Abstract
Being gated by high-energy nucleotides, cardiac ATP-sensitive potassium (KATP) channels are exquisitely sensitive to changes in cellular energy metabolism. An emerging view is that proteins associated with the KATP channel provide an additional layer of regulation. Using putative sulfonylurea receptor (SUR) coiled-coil domains as baits in a 2-hybrid screen against a rat cardiac cDNA library, we identified glycolytic enzymes (GAPDH and aldolase A) as putative interacting proteins. Interaction between aldolase and SUR was confirmed using GST pulldown assays and coimmunoprecipitation assays. Mass spectrometry of proteins from KATP channel immunoprecipitates of rat cardiac membranes identified glycolysis as the most enriched biological process. Coimmunoprecipitation assays confirmed interaction for several glycolytic enzymes throughout the glycolytic pathway. Immunocytochemistry colocalized many of these enzymes with KATP channel subunits in rat cardiac myocytes. The catalytic activities of aldolase and pyruvate kinase functionally modulate KATP channels in patch-clamp experiments, whereas d-glucose was without effect. Overall, our data demonstrate close physical association and functional interaction of the glycolytic process (particularly the distal ATP-generating steps) with cardiac KATP channels.—Hong, M., Kefaloyianni, E., Bao, L., Malester, B., Delaroche, D., Neubert, T. A., Coetzee, W. A. Cardiac ATP-sensitive K+ channel associates with the glycolytic enzyme complex.
Keywords: sulfonylurea receptor, energy metabolism, Kir6.2, SUR2A
ATP-sensitive K+ (KATP) channels are present in a wide variety of cells, including pancreatic β cells, skeletal muscle, vascular smooth muscle, vascular endothelium, and brain (1). They are comprised of 2 types of subunits, namely Kir6.x pore-forming subunits (Kir6.1 and Kir6.2) and SURx regulatory subunits (SUR1, SUR2A, and SUR2B; ref. 2). Each channel is a hetero-octamer composed of 4 Kir6.x and 4 SURx subunits. The KATP channel forms a link between the metabolic state of a cell and membrane excitability (reviewed in ref. 2). This link is typically exemplified in the pancreatic β cell, where increased intracellular ATP closes the KATP channel, resulting in membrane depolarization, increased intracellular Ca2+ levels, and consequently secretory insulin release (1).
It has been recognized for some time that KATP channel activity is preferentially regulated by glycolytically derived ATP. In an elegant series of experiments, Weiss and Lamp (3) demonstrated that, in both the open-cell and inside-out patch-clamp configurations, cardiac KATP channels are closed equally well by ATP production from oxidative phosphorylation, the creatine kinase shuttle system, and glycolysis. However, when intracellular ATP consumption was stimulated, glycolytic enzymes were far more efficient in blocking KATP channel activity, suggesting that glycolytically derived ATP (or intermediates) formed in a membrane subspace preferentially regulates KATP channel activity. These data have been interpreted to suggest that key glycolytic enzymes are located near KATP channels at the plasma sarcolemma (3). The regulation of KATP channels by glycolysis has also been demonstrated in tissues other than the heart. For example, the activity of KATP channels in the basolateral membranes of Necturus enterocytes is inhibited by ATP formed by the glycolytic enzyme pyruvate kinase, which was suggested to be associated with the plasma membrane (4). A structural basis for these data was provided by our finding that KATP channel subunits physically associate with some of the glycolytic enzymes (5). Using a proteomics screen and coimmunoprecipitation assays, we identified GAPDH, triosephosphate isomerase, and pyruvate kinase as de facto KATP channel-interacting proteins. We also demonstrated that the catalytic activity of these enzymes is sufficient to regulate KATP channel activity in the open-cell or inside-out membrane patch-clamp configuration (5). The finding that GAPDH interacts with KATP channel subunits has also been reported by others (6).
In the studies described here, we demonstrate that interaction of glycolytic enzymes with KATP channel subunits is a general feature of the channel complex. Using coimmunoprecipitation assays, tandem mass spectrometry peptide analysis, and immunocytochemistry, we demonstrate association of KATP channels with enzymes throughout the glycolytic chain. We further demonstrate with patch-clamp recordings that substrates of aldolase A and pyruvate kinase led to channel inhibition in a manner that depends on their catalytic activity, whereas glucose had no effect. Our data suggest that the glycolytic pathway is an integral component of the KATP channel protein complex and that most of the glycolytic enzymes (especially those involved in the distal ATP-generating steps) are physically and functionally associated with the cardiac KATP channel.
MATERIALS AND METHODS
Two-hybrid protein-protein interaction screen
Using bioinformatic approaches (COILS algorithm: http://www.ch.embnet.org/software/COILS_form.htm), we identified putative SUR1 and SUR2 coiled-coil (CC) domains and isolated them by PCR. Briefly, aa 947–997 of hamster SUR1 (accession number: A56248) were amplified by PCR (forward primer: 5′-CCGGAATTCCGGGGAGAGGAAAGCCTCAGAGC-3′; reverse primer: 5′-CGCGGATCCGCGCGGGATCTTAGCTCGCTGATG-3′). Similarly, the cDNA corresponding to aa 911–977 of rat SUR2 (accession number: NP_037172) was isolated (forward primer: 5′-CGGAATTCCGCCCTCATGAATAGACAGG-3′; reverse primer: 5′-CGCGGATCCGCGCCAGCAGGTCTTCCAGGG-3′) and subcloned (using engineered restriction sites EcoRI at the 5′ end and BamHI at the 3′ end with a stop codon) in frame into the pBT bait vector (BacterioMatch II; Stratagene, La Jolla, CA, USA). We screened ∼3 × 106 clones from a rat heart cDNA library in the pTarget vector. Positive clones were subjected to further tests to rule out that they were false negatives, including growth in dual-selection media, as well as the ability to retransform putative positives with the bait and achieve colony growth (as described in the manufacturer's protocol). These cDNAs were isolated and sequenced.
GST pulldown experiments
The putative SUR1 and SUR2 CC domain cDNAs were subcloned into the pGEX4T-3 vector (Amersham Biosciences, Piscataway, NJ, USA). The C terminus of mouse Kir6.2 was subcloned in a similar fashion. GST fusion proteins were prepared using BL21 Escherichia coli. Protein expression was induced with 1 mM IPTG after cultures reached an OD of 0.6 (at 600 nm). Cells were lysed at room temperature for 20 min in GLTB I buffer (50 mM Tris, 40 mM EDTA, 2.5% sucrose w/v, 0.02% sodium azide, 1 mg/ml lysozyme, and mammalian protease inhibitor cocktail with PMSF), followed by further lysis on ice for 20 min in GLTB II buffer (50 mM Tris, 100 mM MgCl2, 0.2% Triton-X 100, 0.02% sodium azide, and protease inhibitors) and sonication on ice. The lysed cells were centrifuged at 12,000 rpm, and the GST-fusion proteins were recovered by incubating with glutathione-Sepharose B beads for 2 h at 4°C. The beads were washed 3 times in wash buffer (25 mM Tris, 150 mM NaCl, 5 mM EDTA, 1% Triton-X 100, and protease inhibitors), portioned into aliquots, and stored at −80°C. Lysates of COS7L cells containing endogenous aldolase were incubated with each GST-fusion protein-Sepharose bead complex for 4 h at 4°C, washed 3 times in wash buffer, and separated by SDS-PAGE electrophoresis on a 15% polyacrylamide gel.
Cell culture and transfection
HEK-293T or COS7L cells were cultured in DMEM (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% (v/v) FBS and antibiotics in a humidified atmosphere containing 5% CO2 at 37°C. As described previously (5, 7–8), HEK-293T cells were transfected at 30–40% confluence using FuGene6 (Roche, Indianapolis, IN, USA) with the following cDNAs: HA-tagged mouse Kir6.2 (from Dr. S. Seino, Kobe University, Kobe, Japan), Flag-tagged hamster SUR1 (originally from Dr. S.-L. Shyng, Oregon Health and Science University, Portland, OR, USA), Flag-tagged rat SUR2A, and GFP (Clontech, Palo Alto, CA, USA).
