Abstract
Type 2 diabetes and obesity have emerged as global public health crises. Adipose tissue expansion in obesity promotes accumulation of classically activated macrophages that perpetuate chronic inflammation and sustain insulin resistance. Acute inflammation normally resolves in an actively orchestrated series of molecular and cellular events that ensures return to homeostasis after an inflammatory insult, a process regulated in part by endogenous lipid mediators such as the resolvins. In this study, we sought to determine whether stimulating resolution with resolvin D1 (RvD1) improves insulin sensitivity by resolving chronic inflammation associated with obesity. In male leptin receptor-deficient (db/db) mice, treatment with RvD1 (2 μg/kg) improved glucose tolerance, decreased fasting blood glucose, and increased insulin-stimulated Akt phosphorylation in adipose tissue relative to vehicle-treated mice. Treatment with RvD1 increased adiponectin production, while expression of IL-6 in adipose tissue was decreased. The formation of crown-like structures rich in inflammatory F4/80+CD11c+ macrophages was reduced by >50% in adipose tissue by RvD1 and was associated with an increased percentage of F4/80+ cells expressing macrophage galactose-type C-type lectin 1 (MGL-1), a marker of alternatively activated macrophages. These results suggest that stimulating resolution with the endogenous proresolving mediator RvD1 could provide a novel therapeutic strategy for treating obesity-induced diabetes.—Hellmann, J., Tang, Y., Kosuri, M., Bhatnagar, A., Spite, M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice.
Keywords: lipid mediators, resolution of inflammation, obesity
Resolution of acute inflammation has recently emerged as an active endogenous program that limits excessive and unwarranted inflammation and promotes the return to homeostasis (1). Resolvins are a newly identified family of lipid mediators generated enzymatically from the ω-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) during resolution that have potent and distinct anti-inflammatory vs. proresolving actions (2). For example, DHA-derived resolvin D1 (RvD1) blocks excessive leukocyte infiltration into tissues and blunts the production of proinflammatory cytokines, while promoting noninflammatory macrophage phagocytosis (2–4). Emerging evidence suggests that nonresolving inflammation is a critical underlying component of many prevalent chronic diseases, which is sustained in part by a deficiency of mediators that normally resolve inflammation (5, 6).
Obesity is one of the most prominent risk factors for the development of type 2 diabetes (T2D) and is increasing in prevalence (7, 8). It is now widely recognized that persistent and unchecked inflammation is the key underlying mechanism linking obesity with systemic insulin resistance. This inflammation is established in part by the expansion of the abdominal adipose tissue mass that is associated with an increase in the generation of proinflammatory mediators, such as IL-6 and TNF-α (9, 10). Infiltration of classically activated inflammatory macrophages into adipose tissue has also been shown to contribute to inflammation and insulin resistance (11, 12). Indeed, the accumulation of inflammatory adipose tissue macrophages (ATMs) correlates with hyperinsulinemia and insulin resistance in humans, while targeted disruption of genes regulating macrophage infiltration into tissues (Ccr2), as well as myeloid-specific inflammatory signaling (Ikkβ), alleviates systemic insulin resistance in diet-induced obesity (10, 13, 14).
Adipose tissue expansion is associated with altered ATM phenotypes. Resident ATMs of lean mice consist primarily of alternatively activated (M2) macrophages that serve a homeostatic role and are characterized by distinct cell surface markers, including macrophage-galactose type C-type lectin 1 (MGL-1) and mannose receptor (15). Obesity promotes the infiltration of classically activated (M1) macrophages that express CD11c and have high levels of proinflammatory gene expression (i.e., IL-6). These macrophages form crown-like structures (CLSs) surrounding dying adipocytes (16, 17). In the local environment of the adipose tissue, the balance between M1 and M2 ATMs is reflective of adipose tissue inflammation and local, as well as systemic, insulin resistance.
Recent studies demonstrate that ω-3 fatty acid feeding promotes endogenous production of RvD1 and 17-hydroxy-DHA, a marker of resolvin biosynthesis, in adipose tissue of obese-diabetic mice (18). Increased resolvin biosynthesis is associated with decreases in other arachidonate-derived lipid mediators, such as 12-hydroxyeicosatetraenoic acid (12-HETE), suggesting that ω-3 fatty acid feeding changes substrate utilization, thereby enabling the generation of anti-inflammatory/proresolving lipid mediators (18). Furthermore, transgenic overexpression of fat-1, which encodes a desaturase enzyme enabling endogenous conversion of ω-6 to ω-3 fatty acids, partially protects against obesity-induced insulin resistance in mice and is associated with an increase in the resolvin biosynthetic pathway marker 17-hydroxy-DHA (19). However, the individual role of RvD1 in obesity-induced diabetes has not been examined. Here, we report that RvD1 decreases obesity-induced insulin resistance in vivo in part by regulating the accumulation of inflammatory macrophages in the adipose tissue.
