Abstract
Airway surface liquid (ASL) volume depletion and mucus accumulation occur in cystic fibrosis (CF). The ASL comprises a superficial mucus layer (ML) overlying a periciliary fluid layer (PCL) that contacts surface epithelial cells. We measured viscosity of the ML and PCL from the diffusion of FITC-dextran dissolved in the ASL of unperturbed, well-differentiated primary cultures of human bronchial epithelia grown at an air-liquid interface. Diffusion was measured by fluorescence recovery after photobleaching, using a perfluorocarbon immersion lens and confocal fluorescence detection. Bleaching of an in-plane 6-μm-wide region was done in which diffusion coefficients were computed using solution standards of specified viscosity and finite-element computations of 2-layer dye diffusion in 3 dimensions. We found remarkably elevated viscosity in both ML and PCL of CF vs. non-CF bronchial epithelial cell cultures. Relative viscosities (with saline=1) were in the range 7–10 in the non-CF ML and PCL, and 25–30 in both ML and PCL in CF, and greatly reduced by amiloride treatment or mucin washout. These data indicate that the CF airway surface epithelium, even without hyperviscous secretions from submucosal glands, produces an intrinsically hyperviscous PCL and ML, which likely contributes to CF lung disease by impairment of mucociliary clearance. Our results challenge the view that the PCL is a relatively watery, nonviscous fluid layer in contact with a more viscous ML, and offer an explanation for CF lung disease in the gland-free lower airways.—Derichs, N., Jin, B. -J., Song, Y., Finkbeiner, W. E., Verkman, A. S. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching.
Keywords: CFTR, mucins, diffusion, FRAP
The airway surface liquid (ASL) is the fluid layer covering the airway surface at the interface between surface epithelial cells and the air space. ASL depth, composition, and viscosity are important determinants of mucociliary clearance, bacterial killing, and epithelial and immune cell functions (1, 2). Abnormalities in ASL depth and composition are thought to contribute to lung disease pathogenesis in cystic fibrosis (CF; reviewed in refs. 2, 3). Measurements in well-differentiated primary cultures of human airway epithelia show reduced ASL volume (depth) in cultures from CF vs. control (non-CF) human subjects, possibly a consequence of defective CFTR Cl− channel function and/or ENaC Na+ channel hyperactivity (4–7). Though early studies suggested that the non-CF ASL is hypotonic compared to that in CF, subsequent direct measurements indicated near-isotonic fluid composition in both the non-CF and CF ASL (4, 8). Defining ASL abnormalities in CF is important in understanding the pathogenesis of CF lung disease and in establishing surrogate markers for evaluation of ion channel-targeted CF therapies (9, 10).
The ASL comprises two layers: a mucus layer (ML) contacting the air, which overlies a periciliary layer (PCL) that bathes surface cilia and contacts the surface of epithelial cells lining the airways. The ML and PCL contain a complex mixture of soluble and bound mucins thought to increase viscosity and hence impair mucociliary clearance. Fluid secretion from surface epithelial cells and airway submucosal glands generate ASL. The surface epithelial cells can absorb fluid as well, which is thought to regulate ASL depth by a mechanism involving proteases and purinergic signaling (2). Fluid secretion by airway submucosal glands is impaired in CF, with secretion of a reduced volume of a hyperviscous and hyperacidic fluid (11–15). We reported that impaired gland fluid secretion is an intrinsic defect in CF submucosal glands, with severalfold increased viscosity and reduced pH in gland secretions from nasal biopsies of pediatric CF subjects with minimal CF lung disease (14, 15). It is not known, however, whether the increased viscosity in gland fluid secretions translates to increased ASL viscosity, or whether the airway epithelial cell surface in CF can itself generate a hyperviscous ASL, which might be important in the pathogenesis of CF lung disease in the distal airways that do not contain submucosal glands. It has been assumed, without direct evidence, that the PCL is a relatively nonviscous fluid layer compared to the ML, though more recent data showing the presence of soluble and tethered mucins in the PCL (see Discussion) suggest that the PCL might be more viscous than originally assumed. A major consequence of increased ASL viscosity is impairment of mucociliary clearance by increasing mechanical resistance to ciliary motion (16, 17).
Here, we measured the viscosity of the ASL from the diffusion of a fluorescent dextran by fluorescence recovery after photobleaching. Measurement of depth-resolved viscosity was accomplished by confocal fluorescence detection, together with solution standards and mathematical modeling. Measurements were made in well-differentiated primary cultures of non-CF and CF human bronchial epithelia. Our measurements address the questions of whether the PCL is a watery fluid; ML and PCL viscosity differ; ML and PCL viscosity are increased in CF; and ASL depth-enhancing agents (amiloride, cAMP agonists) reduce ASL viscosity.