Animals
The investigation conformed to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal procedures were approved by the New York University Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats (250–300 g; Charles River, Wilmington, MA, USA) were killed by pentobarbital overdose, and the hearts were rapidly removed for either cell isolation or membrane preparation procedures.
Membrane preparations
For the mass spectrometry analysis, a crude membrane fraction was prepared using a modification of a previously described method (9). In brief, rat heart ventricles were minced on ice in a buffer (250 mM sucrose, 2 mM EGTA, and 20 mM HEPES, pH 7.4, with protease inhibitor cocktail (Roche Applied Science) and 300 μM PMSF] and homogenized on ice using a Polytron (Kinematica, Inc., Bohemia, NY, USA) followed by a Dounce homogenizer (30 strokes; Wheaton, Millville, NJ, USA). After brief centrifugation (4000 g for 10 min at 4°C), the supernatant was subjected to ultracentrifugation (190,000 g for 1 h at 4°C), and the pellet (crude membrane fraction) was solubilized overnight at 4°C in 20 mM HEPES (pH 7.4) and 0.5% Triton X-100.
For coimmunoprecipitation experiments, rat hearts were rinsed in PBS (Invitrogen, Carlsbad, CA, USA) and homogenized in homogenization buffer (100 mM sucrose, 10 mM EDTA, 46 mM KCl, 5 mM NaN3, and 100 mM Tris-HCl, pH 7.4, with protease inhibitor cocktail and 300 μM PMSF) using a Polytron homogenizer, followed by 30 strokes in a loose-fitting Dounce homogenizer, and finally 10 strokes in a tight-fitting Dounce homogenizer. The homogenate was centrifuged (4000 g for 13 min at 4°C), and the supernatant was used for ultracentrifugation. Membrane fractionation was performed using Optiprep gradients (Sigma-Aldrich, St. Louis, MO, USA) as described previously (10) with modifications. In brief, the supernatant was brought to 5% Optiprep, overlaid with working solution (in mM: 250 sucrose, 1 EDTA, and 10 Tris-HCl, pH 7.4) and loaded on top of 15% Optiprep in the working solution. After centrifugation at 200,000 g for 2.5 h, 7 distinct fractions were collected. Since KATP channel subunits were found in the first, third, and fourth fractions from the bottom (data not shown), these fractions were pooled and diluted with 10 mM Tris-HCl and 1 mM EDTA (pH 7.5), and membranes were recovered by centrifugation (15 min at 310,000 g).
Mass spectrometry
Membrane proteins immunoprecipitated using anti-Kir6.2 antibodies (W-62 and G-16; see below) and analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The immunoprecipitates were briefly run into 3 separate lanes of an 8% SDS/PAGE gel (Thermo Scientific, Waltham, MA, USA). The gel was fixed and stained with EZ-Run protein gel staining solution containing Coomassie brilliant blue G-250 (Fisher Scientific, Pittsburgh, PA, USA). The gel slices (∼1 cm) containing the immunoprecipitates were subsequently removed. The samples were reduced and alkylated and digested in gel using mass spectrometry grade trypsin (Promega, Madison, WI, USA) at 12.5 ng/μl in 25 mM NH4HCO3 buffer. The resulting peptides were extracted and dried under vacuum and then resuspended in 0.1% formic acid. Samples were applied directly to a C18 (Reprosil; Dr. Maisch, Ammerbuch, Germany) 12 cm in-house-packed column (PicoFrit; New Objective, Woburn, MA, USA) using a nanoflow 2D HPLC (Eksigent; AB Sciex, Dublin, CA, USA) coupled directly to an Orbitrap LTQ (Thermo Electron, Bremen, Germany) mass spectrometer. A binary solvent system was used to separate the peptides with solvent A (0.1% formic acid aqueous solution) and solvent B (0.1% formic acid in 90% acetonitrile). A gradient from 1–45% B over 90 min was applied for separation. Data-dependent acquisition was performed, looping a survey scan followed by 3 consecutive MS/MS scans for the 3 most intense ions throughout the time of the gradient. Raw MS data were subsequently processed using the DTA supercharge software and used to search a SwissProt rat_mouse database (July 15, 2010 release; http://www.ebi.ac.uk/swissprot/) with the Mascot 2.2.1 (Matrix Science, Boston, MA, USA) search engine. Carbamidomethyl was set as a fixed modification of Cys residues and oxidation of Met and phosphorylation of Ser, Thr, and Tyr residues as variable modifications. The tolerance window for precursor ions was set at 20 ppm and for MS/MS to 0.6 Da.
Coimmunoprecipitation assays and immunoblotting
Coimmunoprecipitation assays with transfected cells were performed as described previously (5, 8). For native coimmunoprecipitation assays, rat heart membrane proteins were solubilized overnight at 4°C in a solution consisting of 150 mM NaCl, 5 mM EDTA, 1% (v/v) Triton X-100, and 25 mM Tris-HCl (pH 7.4). Immunoprecipitation was performed using a commercial system (Thermo Scientific Pierce, Rockford, IL, USA), in which antibodies were prebound to an amine-reactive resin. The washing buffer consisted of 150 mM NaCl, 5 mM EDTA, 0.2% (v/v) Triton X-100, and 25 mM Tris-HCl (pH 7.4). Proteins were eluted with 100 μl of elution buffer and resolved by 8% SDS-PAGE. It should be noted that samples were not boiled. Proteins were transferred to PVDF membranes, and blocking was performed with 5% fat-free cow milk in TBST (TBS plus 0.05% Tween 20) overnight at 4°C. Primary and secondary antibodies (see later) were dissolved in TBST containing 5% milk. Chemiluminescent Western blotting substrate (Pierce) was used for peroxidase detection and the resulting signal was detected on Kodak Biomax light films (Eastman Kodak, Rochester, NY, USA).
Enzymatic isolation of ventricular myocytes
Ventricular myocytes were isolated from male Sprague-Dawley rats (∼250 g; ref. 5). Hearts were rapidly excised after pentobarbital overdose, rinsed with ice-cold Tyrode's solution (in mM: 137 NaCl, 5.4 KCl, 10 HEPES, 1 MgCl2, 0.33 NaH2PO4, 1.8 CaCl2, and 10 glucose, pH 7.4) and then cannulated and perfused in Langendorff mode with oxygenated Tyrode's solution at 37°C for 4 min. The perfusate was then switched to oxygenated nominally Ca2+-free Tyrode's buffer for 5 min, followed by perfusion for 11–13 min with the same solution containing collagenase (type I, 3 mg/ml; Sigma) and protease (type XIV, 0.44 U/ml; Sigma). The enzyme was washed out by 3 min perfusion with KB solution (in mM: 20 taurine, 50 l-glutamic acid, 10 HEPES, 0.5 EGTA, 3 MgSO4, 30 KH2PO4, 30 KCl, and 78 KOH, pH 7.2, adjusted with KOH). The heart was removed from the cannula, and cells were isolated by titration of the left and right ventricle free walls. The procedure resulted in a 90% yield of rod-shaped myocytes. Myocytes exhibiting a brick-like shape and clear cross-striations were used for electrophysiology and immunocytochemistry experiments.
Immunocytochemistry
Isolated cardiac ventricular myocytes were allowed to attach to laminin-coated coverslips for 30 min at 37°C. Cells were fixed in 3.7% paraformaldehyde (in PBS, pH 7.4) for 15 min at room temperature and permeablized with 0.1% Triton X-100 in PBS for 15 min and washed 3 × 5 min with PBS, followed by a blocking step with 5% donkey serum in PBS for 40 min. Primary antibodies, diluted in PBS, were applied overnight at 4°C. After being washed (3×5 min) with PBS, secondary antibodies were applied for 45 min at room temperature. Cells were further washed (4×10 min), first with PBS containing 1% DAPI (Sigma) and then an additional 3 times with PBS. Coverslips were mounted with Prolong Gold antifade reagent (Invitrogen) and cured overnight at room temperature. Images were obtained using a Leica TCS SP5 II laser scanning confocal microscope fitted with a ×63/1.40 oil objective. (Leica Microsystems, Wetzlar, Germany).