MATERIALS AND METHODS
Animals and treatment
Male leptin receptor-deficient mice [B6.BKS(D)-Leprdb/J] were purchased from Jackson Laboratories (Bar Harbor, ME, USA) at 8 wk of age. Resolvin D1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid) was obtained from Cayman Chemical (Ann Arbor, MI, USA). Mice received a daily intraperitoneal injection of either RvD1 (2 μg/kg body weight in 100 μl of 0.1% ethanol in sterile saline) or vehicle (100 μl of 0.1% ethanol in sterile saline) for 8 or 16 d. All procedures were approved by University of Louisville Animal Care and Use Committee.
Glucose tolerance tests
Glucose tolerance tests were performed following 6 h of food deprivation by intraperitoneal injection of d-glucose (1 mg/g) in sterile saline. Blood samples were obtained from the tail, and glucose levels were measured at indicated time points using an Aviva Accu-Chek glucometer (Roche Diagnostics, Manheim, Germany).
Stromal vascular cell (SVC) isolation and flow cytometry analysis
SVCs were isolated from the adipose tissue as previously reported (15). SVC pellets were suspended in FACS buffer (1% FBS in PBS) and incubated with Fc Block (BD Biosciences, San Jose, CA, USA) for 10 min at 4°C before being stained with fluorescent-conjugated primary antibodies or appropriate isotype controls for 30 min at 4°C. Antibodies used included AlexaFluor 647-conjugated anti-Mgl-1 (CD301a; AbD Serotec, Raleigh, NC, USA), FITC-conjugated anti-CD11c (BD Biosciences), and phycoerythrin (PE)-conjugated anti-F4/80 (Biolegends, San Diego, CA, USA). Flow cytometry was carried out using a BD LSRII flow cytometer equipped with FACSDiva v.6.0 (BD Biosciences). Analysis was performed with FlowJo V.7.6 software (Tree Star, Inc., Ashland, OR, USA).
Immunohistochemistry and quantification of CLSs
Formalin-fixed, paraffin-embedded epididymal adipose tissue sections were incubated with PE-conjugated anti-F4/80 and FITC-conjugated anti-CD11c antibodies for 1 h at room temperature. Slides were mounted with SlowFade Gold antifade reagent containing DAPI (Molecular Probes, Eugene, OR, USA). Fluorescent photographs (×10 objective) were obtained using an EVOS fluorescence microscope (Advanced Microscopy Group, Bothell, WA, USA). CLSs were identified as adipocytes completely surrounded by F4/80+ cells and were quantified in 5 random fields in all animals. Hematoxylin and eosin staining was performed following standard procedures.
Biochemical analyses
Plasma insulin, adiponectin, and resistin levels were measured using commercially available Luminex kits (Millipore, Billerica, MA, USA) according to the manufacturer's guidelines. Total plasma cholesterol, HDL and LDL, triglycerides, total protein, albumin (Cholesterol CII enzymatic kit; L-Type TG-H kit; Bradford reagent, bromocresol green; Wako, Richmond, VA, USA), and alanine aminotransferase and aspartate aminotransferase (Infinity, ThermoElectron, Louisville, CO, USA) levels were measured using commercially available assay reagents as indicated. Assays were performed using a Cobas Mira Plus 5600 Autoanalyzer (Roche, Indianapolis, IN, USA). The calculated homeostasis model assessment of insulin resistance (HOMA-IR) score [glucose (mmol)×insulin (mU/ml)/22.5] was used as a derived measure of insulin resistance that reflects a combination of systemic insulin resistance and β-cell deficiency (20).
Immunoblotting and PCR
Tissue lysates were prepared using a standard protocol (18). Equal amounts of protein were separated by SDS-PAGE, transblotted, and probed for phospho-Akt (Ser473, total Akt, phospho-AMPKα, and total AMPKα; Cell Signaling Technology, Beverly, MA, USA). Western blots were developed using ECL-plus reagent followed by luminescence detection using a Typhoon 9400 variable mode imager (GE Healthcare, Chalfont St. Giles, UK). Quantification of band intensities was performed using Image Quant TL software (GE Healthcare).
For quantitative RT-PCR, RNA was extracted from tissues using the RNeasy lipid tissue kit (Qiagen, Germantown, MD, USA), followed by cDNA synthesis. Real-time PCR amplification was performed with SYBR-Green qPCR Master Mix (SA Biosciences, Fredrick, MD, USA) using a 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) and commercially available primers for Emr-1, IL-10, IL-6, and PPAR-γ (SA Biosciences). The following primers were also used (Integrated DNA technologies, Coralville, IA, USA): Ccl2, F 5′-ATGCAGGTCCCTGTCATG-3′ and R 5′-GCTTGAGGTGGTTGTGGA-3′; Fpr2, F 5′-GCCAGGACTTTCGTGGAGAGAT-3′ and R 5′-GATGAACTGGTGCTTGAATCACT-3′ (21). Relative expression was determined by the 2−ΔΔCT method (22) after internal normalization to hprt.
Statistical analysis
Data are expressed as means ± se. Multiple group comparisons were made using 1-way or 2-way ANOVA, followed by the Bonferroni post hoc test. For direct comparisons, an unpaired Student's t test was used. A value of P < 0.05 was considered significant.
