cell Calcium 32, 321–334 [DOI] [PubMed] [Google Scholar]
- 35. Deng X., Wang Y., Zhou Y., Soboloff J., Gill D. L. (2009) STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals. J. Biol. Chem. 284, 22501–22505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Putney J. W. (2009) Capacitative calcium entry: from concept to molecules. Immunol. Rev. 231, 10–22 [DOI] [PubMed] [Google Scholar]
- 37. Harrisingh M. C., Wu Y., Lnenicka G. A., Nitabach M. N. (2007) Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J. Neurosci. 27, 12489–12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stengl M. (2010) Pheromone transduction in moths. Front. Cell. Neurosci. 4, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee H.-G., Seong C.-S., Kim Y.-C., Davis R. L., Han K.-A. (2003) Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev. Biol. 264, 179–190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.