Antibodies used
We used the following antibodies against KATP channel subunits: goat anti-Kir6.2 (G-16; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-Kir6.2 (Lee-62; obtained from Dr. Hon-Chi Lee, Mayo Clinic, Rochester, MN, USA), rabbit anti-Kir6.2 (W-62; ref. 11), chicken anti-Kir6.2 (C62; developed against the N-terminal sequence of mouse Kir6.2, CVAKAKPKFSISPDSLS), and goat anti-SUR2A (M-19; Santa Cruz Biotechnology). Antibodies used against glycolytic enzymes included a goat polyclonal anti-aldolase antibody recognizing the C terminus of human aldolase A (sc-12061; Santa Cruz Biotechnology) as well as the following monoclonal antibodies: anti-aldolase A (clone 3D9–6F3), anti-enolase 1 (clone 8G8), or anti-hexokinase 1 (clone 5G9; all from Sigma) or anti-fructose 6 phosphate kinase (Abcam, Cambridge, MA, USA). As a negative control for native coimmunoprecipitation experiments, we used an unrelated IgG (rabbit polyclonal anti-myc). A monoclonal anti-HA antibody (Covance, Princeton, NJ, USA) was used for immunoblotting. Secondary antibodies used for Western blotting included horseradish peroxidase-conjugated donkey anti-goat (Jackson ImmunoResearch, West Grove, PA, USA) and donkey anti-mouse (Santa Cruz Biotechnology) antibodies. Secondary antibodies used in immunocytochemistry included DyLight-549 donkey anti-chicken and DyLight-488 donkey anti-mouse antibodies (Jackson ImmunoResearch).
Electrophysiology
Isolated rat ventricular myocytes were plated on laminin-coated coverslips, mounted in a recording chamber (RC-21BRW; Warner Instruments, Hamden, CT, USA) and placed on the stage of an inverted microscope (Nikon TE2000-V; Morrell, Auburn Hills, MI, USA). Single-channel recordings were performed in the inside-out configuration at room temperature using standard patch-clamp techniques (Axopatch-200B amplifier; Axon Instruments, Foster City, CA, USA; ref. 12). Patch pipettes were pulled using borosilicate glass (OD 1.5 mm; DMZ-Universal Puller; Zeitz-Instrumente, Augsburg, Germany) and had resistances between 2 and 3 MΩ when filled with the following solution (in mM): 110 gluconic acid, 30 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 0.02 ouabain, pH 7.4, adjusted with KOH. The bath solution contained (in mM) 110 gluconic acid, 30 KCl, 1 EGTA, 1.2 MgCl2, and 10 HEPES, pH 7.2, adjusted with KOH. The pretest solution containing glycolytic substrates was prepared in bath solution containing (in mM) 0.09 MgATP, 0.5 KADP, 1 KH2PO4, and 1 NAD+. This solution was used to prepare 3 test solutions, containing (in mM) 5 phosphoenolpyruvate (PEP), 5 fructose-1,6-bisphosphate (FBP), or 10 glucose, respectively. The pH of all solutions was adjusted to 7.2. The pipette potential was +80 mV, and inward current was represented as upward deflections. The current was filtered at 1 kHz and digitized at 5 kHz. Patches typically contained several channels, and the KATP channel current (I/IC) was calculated as the mean patch current (I) relative to the zero current in the presence of 1 mM ATP (IC; when all KATP channels were blocked). Exposure to ATP was bracketed by recordings in 0 ATP to correct for any rundown (when needed).
RESULTS
SUR subunits contain novel CC domains
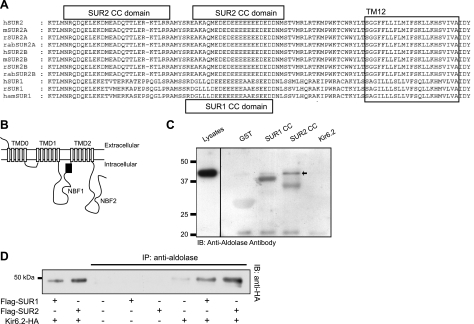
We initiated this study by searching for protein interaction domains within KATP channel subunits. Using bioinformatic approaches (COILS algorithm), we found putative CC domains within both SUR1 and SUR2 subunits (Fig. 1, Supplemental Fig. S1, and Supplemental Table S1). CC domains function as specific interaction domains between 2 proteins, for example, as in our description of the CC domain dependent interaction between SK4 Ca2+-activated K+ channel subunits and the myotubularin lipid phosphatase (13). The putative CC domains with the highest probability of prediction are present within NBF1 close to the 12th transmembrane domain of SURx subunits (Fig. 1). Some of the CC domains exist at equivalent positions of SUR1 and SUR2 (Supplemental Table S1), and the sequence is highly conserved across several species (human, rat, and mouse; Fig. 1). It is likely that these SUR CC domains mediate interactions of KATP channels with other subunits.
Figure 1.
Identification of putative SUR CC domains and interaction with aldolase. A) Using the COILS computer algorithm, we identified several putative CC domains in both SUR1 and SUR2 subunits. See Supplemental Fig. S1 and Supplemental Table S1 for more information. Sequences are shown for human SUR2A (hSUR2), mouse SUR2A (mSUR2A), rabbit SUR2A (rabSUR2A), human SUR2B (hSUR2B), mouse SUR2B (mSUR2B), rabbit SUR2B (rabSUR2B), human SUR1 (hSUR1), rat SUR1 (rSUR1), and hamster SUR1 (hamSUR1). B) SUR subunit topology, depicting the location of the putative CC domains (black box). C) Aldolase interacts with the SUR CC domains. A GST pulldown experiment was performed using GST alone, GST-SUR1 CC domain, GST-SUR2 CC domain, and the GST-Kir6.2 C terminus. Immunoblotting was performed using an anti-aldolase antibody. Left lane: cell lysates (no pulldown). D) COS7 cells were transfected with cDNA combinations as shown. Each protein was expressed in cell lysates (not shown). Immunoprecipitates obtained with an anti-aldolase antibody (C-16) were separated by SDS-PAGE and immunoblotted with an anti-HA antibody.
Two-hybrid screen using the SUR2 CC domain as bait
To identify any proteins interacting with cardiac KATP channel subunits via the identified CC domain, we subcloned the SUR2 CC domain into a bait vector and used it in a bacterial 2-hybrid screen against a rat heart cDNA library. The screen yielded several positive clones, most with unknown relevance to KATP channel function (Table 1). Interestingly, the screen identified the glycolytic enzyme GAPDH as a putative binding partner, which has already been demonstrated to be a KATP channel interacting protein (5–6). Of further interest was the identification of the muscle form of aldolase (aldolase A), which is an enzyme directly upstream of GAPDH in the glycolytic pathway.
Table 1.
Results of the 2-hybid interaction screen against a rat cDNA library when using the putative SUR2 CC domain as bait
| Accession number | Full name |
|---|---|
| NP_058704.1 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) |
| NP_036627.1 | Fructose-bisphosphate aldolase A (muscle-type aldolase) |
| NP_001182174 | Hypothetical protein LOC688786 |
| P01946 | Hemoglobin subunit α-1/2 |
| NP_067599.1 | Myoglobin |
| AAH62062 | Transglutaminase 2, C polypeptide |
| XM_236849 | Similar to Satb1 protein (LOC316164) |
| XP_216546 | Rat protein of unknown function |
| XP_217018.2 | Similar to histone deacetylase 10 |
GST pulldown experiments
Since GAPDH had already been identified as a KATP channel-interacting protein, we focused the next experiments on aldolase. To verify the interaction, we initially performed a GST pulldown experiment. The CC domains of SUR1 and SUR2 were fused to GST proteins using standard subcloning techniques. The Kir6.2 subunit C terminus, also fused to the GST protein, was used as a negative control. Recombinant proteins were expressed and purified using an E. coli expression system and glutathione affinity (GST Sepharose) beads. As further negative controls, we also used the GST protein alone and a reaction containing no added GST proteins (beads only; not illustrated). The lysates of COS7L cells, which contain endogenous aldolase, were incubated with the Sepharose beads/GST-fusion protein complex. The GST proteins and interacting proteins were dissociated from the Sepharose beads by competition with excess reduced glutathione. Interacting aldolase was identified by immunoblotting with an anti-aldolase antibody (Fig. 1C). Aldolase was detected as an ∼40 kDa band in the reactions with the SUR2 CC domain (arrow) and to a lesser extent with the SUR1 CC domain. There was no interaction of aldolase with the Kir6.2 C terminus, suggesting that aldolase directly and specifically interacts with SUR CC domains.