MATERIALS AND METHODS
Primary bronchial epithelial cell culture
Primary cultures of human non-CF and CF bronchial epithelial cells were grown at an air-liquid interface, as described previously (18). Cells were plated at a density of 5 × 105/cm2 onto 12-mm-diameter, 0.4-μm-pore polycarbonate cell culture inserts (Snapwell; Corning, Lowell, MA, USA) precoated with human placental collagen (15 μg/cm2; Sigma, St. Louis, MO, USA). Cultures were grown at an air-liquid interface in ALI medium at 37°C, 5% CO2/95% air, to develop functionally differentiated cell sheets. ALI medium consists of 1:1 LHC medium:Dulbecco's modified Eagles' medium with BSA, 0.5 mg/ml; bovine pituitary extract, 10 μg/ml; insulin, 0.87 μM; transferrin, 0.125 μM; hydrocortisone, 0.1 μM; triiodothyronine, 0.01 μM; epinephrine, 2.7 μM; epidermal growth factor, 0.5 ng/ml; retinoic acid, 50 nM; phosphorylethanolamine, 0.5 μM; ethanolamine, 0.5 μM; ZnSO4, 3.0 μM, FeSO4 1.5 μM; MgCl2, 0.11 mM; CaCl2, 1.0 μM; SeCl2, 3 μM; MnCl2, 0.1 μM; SiCl4, 50 μM; MoCl2, 0.1 μM; VCl4, 0.5 μM; NiSO4, 0.1 mM; SnCl4, 0.5 μM; penicillin G 100 U/ml; and streptomycin, 100 μg/ml. Medium was changed every 2–3 d. Cultures were used 21–30 d after plating, when transepithelial resistance was 400–1000 Ω/cm2 and an ASL film was seen. Cultures from 5 different non-CF and 4 different CF subjects were used for studies here.
Perfusion chamber for ASL confocal microscopy
As described previously (5), custom-built stainless steel perfusion chambers were used to perfuse the serosal surface of cell cultures in which the fluorescently stained ASL at the apical (mucosal) surface is observed from the top using an objective lens. The chambers were designed to fit 12-mm-diameter polycarbonate cell culture inserts, with a bottom component for basolateral perfusion and a top component to accommodate the cell culture insert for observation using an immersion objective lens. The perfusion chambers containing cell culture inserts were maintained in a cell culture incubator or a 37°C microincubator (Harvard Apparatus, Holliston, MA, USA) on the microscope stage.
ASL depth measurement by z-scanning confocal microscopy
ASL depth measurements were done in unperturbed cell cultures (without prior washing) and in washed cultures in which an ASL was allowed to form by 12 h incubation in a cell culture incubator. The ASL was stained with FITC-dextran (70 kDa; Sigma) by depositing a small amount of solid (powder) dye (<2 μg) on the culture mucosal surface 12 h before initial measurements. For measurements, the culture inserts were covered with 150 μl high-boiling-point perfluorocarbon FC-70 (3M, St. Paul, MN, USA) to prevent evaporation. ASL depth was measured by laser z-scanning confocal microscopy as described (5), using a Nikon C1 confocal microscope (Nikon, Tokyo, Japan) equipped with an ×40 water-immersion objective (numerical aperture 0.8). ASL depth was determined from z-image stacks as reported previously (5). Between measurements, the perfusion chamber was maintained at 37°C in an incubator. In some studies, cell cultures were incubated at 37°C for 2 h with amiloride (100 μM) or forskolin (10 μM) in the basolateral perfusate.
ASL viscosity measurement by fluorescence recovery after photobleaching
Photobleaching was done just after ASL depth measurement without changing the optical configuration. Photobleaching of an in-plane (x, y) 6- × 18-μm rectangular region was done in which relative diffusion was determined using solution standards of specified viscosity and finite-element computations of 2-layer dye diffusion in 3 dimensions (see below). Photobleaching was accomplished by increasing laser intensity by 2000- to 4000-fold for 400 ms to reduce fluorescence by 25–35% in the rectangular bleach zone. Generally, 4–5 recovery curves from different locations in each filter were obtained and averaged. In ∼10% of recovery measurements (mostly in CF cultures), little fluorescence recovery occurred (<10%), which might be a consequence of incompletely dissolved dye or very thick mucus. These data were not included in the average. Serial confocal images (z resolution ∼2 μm) prior to and after photobleaching were acquired at 2.5 Hz at z focal planes (in separate measurements) in the fluorescently stained PCL (+4 μm above cell surface) and ML (+12 μm above cell surface and 4 μm below the upper surface of the ML). For each set of studies, control (no-bleach) measurements were done to ensure insignificant photobleaching by the probe beam (<1% total). For determination of relative viscosities, photobleaching was done on thin, 20-μm layers of solution standards between glass coverslips (PBS containing 0, 20, 38, and 46% sucrose; relative viscosity of 1, 1.79, 4.13, and 8.14, respectively, as measured by time-flow viscometry). For computation of relative viscosity in the ASL and solution standards, quarter- and half-times (t1/4, t1/2) were determined by regression analysis from fluorescence recovery curves, as described previously (19). Statistical differences among groups were determined using ANOVA.