RESULTS
RvD1 enhances glucose tolerance and insulin sensitivity in obese-diabetic mice
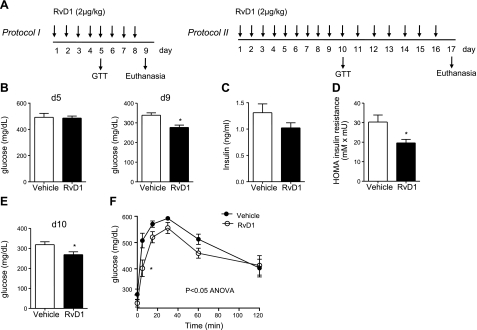
To determine whether RvD1 alters metabolic parameters in obese mice with established insulin resistance (23), RvD1 was administered to 10-wk-old db/db mice for 8 or 16 d (Fig. 1A, protocols I and II). We chose db/db mice for our studies because they develop severe insulin resistance, as well as hyperglycemia, and are widely used as a model of obesity and T2D. During the treatment protocol, no differences in body weight, organ/body weight ratios, total cholesterol, HDL, LDL, or liver enzymes were evident between vehicle and RvD1-treated mice, although a trend toward an increased spleen-to-body weight ratio and decreased glycosylated hemoglobin A1C levels was observed with RvD1 treatment (Table 1). A small increase in plasma triglycerides was evident with short-term treatment, although this increase normalized over time (Table 1). In mice treated according to protocol I (Fig. 1A), fasting blood glucose was determined at d 5 and on euthanasia at d 9. Consistent with previous reports (23), the db/db mice were markedly hyperglycemic at this age, with fasting blood glucose levels of 338 ± 12.3 mg/dl. RvD1 treatment decreased fasting blood glucose levels compared with vehicle-treated mice, reaching statistical significance by the end of the study (d 9; Fig. 1B). Consistent with the modest change in blood glucose at d 5, RvD1 did not alter glucose tolerance at this time point (data not shown). RvD1-treated animals were also significantly less insulin resistant, as assessed by the HOMA of insulin resistance (Fig. 1D).
Figure 1.
RvD1 improves glucose tolerance and insulin sensitivity in obese-diabetic mice. A) Study protocols of db/db mice treated daily with RvD1 intraperitoneally. GTT, glucose tolerance test. B) Fasting blood glucose at d 5 and 9 in treatment protocol I. C) Plasma insulin levels on euthanasia. D) Calculated HOMA for insulin resistance; n = 5/group. E) Fasting blood glucose at d 10 in treatment protocol II. F) Glucose tolerance tests performed at d 10; n = 6/group. Data are means ± se. *P < 0.05; Student's t test (B–E) or 2-way ANOVA with Bonferroni posttest (F).
Table 1.
Body weight, organ weight, and serum biochemistry in RvD1-treated db/db mice
| Parameter | 9 d |
17 d |
||
|---|---|---|---|---|
| Vehicle | RvD1 | Vehicle | RvD1 | |
| BWT (g) | 43.4 ± 0.8 | 45.8 ± 0.7 | 48.6 ± 1.6 | 46.3 ± 1.6 |
| Liver (g) | 2.98 ± 0.1 | 3.33 ± 0.1 | 3.70 ± 0.2 | 3.4 ± 0.2 |
| Spleen (g) | ND | ND | 0.065 ± 0.010 | 0.102 ± 0.039 |
| Heart (g) | 0.130 ± 0.010 | 0.135 ± 0.004 | 0.142 ± 0.003 | 0.123 ± 0.010 |
| Liver/BWT (%) | 6.8 ± 0.2 | 7.3 ± 0.1 | 7.6 ± 0.3 | 7.3 ± 0.3 |
| Heart/BWT (%) | 0.297 ± 0.012 | 0.295 ± 0.004 | 0.293 ± 0.009 | 0.266 ± 0.020 |
| Spleen/BWT (%) | ND | ND | 0.132 ± 0.020 | 0.235 ± 0.090 |
| Cholesterol (mg/dl) | 185.7 ± 11.2 | 180.5 ± 5.2 | 199.4 ± 6.7 | 222.8 ± 9.8 |
| HDL (mg/dl) | 149.9 ± 8.0 | 137.9 ± 4.4 | 136.7 ± 3.8 | 149.7 ± 5.1 |
| LDL (mg/dl) | 24.1 ± 2.1 | 24.1 ± 1.2 | 28.3 ± 2.3 | 33.4 ± 6.0 |
| HDL/LDL | 6.3 ± 0.2 | 5.8 ± 0.3 | 5.0 ± 0.5 | 5.0 ± 0.7 |
| Triglycerides (mg/dl) | 36.4 ± 5.0 | 51.3 ± 3.9* | 63.7 ± 12.4 | 57.5 ± 11.2 |
| ALT (U/L) | 153.6 ± 27.1 | 151.1 ± 22.3 | 216.8 ± 20.1 | 224.6 ± 34.4 |
| AST (U/L) | 137.1 ± 14.9 | 134.8 ± 19.8 | 164.5 ± 13.2 | 175.1 ± 21.0 |
| Total protein (g/dl) | 5.94 ± 0.1 | 5.87 ± 0.1 | 5.3 ± 0.2 | 5.7 ± 0.2 |
| Albumin (g/dl) | 4.0 ± 0.1 | 4.0 ± 0.1 | 3.6 ± 0.1 | 3.8 ± 0.1 |
| HbA1C (%) | 7.1 ± 0.2 | 7.2 ± 0.2 | 8.1 ± 0.1 | 7.4 ± 0.4 |
Data are means ± se; n = 5–6/group. BWT, body weight; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, hemoglobin A1c (% glycosylated); ND, not determined.