The COILS algorithm predicts that aldolase contains 3 putative CC domains (CC1: E50-L63; CC2: L128-D141; and CC3: K317-K330). These CC domains are located on surface helices and may therefore interact with other proteins. The aldolase CC3 domain is predicted with 80% probability. Using standard PCR cloning techniques, we generated 2 aldolase constructs (C-terminal tagged with HA epitopes). The full-length aldolase contains all 3 CC domains, and CC3 was truncated in another (ΔCC3 aldolase). The 2 cDNAs were transfected into COS7L cells, and cell lysates were subjected to pulldowns using the GST-bound SUR2 CC domain. The precipitated product was separated by SDS-PAGE and immunoblotted with an anti-HA antibody, which demonstrated that interaction is lost when the aldolase CC-3 domain was not present (Supplemental Fig. S2).
Coimmunoprecipitation of aldolase and KATP channel subunits in cell lines
We next verified that aldolase interacts with full-length KATP channel subunits and that interaction occurs in mammalian cells. HEK-293T cells were transfected with Kir6.2/SUR1 or Kir6.2/SUR2A cDNAs. To facilitate these biochemistry experiments, the Kir6.2 subunit was C-terminally tagged with an HA epitope, and SUR subunits were N-terminally tagged with the Flag epitope, as described previously (8). Kir6.2-HA migrated as a 44-kDa band in immunoblot of cell lysates (Fig. 1D). Immunoprecipitation was performed using an anti-aldolase antibody. The Kir6.2 protein was readily detected in the aldolase immunoprecipitates when lysates from cells coexpressing Kir6.2-HA/SURx subunits were used. Interaction of aldolase A with KATP channel subunits was robust (notably, the interaction appears to be stronger with SUR2).
MS analysis identified several glycolytic enzymes as KATP channel-interacting proteins
To obtain a broader picture of proteins that are putative candidates associating with the KATP channel complex, analysis by MS was performed. Using rat heart ventricle membrane fractions, we immunoprecipitated Kir6.2 subunits with 2 separate antibodies. Each immunoprecipitate was separated on a 1D SDS gel, digested with trypsin in gel, and analyzed by LC-MS/MS. The spectra were used to identify proteins with Mascot searches against rat and mouse SwissProt databases (mouse was included since the database for this species is well annotated). Three separate experiments were performed, and Supplemental Table S2 lists 168 proteins that were identified in ≥2 experiments (most were present in all 3 reactions). This list was subjected analyzed with Pathway Studio software to find biological pathways statistically enriched in these proteins (Table 2). This analysis shows the most significantly represented pathway to be glycolysis, with 16 proteins belonging to this group. Other biological pathways that were well represented include, among others, the tricarboxylic acid cycle, fatty acid metabolic processes, transport, heart development, and responses to hypoxia. Although these experiments were not designed to be quantitative, it is of interest for the present study that proteins involved in glycolytic energy metabolism were identified from immunoprecipitates by using separate anti-Kir6.2 antibodies. The Mascot data for these proteins are given in Table 3. Note that enzymes involved in the proximal, ATP-consuming glycolytic steps (e.g., hexokinase and glucose-6-phosphate isomerase) had low Mascot scores and low (<10%) peptide coverage, suggesting that these proteins may not tightly associate with the KATP channel complex.
Table 2.
Pathways that are enriched for the GO category “biological function”
| Pathway | Proteins in pathway |
P value | |||
|---|---|---|---|---|---|
| Total (n) | Identified (n) | Coverage (%) | Names | ||
| Glycolysis | 68 | 15 | 22 | Hk1, GAPDH, ENO1, OGDH, PGK1, GPI, LDHA, DLAT, PKM2, ALDOA, MDH2, LDHB, ENO3, PDHA1, PDHB | 2.04E-21 |
| Metabolic process | 858 | 28 | 3 | SCCPDH, GAPDH, ATP1A1, ATP2A2, ACAT1, ALDH2, ACSL1, ACO2, OGDH, HSD17B4, DLAT, ALDOA, ACADM, HADH, IDH2, SUCLG1, DLST, ACADVL, MDH2, HADHA, ACADL, ECHS1, UGP2, HADHB, ACAA2, PDHA1, PDHB, PDHX | 4.89E-16 |
| ATP catabolic process | 21 | 7 | 33 | ATP1A1, ATP5O, MYH6, MYH7, ATP5A1, ATP5C1, ATP5B | 4.89E-12 |
| Tricarboxylic acid cycle | 24 | 7 | 29 | ACO2, CS, IDH2, SUCLG1, DLST, MDH2, PDHB | 1.44E-11 |
| Ventricular cardiac muscle morphogenesis | 25 | 7 | 28 | TNNI3, TNNT2, MYL2, TPM1, MYH6, MYH7, MYL3 | 1.99E-11 |
| ATP biosynthetic process | 74 | 9 | 12 | ATP1A1, ATP2A2, PKM2, ATP5O, ATP1B1, ALDOA, ATP5A1, ATP5C1, ATP5B | 7.53E-11 |
| Fatty acid metabolic process | 110 | 10 | 9 | ACSL1, HSD17B4, ACADM, HADH, ACADVL, HADHA, ACADL, ECHS1, HADHB, ACAA2 | 1.22E-10 |
| Oxidation reduction | 702 | 19 | 2 | SCCPDH, GAPDH, ALDH2, OGDH, HSD17B4, DLD, LDHA, NDUFS1, ACADM, HADH, IDH2, ACADVL, MDH2, LDHB, HADHA, ACADL, NDUFV1, PDHA1, PDHB | 9.06E-10 |
| ATP metabolic process | 27 | 6 | 22 | NDUFS1, ATP5O, ATP5F1, ATP5A1, ATP5C1, ATP5B | 2.69E-09 |
| Cardiac muscle contraction | 27 | 6 | 22 | TNNI3, TNNT2, MYL2, TPM1, ACTC1, MYL3 | 2.69E-09 |
| Cardiac myofibril assembly | 9 | 4 | 44 | Murc, MYH10, MYL2, ACTC1 | 5.87E-08 |
| Lipid metabolic process | 342 | 12 | 3 | ACSL1, HSD17B4, ACADM, HADH, ACADVL, ATP5A1, HADHA, ACADL, ECHS1, HADHB, ACAA2, ATP5B | 8.69E-08 |
| Transport | 1807 | 25 | 1 | UQCRC2, TMEM38A, ENSMUSG00000058927, ATP1A1, ATP2A2, VDAC1, SLC2A4, TF, SLC25A3, VDAC2, SLC25A4, MYO1C, NDUFS1, ATP5O, SLC16A1, ATP1B1, AP2A1, GOT2, ATP5F1, ATP5A1, RAB10, ATP5C1, NDUFV1, UQCRC1, ATP5B | 1.08E-06 |
| Acetyl-CoA biosynthetic process from pyruvate | 6 | 3 | 50 | DLD, DLAT, PDHB | 2.03E-06 |
| ADP biosynthetic process | 6 | 3 | 50 | ATP5A1, ATP5C1, ATP5B | 2.03E-06 |
| Cellular carbohydrate metabolic process | 25 | 4 | 16 | LDHA, CS, MDH2, LDHB | 5.55E-06 |
| Positive regulation of ATPase activity | 9 | 3 | 33 | TNNT2, TPM1, MYL3 | 8.46E-06 |
| ATP synthesis coupled proton transport | 62 | 5 | 8 | ATP5O, ATP5F1, ATP5A1, ATP5C1, ATP5B | 1.13E-05 |
| Response to drug | 295 | 9 | 3 | ATP1A1, ACSL1, LDHA, YWHAZ, ACADM, ACTC1, HADH, HADHA, ENO3 | 1.17E-05 |
| Oxidative phosphorylation | 10 | 3 | 30 | UQCRC2, ATP5C1, UQCRC1 | 1.20E-05 |
| Proton transport | 69 | 5 | 7 | ATP5O, ATP5F1, ATP5A1, ATP5C1, ATP5B | 1.92E-05 |
| Response to hypoxia | 184 | 7 | 3 | TFRC, TF, LDHA, PYGM, PKM2, ATP1B1, ALDOA | 2.81E-05 |
| Ion transport | 626 | 12 | 1 | TMEM38A, ATP1A1, ATP2A2, VDAC1, TF, VDAC2, ATP5O, ATP1B1, ATP5F1, ATP5A1, ATP5C1, ATP5B | 4.34E-05 |
| Cellular calcium ion homeostasis | 87 | 5 | 5 | Pln, ITGB1, TNNI3, ATP2A2, PYGM | 5.88E-05 |
| Phosphocreatine metabolic process | 4 | 2 | 50 | CKM, CKMT2 | 0.00013183 |
| Anaerobic glycolysis | 5 | 2 | 40 | LDHA, LDHB | 0.0002 |
| Response to nutrient | 117 | 5 | 4 | TFRC, ACSL1, LDHA, PKM2, ACADM | 0.0002 |
| Electron transport chain | 118 | 5 | 4 | UQCRC2, ENSMUSG00000058927, NDUFS1, NDUFV1, UQCRC1 | 0.0002 |
| Clathrin coat assembly | 7 | 2 | 28 | PICALM, AP2B1 | 0.0005 |
| Gluconeogenesis | 33 | 3 | 9 | GAPDH, PGK1, GPI | 0.0005 |
| Creatine metabolic process | 9 | 2 | 22 | CKM, CKMT2 | 0.0008 |
| Mitochondrial electron transport, NADH to ubiquinone | 39 | 3 | 7 | DLD, NDUFS1, NDUFV1 | 0.0008 |
| Glycogen metabolic process | 41 | 3 | 7 | PYGM, GYS1, PYGB | 0.0009 |
| Glycogen catabolic process | 10 | 2 | 20 | PYGM, PYGB | 0.0009 |
| Response to activity | 10 | 2 | 20 | HADH, UQCRC1 | 0.0009 |
| Response to stress | 239 | 6 | 2 | SERPINH1, ERN1, HSPA5, HSPA8, CRYAB, IDH2 | 0.0009 |
Only entries with a value of P < 0.001 are shown. Some entries were deleted in the interest of space due to functional overlap between categories. Listings show the total number of proteins present in a particular GO biological function category as well as the identified proteins (Supplemental Table S2) present in that pathway.
Table 3.
Mascot data for the subgroup of proteins identified as belonging to the GO biological function category “glycolysis”
| Gene | SwissProt | Description | Mascot score | Mass (Da) | Peptides | Coverage (%) |
|---|---|---|---|---|---|---|
| ALDOA | ALDOA_RAT | Fructose-bisphosphate aldolase A | 494 | 39,327 | 16 | 48.1 |
| ENO1 | ENOA_RAT | α-Enolase | 451 | 47,098 | 16 | 37.4 |
| ENO3 | ENOB_RAT | β-Enolase | 374 | 46,984 | 14 | 32.5 |
| GAPDH | G3P_RAT | Glyceraldehyde-3-phosphate dehydrogenase | 1150 | 35,805 | 47 | 69.1 |
| GPI | G6PI_RAT | Glucose-6-phosphate isomerase | 151 | 62,787 | 4 | 5.6 |
| Hk1 | HXK1_RAT | Hexokinase-1 | 71 | 102,342 | 2 | 2.9 |
| PKM2 | KPYM_RAT | Pyruvate kinase isozymes M1/M2 | 840 | 57,781 | 29 | 52.9 |
| LDHA | LDHA_RAT | l-Lactate dehydrogenase A | 282 | 36,427 | 12 | 36.5 |
| LDHB | LDHB_RAT | l-Lactate dehydrogenase B | 633 | 36,589 | 24 | 49.6 |
| MDH2 | MDHM_RAT | Malate dehydrogenase, mitochondrial | 807 | 35,661 | 22 | 66.3 |
| OGDH | ODO1_RAT | 2-Oxoglutarate dehydrogenase, mitochondrial | 396 | 116,221 | 19 | 15.3 |
| DLAT | ODP2_RAT | Dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex, | 490 | 67,123 | 18 | 31.1 |
| PDHA1 | ODPA_MOUSE | Pyruvate dehydrogenase E1 component subunit α, somatic form, mitochondrial | 375 | 43,204 | 20 | 37.2 |
| PDHB | ODPB_RAT | Pyruvate dehydrogenase E1 component subunit β, mitochondrial | 444 | 38,957 | 15 | 35.3 |
| PGK1 | PGK1_RAT | Phosphoglycerate kinase 1 | 95 | 44,510 | 7 | 19.8 |
| TPI1 | TPIS_RAT | Triosephosphate isomerase | 95 | 26,832 | 6 | 22.5 |
Each protein was identified multiple times in the 3 mass spectrometry experiments performed. Representative examples for various proteins for a given experiment are listed. Peptides column shows the number of peptide matches with a protein; Coverage column shows the percentage of the protein amino acid sequence matched by the peptides.
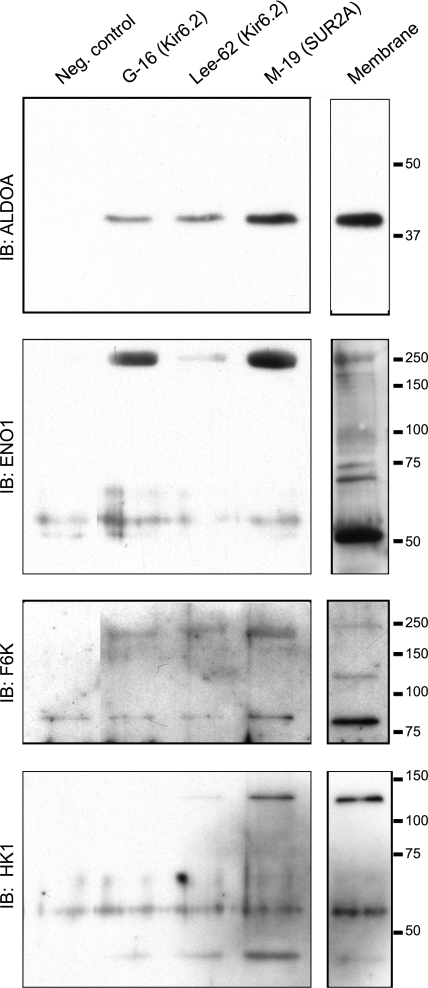
Coimmunoprecipitation assays with rat cardiac membranes
The MS-MS experiments suggest that several enzymes involved in the glycolytic pathway may interact with KATP channel subunits. To independently verify these data, we performed coimmunoprecipitation assays using rat heart membrane fractions. We focused on aldolase A (ALDOA), enolase 1 (ENO1), fructose 6 phosphate kinase (F6K), and hexokinase 1 (HK1) because of antibody availability. Each of these glycolytic enzymes was present in rat heart membranes (Fig. 2, right panels). Moreover, these enzymes were also present in immunoprecipitates obtained with antibodies against either SUR2A subunits or with 2 separate antibodies against Kir6.2. The molecular sizes of the immunoreactive bands were higher than expected in the case of ENO-1, F6K, and HK1, which may be due to incomplete denaturing (samples were not boiled prior to SDS-PAGE), post-translational modifications, or complex formation with other proteins (such as KATP channel subunits). These experiments demonstrate that diverse glycolytic enzymes throughout the glycolytic pathway interact with the KATP channel protein complex.
Figure 2.