P < 0.05.
Because RvD1 treatment was associated with a time-dependent improvement in glucose homeostasis, we examined whether longer treatment would improve glucose tolerance. Accordingly, db/db mice were treated with RvD1 for 16 d, and fasting blood glucose levels were measured at d 10 and on euthanasia at d 17. Glucose tolerance was tested on d 10 (Fig. 1A, protocol II). As shown in Fig. 1E, RvD1-treated animals showed a marked improvement in fasting blood glucose levels by d 10, consistent with the decrease at d 9 as determined in protocol I. Notably, this duration of RvD1 treatment significantly improved glucose tolerance in response to a bolus of exogenous glucose (Fig. 1F; P<0.05; ANOVA).
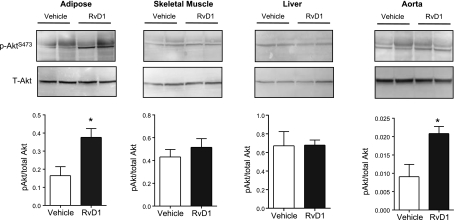
We next asked whether insulin signaling was modulated by RvD1. For this, insulin was administered (1.5 U/kg) to mice before euthanasia, and the phosphorylation of Akt, a downstream target of insulin signaling, was measured in tissues known to become insulin resistant in db/db mice at this age (23). This analysis revealed that RvD1-treated mice had enhanced insulin-stimulated Akt phosphorylation in adipose tissue and in the vasculature compared with vehicle-treated mice, while no significant modulation was evident in skeletal muscle, liver (Fig. 2), or heart (data not shown) with this duration of RvD1 treatment. Collectively, these data demonstrate that RvD1 improves metabolic derangements associated with established obesity-induced diabetes.
Figure 2.
Insulin sensitivity is improved in RvD1-treated db/db mice. Insulin-stimulated phosphorylation of Akt (S473) in adipose tissue, skeletal muscle, liver, and aorta of mice treated for 16 d with RvD1. Bottom panels: quantification of the ratio of phosphorylated Akt to total Akt; n = 6/group. Data are means ± se. *P < 0.05.
Macrophage-rich CLS and inflammatory cytokine production are decreased by RvD1 in adipose tissue of db/db mice
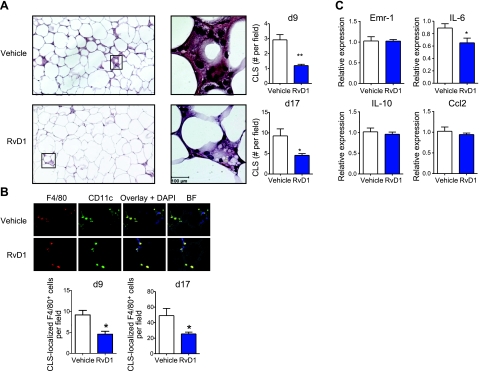
Previous studies show that the accumulation of inflammatory ATMs is a critical component linking obesity with systemic insulin resistance (12, 24). As RvD1 treatment was associated with a robust increase in adipose tissue insulin-signaling and whole-body glucose tolerance, we asked whether RvD1 affects ATM accumulation. As shown in Fig. 3A, RvD1 treatment drastically reduced the amount of CLSs in adipose tissue of db/db mice in both study protocols (d 9 and 17). Consistent with previous reports localizing proinflammatory F4/80+/CD11c+ macrophages to CLSs (16), immunofluorescence microscopy confirmed the presence of this macrophage population in CLSs (Fig. 3B). With the use of these criteria, the total amount of F4/80+ CLS-localized macrophages was determined and found to significantly decrease in RvD1-treated mice when normalized to total CLS content (Fig. 3B; bottom panels). Notably, adipose tissue mRNA expression of pan-macrophage marker Emr-1 (F4/80) was not changed with RvD1 treatment, indicating that only CLS-localized macrophage content was affected (Fig. 3C). Consistent with the decrease in CLS-localized ATMs, adipose tissue expression of the proinflammatory cytokine IL-6 was significantly reduced in RvD1-treated animals. Notably, RvD1 treatment did not affect adipose tissue mRNA expression of anti-inflammatory cytokine IL-10 or monocyte chemoattractant Ccl2 (Fig. 3C).
Figure 3.
Adipose tissue macrophage accumulation and inflammation are decreased by RvD1 treatment in db/db mice. A) Left panels: analysis of CLS formation in adipose tissue of db/db mice treated with vehicle or RvD1 for 8 d by hematoxylin and eosin staining (left images: ×10; right images: ×60). Right panels: quantification of the number of CLSs in db/db mice treated without or with RvD1 for 8 or 16 d and euthanized at d 9 or 17, respectively. B) Top panels: representative immunofluorescence microscopy images of adipose tissue CLSs rich in F4/80+ CD11c+ cells. Bottom panels: quantification of CLS-localized F4/80+ cells in mice treated without or with RvD1 as in A. C) Real-time quantitative PCR analysis of adipose tissue mRNA expression of Emr-1, IL-6, IL-10, and Ccl2 at 16 d after RvD1 treatment. Data are means ± se; n = 6/group. *P < 0.05, **P < 0.01.
RvD1 treatment increases the ratio of M2:M1 adipose tissue macrophages in obese-diabetic mice
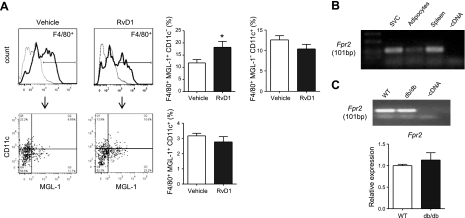
Recent studies demonstrate that MGL-1 is a reliable marker for anti-inflammatory M2 ATMs, while CD11c expression is characteristic of classically activated M1 ATMs (15). To determine whether the phenotype of ATMs was modulated by RvD1 treatment, we isolated SVCs from adipose tissue of db/db mice treated without or with RvD1 for 16 d and assessed the expression of CD11c and MGL-1 on F4/80+ ATMs. As shown in Fig. 4A, the percentage of F4/80+ ATMs expressing MGL-1 and lacking CD11c was significantly increased with RvD1 treatment, suggesting that RvD1 treatment shifted the balance from an M1 to an M2 phenotype in ATMs.
Figure 4.
RvD1 increases the ratio of M2:M1 adipose tissue macrophages in db/db mice: identification of the RvD1 receptor Fpr2. A) Left panels: flow cytometry analysis of CD11c+ and MGL-1+ surface expression on F4/80+ adipose tissue macrophages isolated from vehicle or RvD1-treated (16 d) db/db mice. Right panels: quantification of individual macrophage populations; n = 6/group. B) mRNA expression of the putative RvD1 receptor Fpr2 in isolated adipose tissue SVC, adipocytes, and spleen, n = 3. C) Top panel: expression of Fpr2 in resident peritoneal macrophages isolated from naive WT and db/db mice. Bottom panel: quantitative RT-PCR analysis; n = 3/group. Data are means ± se. *P < 0.05.
RvD1 receptor, Fpr2, is expressed in adipose tissue
The biological actions of RvD1 are mediated by specific binding to GPCRs, denoted GPR32 and FPR2 (also termed ALX; ref. 3). As there is currently no known murine homologue of GPR32, we sought to determine whether the murine counterpart of FPR2 is expressed in adipose tissue. As shown in Fig. 4B, Fpr2 was identified in the adipose tissue SVC fraction, which contains macrophages (see above). Interestingly, Fpr2 expression was also observed in isolated adipocytes. In accordance with previous reports, high levels of Fpr2 expression were observed in the spleen, which was used as a positive control for identification (25). Notably, Fpr2 was also expressed in resident peritoneal macrophages isolated from wild-type (WT) and db/db mice (Fig. 4C). Quantitative RT-PCR analysis revealed no significant differences between WT and db/db mice, indicating that the systemic metabolic derangements in db/db mice do not alter mRNA expression of Fpr2 in macrophages (Fig. 4C, bottom panel).
Insulin-sensitizing adipokine, adiponectin, is increased in RvD1-treated db/db mice
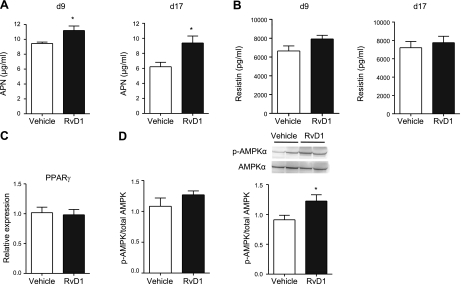
Obesity and diabetes are inversely correlated with circulating levels of the insulin-sensitizing adipokine adiponectin (APN; ref. 26). Given that RvD1 treatment was associated with decreased adipose tissue inflammation, we questioned whether APN levels might be restored in RvD1-treated db/db mice. Indeed, circulating plasma levels of APN were significantly increased in RvD1-treated db/db mice at both d 9 and 17, whereas the levels of resistin were not affected (Fig. 5A, B, respectively). We next probed whether expression of PPARγ was modulated by RvD1 treatment, which could be in part responsible for the observed increase in APN. However, this analysis revealed that PPARγ transcripts were not affected by RvD1 treatment of db/db mice (Fig. 5C).
Figure 5.