Coimmunoprecipitation of glycolytic enzymes and KATP channel subunits in rat heart membranes. KATP channel subunits were immunoprecipitated using anti-Kir6.2 antibodies (G-16 or Lee-62) or antibodies directed against the SUR2A subunit (M-19) or an unrelated IgG as a negative control (see Materials and Methods for full details). Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with monoclonal antibodies against the glycolytic enzymes ALDOA, ENO1, F6K, or HK1. Right lanes: depiction of the presence of the proteins in membranes (no immunoprecipitation).
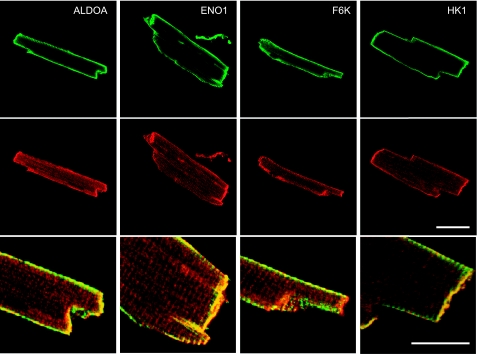
Colocalization of glycolytic enzymes and KATP channel subunits
We previously demonstrated that GAPDH and pyruvate kinase are membrane bound and that they strongly colocalize with KATP channel subunits in cardiac myocytes (5). We therefore performed a similar analysis for some of the other glycolytic enzymes indentified in this study. Using enzymatically isolated rat ventricular myocytes, we performed immunocytochemistry with 2 separate antibodies against the Kir6.2 subunit. Cells were costained with monoclonal antibodies against ALDOA, ENO1, F6K, and HK1. Figure 3 demonstrates strong colocalization of Kir6.2 with each of these glycolytic enzymes. Costaining was most notable at the cell periphery (particularly at the cell ends) and to a lesser extent along the z lines. Both anti-Kir6.2 antibodies generated a similar colocalization pattern (data not shown). Staining was not observed in negative controls in which the primary antibody was omitted (not shown). These data demonstrate strong colocalization of several glycolytic enzymes with KATP channel subunits.
Figure 3.
Coimmunostaining of glycolytic enzymes and Kir6.2 in rat cardiomyocytes. Top panels: localization of ALDOA, ENO1, F6K, and HK1 using monoclonal antibodies. Detection was in the green channel (DyLight 488). Middle panels: staining with the C-62 anti-Kir6.2 antibody (detected in the red channel; DyLight 549). Bottom panels: overlays of the red and green channels. Overlay image is a magnified region of the top 2 images to show more detail. Scale bars = 40 μm (top and middle panels); 20 μm (bottom panels).
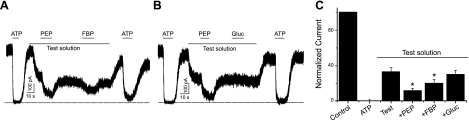
Functional consequences of interaction
The activity of KATP channels was recorded in the inside-out patch-clamp configuration to investigate the physiological relevance of their interaction with glycolytic enzymes. Channel opening occurred spontaneously following patch excision, and the KATP channels were reversibly inhibited by 1 mM ATP (Fig. 4). The maximal current in the absence of ATP is referred to as Imax. A pretest solution containing ATP (90 μM), ADP, inorganic phosphate, and NAD+ led to a partial inhibition of channel activity (67.3±4.6% of Imax; n=18; P<0.05). Consistent with our previous report (5), further application of PEP (5 mM) effectively and reversibly decreased KATP channel activity in 13 of 18 patches (P<0.05; Fig. 4C). In 8 of these patches, we applied FBP (the aldolase A substrate), which also reversibly diminished KATP channel activity (Fig. 4C). These glycolytic substrates did not inhibit KATP channel activity in the absence of ADP or inorganic phosphate (data not shown). d-Glucose (10 mM) had no inhibitory effect on KATP channel current (n=13). These data demonstrate that KATP channel activity in most (but not all) patches is sensitive to the distal catalytic activity of the glycolytic cascade (aldolase A and pyruvate kinase) but not to that of the entire glycolytic cascade.
Figure 4.
Substrates of PK and ALDOA, but not glucose, suppress KATP channel in excised, inside-out membrane patches. A) Channel activity was blocked by ATP (1 mM) and was partially inhibited by the test solution (containing 90 μM ATP, 0.5 mM ADP, 1 mM KH2PO4, and 1 mM NAD+). Further addition of PEP (5 mM) and FBP (5 mM) reversibly inhibited channel activity. B) Similar experiment depicting the effects of PEP and glucose (Gluc; 10 mM). C) Summary data (means±se) normalized to control current (in the absence of ATP). Dotted lines indicate zero current.
DISCUSSION
Collectively, our data demonstrate that several enzymes in the glycolytic cascade interact with KATP channel subunits. The data supporting this concept are identification of aldolase A as a putative interacting protein in 2-hybrid assays; interaction of aldolase A with a putative CC domain within the SUR subunit using GST-pulldown experiments; interaction with aldolase A with full-length SUR subunits in mammalian heterologous expression systems; identification of several components of the glycolytic pathway in KATP channel subunit immunoprecipitates from rat heart membrane fractions using mass spectrometric approaches; independent demonstration of coimmunoprecipitation of ALDOA, ENO1, F6K, and HK1 with KATP channel subunits from rat heart membrane fractions; colocalization of these glycolytic enzymes with KATP channel subunits in isolated rat ventricular myocytes; and regulation of KATP channels by catalytic activity of aldolase A and pyruvate kinase but not by d-glucose.
Glycolytic ATP production
ATP generation from carbohydrate catabolism occurs successively through glycolysis and oxidative phosphorylation. Most of the high-energy phosphate production occurs in the mitochondria by oxidative phosphorylation, which supplies ATP for energy demanding processes such as muscle contraction. Glycolysis produces a relatively small amount of ATP for every glucose molecule that is converted to pyruvate and NADH. Nevertheless, it is well established that many membrane-bound biological processes are preferentially fueled by the glycolytic ATP flux. For example, there is strong evidence for regulation by glycolysis of the Na+-K+-ATPase, the V-type H+-ATPase, plasmalemmal Ca2+-ATPase, sarcoendoplasmic reticulum Ca2+-ATPase, KATP channel, some of the TRP channels, the L-type Ca2+ channel, and the sarcoplasmic reticulum Ca2+ release channel (ryanodine receptor) (14). In several of these cases, direct association of glycolytic enzymes with the ion transporter subunits has been demonstrated. The concept has emerged that glycolytic ATP production is compartmentalized and that ATP is produced in close proximity of these target proteins, which mechanistically allows for their preferential regulation by glycolysis. The functional compartmentalization of both oxidative and glycolytic metabolism has been demonstrated in diverse tissues, including heart (15–17), smooth muscle (18–19), neural tissue (20), skeletal muscle (21), and pancreatic β cells (22). There is, of course, interaction between cytosolic ATP pools (derived from mitochondria) and the glycolytic subspace, and this communication is provided by energy transfer systems mediated by adenylate kinase and creatine kinase, which in turn have also been demonstrated to interact with the KATP channel complex (23).
Interaction of glycolytic enzymes with KATP channels
We are not the first to demonstrate functional interaction of glycolytic enzymes with the KATP channel protein complex (see introduction). Previous studies (24) have clearly indicated that substrates of the glycolytic process (e.g., FBP or PEP) effectively inhibit KATP channel activity These studies suggested that under conditions of high intrinsic ATP consumption, glycolysis is more effective than mitochondrial metabolism in regulating the activity of KATP channels. Such compartmentalization of glycolytic ATP production in a membrane subspace has long been proposed as a protective mechanism in metabolically stressed heart muscle (e.g., during hypoxia and ischemia; ref. 25). Functional compartmentalization of ATP in a membrane subspace has also been demonstrated to be involved in receptor signaling (e.g., angiotensin II-mediated closure of cardiac KATP channels; ref. 26). We provided a structural basis for these concepts with our demonstration that 3 glycolytic enzymes (GAPDH, TPI, and pyruvate kinase) physically interact with the KATP channel protein complex (5). The data provided in this study extends this observation with the finding that the KATP channel complex and the glycolytic process are richly interwoven. The mass spectrometry experiments have demonstrated statistically that the biological pathway most significantly represented by KATP channel associated proteins is glycolysis, with the possibility that many different proteins throughout the glycolytic process may couple (directly or indirectly) with the KATP channel complex. Aldolase A is a very interesting candidate, since we identified this enzyme (along with GAPDH) in 2 separate proteomic screens (Tables 1 and 2). Moreover, the possibility that aldolase directly interacts with the KATP channel complex via the identified putative CC domains is very real. First, we demonstrated with GST pulldown experiments that a putative CC domain within the SUR2 subunit directly interacts with aldolase. Second, this interaction was disrupted when we deleted a putative CC domain (K317–K330) present in aldolase. Interestingly, another study also implicated an amino acid residue in this region as being responsible for interaction between aldolase and the V-ATPase B-subunit (27). At present, we cannot make definitive conclusions regarding the nature of interaction of the other glycolytic enzymes with KATP channel subunits. It is interesting to note, however, that the mass spectrometric peptide coverage and Mascot scores were in general higher (suggesting greater relative protein abundance) for enzymes involved in the second (energy-producing) phase of the glycolytic process and lower for enzymes involved in the primary (energy-consuming) phase. This raises the possibility that there is preferential interaction of KATP channel subunits with enzymes involved in the ATP-generating steps (such as GAPDH, phosphoglycerate kinase, phosphoglycerate mutase, enolase, and pyruvate kinase). Indeed, some of these enzymes were well represented in our proteomic screens. Moreover, substrates of these enzymes effectively regulate the activity of KATP channels, even in excised membrane patches, in a manner that depends on their catalytic activity (see also refs. 3, 5, 24). In contrast, substrates for enzymes acting upstream in the glycolytic pathway are less effective (24). In our studies, for example, d-glucose had no effect on KATP channels in excised patches; a finding that is best reconciled with the assumption that key catalytic enzymes are loosely associated with the KATP channel or that interaction with these enzymes does not survive the process of patch excision. Nevertheless, the physiological involvement of these key steps is clear when considering that elevated glucose levels can reverse the action potential shortening and KATP channel activation caused by metabolic impairment (such as hypoxia) (28–29).
Pathophysiological implications
The biological consequences of the glycolytic enzyme interaction with KATP channels in the context of cardiovascular function and pathology are not entirely clear. Seemingly, there is a contradictory phenomenon described in the literature: glycolysis has been described to protect the myocardium from ischemia, in part by maintenance of the cardiac action potential (25). From this perspective, the inhibition of KATP channel activity by an increased glycolytic rate is entirely consistent with the maintenance of the action potential duration. However, there is also strong evidence in the literature that pharmacological opening of KATP channels is cardioprotective against ischemic events (30). Moreover, KATP blockers (which reduce ischemic preconditioning; ref. 31) actually enhance glycolysis (32–34). One can therefore imagine the presence of a 2-way signaling pathway with an equilibrium optimum between KATP activity and glycolytic flux, where the maximal potential protective effect of enhanced glycolytic flux during ischemia is blunted by the inhibitory effects on KATP channels. Conversely, the maximal potential protection afforded by KATP channel opening during ischemia may be mitigated by channel inhibition due to the enhanced glycolytic flux. This should be balanced with the strong possibility that the increased glycolytic flux may regulate other important membrane processes during ischemia, such as the Na+/K+ pump and proteins involved in Ca2+ homeostasis (14). It may even be possible that functional units of groups of membrane proteins are independently regulated by the glycolytic pathway. Answers to these intriguing questions would require new approaches to this problem.
Other putative interacting proteins
Although the focus of this study is on glycolysis, it should be noted that the mass spectrometry experiments have identified many other proteins as putatively interacting with the KATP channel complex. With the caveat that these proteins were not verified for their interaction with KATP channel subunits, we analyzed these for enrichment in biological processes (Table 2); it is interesting that groups of proteins in categories such as ATP catabolic process, ATP biosynthetic process, ADP biosynthetic process, fatty acid β-oxidation, tricarboxylic acid cycle, and oxidative phosphorylation are statistically enriched. Overall, this suggests unanticipated roles for KATP channels in energy metabolism. Indeed, a recent report that KATP channels control energy expenditure and determine body weight (35) is entirely consistent with this observation. A large number of other identified proteins are denoted to be involved in oxidation/reduction processes (Table 2). This observation is in keeping with the known regulation of KATP channels by redox state (e.g., ref. 36). The proteins represented in the category of clathrin coat assembly is reminiscent of reports of KATP channels interacting with (and being regulated by) vesicle-associated proteins (such as syntaxin-1A; ref. 37) and the role that cardiac KATP channels have in secretory release of ANP (38) and neurotransmitters (39). Finally, it would not escape the reader's attention that in many cases the subcellular localization of the identified proteins is intracellular (often mitochondrial). Clearly, KATP channels can have important intracellular roles; for example, by being present in intracellular endosomal compartments, from where they can translocate to the sarcolemma and thus function as a reservoir to increase surface KATP channel current density after an ischemic event (40). However, the localization of KATP channels and their roles in these compartments remain to be confirmed.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Hon-Chi Lee (Mayo Clinic, Rochester, MN, USA) for the generous gift of a rabbit polyclonal anti-Kir6.2 antibody, Dr. Åsa Wåhlander for mass spectrometry, and Dr. Guoan Zhang and Steven Blais for assistance with mass spectrometry data analysis.
This work was supported by U.S. National Institutes of Health grant HL-085820 (to W.A.C. and T.A.N.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Ashcroft F. M. (1988) Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 11, 97–118 [DOI] [PubMed] [Google Scholar]
- 2. Nichols C. G. (2006) KATP channels as molecular sensors of cellular metabolism. Nature 440, 470–476 [DOI] [PubMed] [Google Scholar]
- 3. Weiss J. N., Lamp S. T. (1989) Cardiac ATP-sensitive K + channels - evidence for preferential regulation by glycolysis. J. Gen. Physiol. 94, 911–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dubinsky W. P., Mayorga-Wark O., Schultz S. G. (1998) Colocalization of glycolytic enzyme activity and KATP channels in basolateral membrane of Necturus enterocytes. Am. J. Physiol. 275, C1653–C1659 [DOI] [PubMed] [Google Scholar]