Adiponectin production and adipose tissue AMPK phosphorylation are increased in RvD1-treated db/db mice. A) Plasma adiponectin (APN) levels in db/db mice treated with RvD1 for 8 or 16 d and euthanized at d 9 or 17, respectively (n=5–6/group). B) Plasma resistin levels. C) mRNA expression of PPARγ in adipose tissue. D) Phosphorylation of AMPKα in adipose tissue of db/db mice treated with RvD1 for 8 or 16 d and euthanized at d 9 or 17, respectively. Data are means ± se; n = 5–6/group. *P < 0.05.
One of the primary targets of APN is AMPK, which in part mediates the insulin-sensitizing effects of APN (26). Accordingly, the phosphorylation of AMPK was measured in adipose tissue of mice treated without or with RvD1. Levels of phosphorylated AMPK were not significantly increased in adipose tissue of RvD1-treated mice at d 9, a time point when the plasma APN levels were increased. However, a significant increase in AMPK phosphorylation was observed after longer treatment with RvD1 (Fig. 5D).
DISCUSSION
The results presented here demonstrate that in leptin receptor-deficient mice, an established animal model of obesity-induced diabetes, RvD1 prevents the accumulation of macrophages in adipose tissue and restores systemic insulin sensitivity. These beneficial effects were associated with decreased fasting blood glucose, improved glucose tolerance, and increased circulating APN levels, and they were independent of changes in body weight. As RvD1 is an endogenous mediator generated from ω-3 fatty acid DHA, these results suggest that resolvins could potentially mediate some of the beneficial actions attributed to ω-3 fatty acids in obesity-induced diabetes.
ω-3 fatty acids have anti-inflammatory and insulin-sensitizing actions that have been demonstrated in animal models of obesity-induced diabetes (27). Recently, a diet rich in ω-3 fatty acids (EPA and DHA) was found to improve insulin-sensitivity in leptin-deficient mice (ob/ob), in part via increases in insulin-sensitizing genes and APN expression specifically in adipose tissue. Notably, dietary intake of ω-3 fatty acids in this study was associated with endogenous production of RvD1 and 17-hydroxy-DHA, a marker of resolvin biosynthesis, in adipose tissue (18). We used db/db mice, which develop severe obesity and T2D to a similar extent as ob/ob mice. Thus the results presented here are consistent with and extend the findings reported in the aforementioned study and highlight that RvD1 may underlie some of the protective actions of ω-3 fatty acid feeding in this context. Notably, a recent report documented that transgenic overexpression of fat-1, which encodes a desaturase enzyme that enables endogenous conversion of ω-6 fatty acids to ω-3 fatty acids, protects mice from the development of obesity-induced insulin-resistance (19). Interestingly, these studies demonstrated that high-fat feeding results in a deficit of endogenous resolvin and protectin biosynthesis and that these deficits are restored in fat-1 transgenic mice. For example, levels of the RvD1 biosynthetic pathway marker 17-hydroxy-DHA was increased 138% in adipose tissue of fat-1 transgenic mice on a high-fat diet (19). It is important to note that, in addition to RvD1, DHA is converted into other bioactive mediators with potent anti-inflammatory/proresolving actions, such as the protectins and the maresins (28). Thus, although our studies highlight that the protective actions of DHA in obesity and T2D could be mediated in part by RvD1, further studies are required to delineate the overall contribution of different lipid mediator families to the effects of DHA.
ATM accumulation has emerged as a critical underlying component linking obesity with systemic insulin resistance (12, 16, 24, 29). Infiltration of classically activated M1 macrophages into abdominal adipose tissue promotes inflammation and suppresses insulin sensitivity. It is now widely accepted that an altered balance between alternatively activated M2 and classically activated macrophages within adipose tissue promotes inflammation and insulin resistance in obesity-induced diabetes, while decreasing inflammatory ATMs has proved to be beneficial (29). Several reports have rigorously defined surface markers representing these ATM populations, which characterize M1 ATMs as F4/80+ CD11c+ MGL-1−, whereas M2 ATMs express high levels of MGL-1 and have lower expression of CD11c (15). It should be noted, however, that recent studies have also shown that while MGL-1 is a reliable marker for anti-inflammatory ATMs, CD11c expression is maintained after phenotypic reversion from M1 to M2 macrophages (30). Thus, in the present study, we focused on MGL-1 as a marker for alternatively activated macrophages. As ω-3 fatty acid feeding is associated with decreased adipose tissue macrophage accumulation and inflammation, as well as RvD1 biosynthesis in adipose tissue (18, 19), we questioned whether RvD1-treated mice became insulin sensitive because of alterations in ATM accumulation. Indeed, our results demonstrate that RvD1 significantly reduces (50–60%) the formation of CLSs, which are rich in F4/80+ CD11c+ macrophages, and this was associated with increased insulin sensitivity in adipose tissue. Of importance, the percentage of ATMs expressing the M2 marker MGL-1 was significantly increased in animals receiving RvD1 treatment. It is important to note that the overall ATM content, as assessed using pan-macrophage marker F4/80, was not changed with RvD1 treatment, indicating that only CLS-localized macrophages were reduced in RvD1-treated animals. This observation is consistent with other studies showing that reversion from a high-fat diet to a normal-chow diet decreases inflammatory ATMs and protects against insulin resistance but does not decrease total ATM content (30). Although our results show that RvD1 treatment affects ATMs, we cannot rule out that RvD1 might also affect other SVC populations, such as Th1 and γδ T cells, which have recently been documented to potentiate adipose tissue inflammation (31, 32). Additional studies are needed to elucidate whether these populations are also modulated by resolvins as well.
One prominent established mechanism whereby M1 ATMs contribute to adipose tissue inflammation and systemic insulin resistance is that this macrophage population secretes proinflammatory mediators that suppress the production of the insulin-sensitizing adipokine APN. Indeed, circulating levels of APN negatively correlate with insulin resistance in humans, while mice deficient in APN develop insulin resistance (26, 33). Both TNF-α and IL-6 directly suppress APN production in adipocytes, and high levels of these proinflammatory mediators are associated with obesity-induced insulin resistance (26, 34). Of note, one of the prominent affects of ω-3 fatty acids in the context of obesity is that they increase circulating APN (18, 19). Accordingly, in the studies reported here, we found that RvD1-treated animals have significantly higher plasma APN levels. These results are similar to the previously reported actions of DHA-derived protectin D1, which acutely increases APN transcripts in isolated adipose tissue explants (18). Notably, transcripts of IL-6 were also significantly decreased in adipose tissue of RvD1-treated mice, which could potentially explain the increase in APN in vivo. These results are consistent with the reduction in CLS-localized ATMs, which are a rich source of IL-6 (16), and in line with the well documented role of RvD1 in regulating proinflammatory cytokine generation both in vivo and in isolated macrophages (6, 35, 36). Of interest, IL-10 expression was not increased with RvD1 treatment, in contrast to other proresolving lipid mediators, such as lipoxin A4, which stimulates IL-10 production in macrophages (37). However, it should be pointed out that APN alone has been shown to regulate macrophage phenotype and blunt proinflammatory cytokine generation in macrophages independent of IL-10 (33, 38). Notably, our studies also demonstrate that the levels of activated AMPK were increased in the adipose tissue of RvD1-treated mice, although with delayed kinetics when compared with the increase in APN. This is interesting in light of the fact that APN drives an anti-inflammatory phenotype in macrophages through the activation of AMPK and that AMPK is a central regulator of macrophage phenotype (33, 39). Hence, up-regulation of APN leading to AMPK activation may be one mechanism underlying the insulin-sensitizing and anti-inflammatory effects of RvD1.
It is important to note that while adipose tissue inflammation and insulin resistance were decreased, insulin signaling in the skeletal muscle and liver did not seem to be affected by short-term RvD1 treatment. Although we cannot exclude other tissue-specific actions of RvD1, these results suggest that the adipose tissue may be an important target of exogenously administered RvD1. Moreover, it is well documented that inflammation and insulin resistance in the adipose tissue drives insulin resistance in both the liver and skeletal muscle (40, 41). Thus, ameliorating adipose tissue insulin resistance may delay the development of systemic insulin resistance sustained by insulin resistance in the skeletal muscle and liver.
Because the biological actions of RvD1 are mediated by specific binding to GPCRs, FPR2 (ALX), and GPR32 (3), we examined whether the receptors for RvD1 were expressed in adipose tissue. We sought to identify whether the closely related murine homologue of human FPR2 is expressed in adipose tissue. We found that Fpr2 was expressed in both adipose tissue SVCs and isolated adipocytes. Of interest, Fpr2 was also expressed in peritoneal macrophages isolated from db/db mice. No changes in PPARγ mRNA were evident in adipose tissue of RvD1-treated mice, consistent with previous reports showing that RvD1 is not a direct agonist for PPARγ (3). Of importance, the endogenous anti-inflammatory role of murine Fpr2 was recently demonstrated in Fpr2−/− mice, and murine Fpr2 also binds other endogenous anti-inflammatory mediators, such as lipoxin A4 and annexin I (42).
In summary, the findings of this study demonstrate the potent actions of RvD1 in improving obesity-induced insulin resistance in vivo. Our results indicate that local production of bioactive lipid mediators may underlie some of the beneficial actions of ω-3 fatty acids in animal models of obesity and diabetes and suggest that stimulating resolution of inflammation with proresolving lipid mediators may represent a novel pharmacologic strategy for controlling macrophage-mediated inflammation in obese individuals with T2D.
Acknowledgments
This work was supported by the U.S. National Institutes of Health-sponsored Diabetes and Obesity Center (P20RR024489).