- 5. Dhar Chowdhury P., Harrell M. D., Han S., Jankowska D., Parachuru L., Morrissey A., Srivastava S., Liu W., Malester B., Yoshida H., Coetzee W. A. (2005) The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose phosphate isomerase and pyruvate kinase are components of the KATP channel macromolecular complex and regulate its function. J. Biol. Chem. 280, 38464–38470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jovanovic S., Du Q., Crawford R. M., Budas G. R., Stagljar I., Jovanovic A. (2005) Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal K(ATP) channel. EMBO Rep. 6, 848–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pountney D. J., Sun Z. Q., Porter L. M., Nitabach M. N., Nakamura T. Y., Holmes D., Rosner E., Kaneko M., Manaris T., Holmes T. C., Coetzee W. A. (2001) Is the molecular composition of K(ATP) channels more complex than originally thought? Biochemical and electrophysiological evidence for heteromultimeric assembly of the K ATP channel subunits Kir6.1 and Kir6.2. J. Mol. Cell. Cardiol. 33, 1541–1546 [DOI] [PubMed] [Google Scholar]
- 8. Kang G., Chepurny O. G., Malester B., Rindler M. J., Rehmann H., Bos J. L., Schwede F., Coetzee W. A., Holz G. G. (2006) cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic {beta} cells and rat INS-1 cells. J. Physiol. 573, 595–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suarez E., Bach D., Cadefau J., Palacin M., Zorzano A., Guma A. (2001) A novel role of neuregulin in skeletal muscle. Neuregulin stimulates glucose uptake, glucose transporter translocation, and transporter expression in muscle cells. J. Biol. Chem. 276, 18257–18264 [DOI] [PubMed] [Google Scholar]
- 10. Lei B., Lionetti V., Young M. E., Chandler M. P., d'Agostino C., Kang E., Altarejos M., Matsuo K., Hintze T. H., Stanley W. C., Recchia F. A. (2004) Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J. Mol. Cell. Cardiol. 36, 567–576 [DOI] [PubMed] [Google Scholar]
- 11. Yoshida H., Feig J., Ghiu I. A., Artman M., Coetzee W. A. (2004) K(ATP) channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J. Mol. Cell. Cardiol. 37, 857–869 [DOI] [PubMed] [Google Scholar]
- 12. Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 391, 85–100 [DOI] [PubMed] [Google Scholar]
- 13. Srivastava S., Li Z., Lin L., Liu G., Ko K., Coetzee W. A., Skolnik E. Y. (2005) The phosphatidylinositol 3-phosphate phosphatase myotubularin- related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1. Mol. Cell. Biol. 25, 3630–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhar-Chowdhury P., Malester B., Rajacic P., Coetzee W. A. (2007) The regulation of ion channels and transporters by glycolytically derived ATP. Cell. Mol. Life Sci. 64, 3069–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bricknell O. L., Opie L. H. (1976) Glycolytic ATP and its production during ischemia in isolated Langendorff-perfused rat hearts. Recent Adv. Stud. Cardiac. Struct. Metab. 11, 509–519 [PubMed] [Google Scholar]
- 16. Geisbuhler T., Altschuld R. A., Trewyn R. W., Ansel A. Z., Lamka K., Brierley G. P. (1984) Adenine nucleotide metabolism and compartmentalization in isolated adult rat heart cells. Circ. Res. 54, 536–546 [DOI] [PubMed] [Google Scholar]
- 17. Dizon J., Burkhoff D., Tauskela J., Whang J., Cannon P., Katz J. (1998) Metabolic inhibition in the perfused rat heart: evidence for glycolytic requirement for normal sodium homeostasis. Am. J. Physiol. 274, H1082–H1089 [DOI] [PubMed] [Google Scholar]
- 18. Paul R. J. (1983) Functional compartmentalization of oxidative and glycolytic metabolism in vascular smooth muscle. Am. J. Physiol. 244, C399–C409 [DOI] [PubMed] [Google Scholar]
- 19. Davidheiser S., Joseph J., Davies R. E. (1984) Separation of aerobic glycolysis from oxidative metabolism and contractility in rat anococcygeus muscle. Am. J. Physiol. 247, C335–C341 [DOI] [PubMed] [Google Scholar]
- 20. Andersen B. J., Marmarou A. (1992) Functional compartmentalization of energy production in neural tissue. Brain Res. 585, 190–195 [DOI] [PubMed] [Google Scholar]
- 21. Korge P., Campbell K. B. (1995) The importance of ATPase microenvironment in muscle fatigue: a hypothesis. Int. J. Sports. Med. 16, 172–179 [DOI] [PubMed] [Google Scholar]
- 22. Malaisse W. J., Sener A., Levy J., Herchuelz A. (1976) The stimulus-secretion coupling of glucose-induced insulin release. XXII. Qualitative and quantitative aspects of glycolysis in isolated islets. Acta Diabetol. Lat. 13, 202–215 [DOI] [PubMed] [Google Scholar]
- 23. Alekseev A. E., Hodgson D. M., Karger A. B., Park S., Zingman L. V., Terzic A. (2005) ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J. Mol. Cell. Cardiol. 38, 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weiss J. N., Lamp S. T. (1987) Glycolysis preferentially inhibits ATP-sensitive K + channels in isolated guinea pig cardiac myocytes. Science 238, 67–69 [DOI] [PubMed] [Google Scholar]
- 25. Opie L. H., Tuschmidt R., Bricknell O., Girardier L. (1980) Role of glycolysis in maintenance of the action potential duration and contractile activity in isolated perfused rat heart. J. Physiol. (Paris) 76, 821–829 [PubMed] [Google Scholar]
- 26. Tsuchiya K., Horie M., Watanuki M., Albrecht C. A., Obayashi K., Fujiwara H., Sasayama S. (1997) Functional compartmentalization of ATP is involved in angiotensin II-mediated closure of cardiac ATP-sensitive K+ channels. Circulation 96, 3129–3135 [DOI] [PubMed] [Google Scholar]
- 27. Lu M., Ammar D., Ives H., Albrecht F., Gluck S. L. (2007) Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J. Biol. Chem. 282, 24495–24503 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura S., Kiyosue T., Arita M. (1989) Glucose reverses 2,4-dinitrophenol induced changes in action potentials and membrane currents of guinea pig ventricular cells via enhanced glycolysis. Cardiovasc. Res. 23, 286–294 [DOI] [PubMed] [Google Scholar]
- 29. Shigematsu S., Arita M. (1997) Anoxia-induced activation of ATP-sensitive K+ channels in guinea pig ventricular cells and its modulation by glycolysis. Cardiovasc. Res. 35, 273–282 [DOI] [PubMed] [Google Scholar]
- 30. Hearse D. J. (1995) Activation of ATP-sensitive potassium channels: A novel pharmacological approach to myocardial protection. Cardiovasc. Res. 30, 1–17 [PubMed] [Google Scholar]
- 31. Gross G. J., Fryer R. M. (1999) Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ. Res. 84, 973–979 [DOI] [PubMed] [Google Scholar]
- 32. Hatao K., Kaku K., Matsuda M., Tsuchiya M., Kaneko T. (1985) Sulfonylurea stimulates liver fructose-2,6-bisphosphate formation in proportion to its hypoglycemic action. Diabetes Res. Clin. Pract. 1, 49–53 [DOI] [PubMed] [Google Scholar]
- 33. Schaffer S. W., Tan B. H., Mozaffari M. S. (1985) Effect of glyburide on myocardial metabolism and function. Am. J. Med. 79, 48–52 [DOI] [PubMed] [Google Scholar]
- 34. Kramer J. H., Lampson W. G., Schaffer S. W. (1983) Effect of tolbutamide on myocardial energy metabolism. Am. J. Physiol. 245, H313–H319 [DOI] [PubMed] [Google Scholar]
- 35. Alekseev A. E., Reyes S., Yamada S., Hodgson-Zingman D. M., Sattiraju S., Zhu Z., Sierra A., Gerbin M., Coetzee W. A., Goldhamer D. J., Terzic A., Zingman L. V. (2010) Sarcolemmal ATP-sensitive K(+) channels control energy expenditure determining body weight. Cell Metab. 11, 58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coetzee W. A., Nakamura T. Y., Faivre J. F. (1995) Effects of thiol-modifying agents on K ATP channels in guinea pig ventricular cells. Am. J. Physiol. Heart Circ. Physiol. 269, H1625–H1633 [DOI] [PubMed] [Google Scholar]
- 37. Kang Y., Leung Y. M., Manning-Fox J. E., Xia F., Xie H., Sheu L., Tsushima R. G., Light P. E., Gaisano H. Y. (2004) Syntaxin-1A inhibits cardiac KATP channels by its actions on nucleotide binding folds 1 and 2 of sulfonylurea receptor 2A. J. Biol. Chem. 279, 47125–47131 [DOI] [PubMed] [Google Scholar]
- 38. Saegusa N., Sato T., Saito T., Tamagawa M., Komuro I., Nakaya H. (2005) Kir6.2-deficient mice are susceptible to stimulated ANP secretion: K(ATP) channel acts as a negative feedback mechanism? Cardiovasc. Res. 67, 60–68 [DOI] [PubMed] [Google Scholar]
- 39. Oe K., Sperlagh B., Santha E., Matko I., Nagashima H., Foldes F. F., Vizi E. S. (1999) Modulation of norepinephrine release by ATP-dependent K(+)-channel activators and inhibitors in guinea-pig and human isolated right atrium. Cardiovasc. Res. 43, 125–134 [DOI] [PubMed] [Google Scholar]
- 40. Bao L., Hadjiolova K., Coetzee W. A., Rindler M. J. (2011) Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits. Am. J. Physiol. 300, H262–H270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.