REFERENCES
- 1. Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O'Neill L. A., Perretti M., Rossi A. G., Wallace J. L. (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C. H., Yang R., Petasis N. A., Serhan C. N. (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. U. S. A. 107, 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., Flower R. J., Perretti M., Serhan C. N. (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nathan C., Ding A. (2010) Nonresolving inflammation. Cell 140, 871–882 [DOI] [PubMed] [Google Scholar]
- 6. Merched A. J., Ko K., Gotlinger K. H., Serhan C. N., Chan L. (2008) Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241 [DOI] [PubMed] [Google Scholar]
- 8. Spiegelman B. M., Flier J. S. (2001) Obesity and the regulation of energy balance. Cell 104, 531–543 [DOI] [PubMed] [Google Scholar]
- 9. Kern P. A., Ranganathan S., Li C., Wood L., Ranganathan G. (2001) Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 280, E745–E751 [DOI] [PubMed] [Google Scholar]
- 10. Arkan M. C., Hevener A. L., Greten F. R., Maeda S., Li Z. W., Long J. M., Wynshaw-Boris A., Poli G., Olefsky J., Karin M. (2005) IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 [DOI] [PubMed] [Google Scholar]
- 11. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lumeng C. N., Deyoung S. M., Bodzin J. L., Saltiel A. R. (2007) Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23 [DOI] [PubMed] [Google Scholar]
- 13. Apovian C. M., Bigornia S., Mott M., Meyers M. R., Ulloor J., Gagua M., McDonnell M., Hess D., Joseph L., Gokce N. (2008) Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler. Thromb. Vasc. Biol. 28, 1654–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W., Jr. (2006) CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lumeng C. N., DelProposto J. B., Westcott D. J., Saltiel A. R. (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A. S., Obin M. S. (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 [DOI] [PubMed] [Google Scholar]
- 18. González-Périz A., Horrillo R., Ferre N., Gronert K., Dong B., Moran-Salvador E., Titos E., Martinez-Clemente M., Lopez-Parra M., Arroyo V., Clària J. (2009) Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 23, 1946–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White P. J., Arita M., Taguchi R., Kang J. X., Marette A. (2010) Transgenic restoration of long chain ω-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high fat-fed mice. Diabetes 59, 3066–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 [DOI] [PubMed] [Google Scholar]
- 21. Leedom A. J., Sullivan A. B., Dong B., Lau D., Gronert K. (2010) Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 176, 74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 23. Shao J., Yamashita H., Qiao L., Friedman J. E. (2000) Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J. Endocrinol. 167, 107–115 [DOI] [PubMed] [Google Scholar]
- 24. Lumeng C. N., Deyoung S. M., Saltiel A. R. (2007) Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am. J. Physiol. Endocrinol. Metab. 292, E166–E174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao J. L., Chen H., Filie J. D., Kozak C. A., Murphy P. M. (1998) Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics 51, 270–276 [DOI] [PubMed] [Google Scholar]
- 26. Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fedor D., Kelley D. S. (2009) Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 12, 138–146 [DOI] [PubMed] [Google Scholar]
- 28. Spite M., Serhan C. N. (2010) Novel lipid mediators promote resolution of acute inflammation: Impact of aspirin and statins. Circ. Res. 107, 1170–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olefsky J. M., Glass C. K. (2010) Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 [DOI] [PubMed] [Google Scholar]
- 30. Li P., Lu M., Nguyen M. T., Bae E. J., Chapman J., Feng D., Hawkins M., Pessin J. E., Sears D. D., Nguyen A. K., Amidi A., Watkins S. M., Nguyen U., Olefsky J. M. (2010) Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 285, 15333–15345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rocha V. Z., Folco E. J., Sukhova G., Shimizu K., Gotsman I., Vernon A. H., Libby P. (2008) Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ. Res. 103, 467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zuniga L. A., Shen W. J., Joyce-Shaikh B., Pyatnova E. A., Richards A. G., Thom C., Andrade S. M., Cua D. J., Kraemer F. B., Butcher E. C. (2010) IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 185, 6947–6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohashi K., Parker J. L., Ouchi N., Higuchi A., Vita J. A., Gokce N., Pedersen A. A., Kalthoff C., Tullin S., Sams A., Summer R., Walsh K. (2010) Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 285, 6153–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kristiansen O. P., Mandrup-Poulsen T. (2005) Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 54(Suppl. 2), S114–S124 [DOI] [PubMed] [Google Scholar]
- 35. Schif-Zuck S., Gross N., Assi S., Rostoker R., Serhan C. N., Ariel A. (2010) Saturated-efferocytosis generates pro-resolving CD11b(low) macrophages: Modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun Y. P., Oh S. F., Uddin J., Yang R., Gotlinger K., Campbell E., Colgan S. P., Petasis N. A., Serhan C. N. (2007) Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 282, 9323–9334 [DOI] [PubMed] [Google Scholar]
- 37. Schwab J. M., Chiang N., Arita M., Serhan C. N. (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Folco E. J., Rocha V. Z., Lopez-Ilasaca M., Libby P. (2009) Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J. Biol. Chem. 284, 25569–25575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sag D., Carling D., Stout R. D., Suttles J. (2008) Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 181, 8633–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosen E. D., Spiegelman B. M. (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guilherme A., Virbasius J. V., Puri V., Czech M. P. (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell. Biol. 9, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dufton N., Hannon R., Brancaleone V., Dalli J., Patel H. B., Gray M., D'Acquisto F., Buckingham J. C., Perretti M., Flower R. J. (2010) Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 184, 2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]