Finite-element diffusion computations
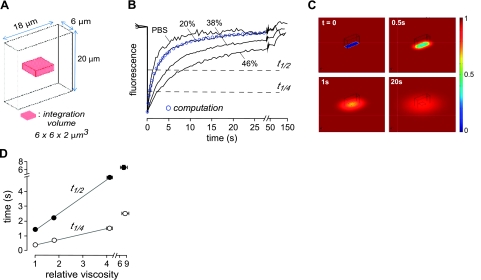
Photobleaching experiments were simulated by solution of the diffusion equation: ∂c/∂t = ∇ · (D ∇c), where D is diffusion coefficient and c is relative concentration. Computations were done by the finite-element method using the 3-dimensional diffusion model in Comsol Multiphysics (Comsol, Inc., Palo Alto, CA, USA). For computation of 1-layer diffusion (as done in validation studies in Fig. 1), diffusion, bleach, and integration volume (see Fig. 1A, top) were chosen to simulate experiments. The entire computation domain size was 0.4 × 0.4 × 0.02 mm, with rectangular bleach volume 6 × 18 × 20 μm, and integration volume 6 × 6 × 2 μm. The initial (postbleach) conditions were c = 0 in the bleach volume and c = 1 elsewhere. Boundary conditions included c = 1 at outer boundary, continuity at the interior boundary, and insulation/symmetry at the bottom and top surfaces.
Figure 1.
Diffusion measured by fluorescence recovery after photobleaching with confocal detection. A) Bleach geometry (rectangular volume 6×18×20 μm) and signal integration volume for 1-layer diffusion. B) Photobleaching recovery for 20-μm-thick layers of PBS containing 2 mg/ml FITC-dextran (70 kDa) with indicted sucrose to increase viscosity. Bleach depth was 25–35% (shown normalized for comparison). Fluorescence was detected in a z plane at the center of the 20-μm-thick solution layer and represents integrated signal in a detection volume of 6 μm × 6 μm × 2 μm. Blue circles represent theoretical fluorescence recovery kinetics for the 20% sucrose solution computed using the finite-element method (number of elements, 272667; relative tolerance, 10−6). C) Pseudocolored concentration profiles of relative concentration at t = 0, 0.5, 1, 20 s for diffusion coefficient 4 × 10−8 cm2/s. D) Fitted t1/4 and t1/2 for photobleaching measurements as in B (means ± se, n=5).
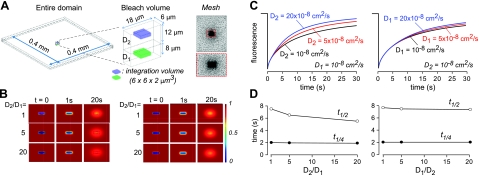
For computations of 2-layer diffusion with a lower layer of 8 μm depth with diffusion coefficient D1 in contact with an upper layer of 12 μm depth with diffusion coefficient D2 (see Fig. 3A), bleach volume was 6 × 18 × 20 μm. Integration volume was 6 × 6 × 2 μm, centered in the lower layer (at z=4 μm) or upper layer (at z=16 μm). The initial and boundary conditions were the same as for the 1-layer diffusion model, with an additional continuity boundary condition at the interface between lower and upper layers. A variable-density computation mesh ∼3 × 105 tetrahedral elements was used (see Fig. 3A for projected mesh in the x–y plane). Computations were carried out using a 2.8 GHz Intel Core CPU with 64-bit operating system and 8 GB memory. Simulation time steps were determined automatically in the Comsol Multiphysics software based on mesh size.
Figure 3.
Finite-element computations of 2-layer diffusion. A) Schematic of bleach geometry for 2-layer diffusion, showing entire computation domain (left panel), 6- × 18- × 20-μm bleach volume and signal integration volume (middle panel), and projected mesh used for computation (right panel), with expanded region in red dashed box. B) Pseudocolored concentration profiles showing relative concentration in bleached rectangular region at t = 0, 1, 20 s in the lower layer (left panel) and upper layer (right panel) for diffusion coefficients: D1 = 10−8 cm2/s, with D2 = 10−8, 5 × 10−8, 20 × 10−8 cm2/s (left panel); and D2 = 10−8 cm2/s, with D1 = 10−8, 5 × 10−8, 20 × 10−8 cm2/s (right panel). C) Computed recovery curves: concentration time course in integration volume in the lower layer (z=4 μm; left panel) and upper layer (z=16 μm; right panel), with indicated D1 and D2. D) Times for t1/4 and t1/2 recovery for simulations in C.
RESULTS
Figure 1A shows the bleach geometry used for photobleaching measurements of FITC diffusion in solution standards. A rectangular bleach volume of in-plane dimensions 6 × 18 μm was chosen in order to determine diffusion coefficients accurately for 2-layer (ML and PCL) diffusion (see below). Measurements were done using a 400-ms bleach time, 2.5-Hz sampling frequency, and ×40 water-immersion lens. The 6- × 18-μm rectangular bleach area was chosen to have smaller width (6 μm) than ASL depth to minimize corrections for analysis of 2-layer diffusion data and to give pseudo-1-dimensional recovery kinetics, which are substantially slower than recovery for a circular bleach area of the same width.
Recovery measurements were made for 20-μm-thick layers of FITC-dextran in saline containing different percentages of sucrose to increase solution viscosity (Fig. 1B). The plotted fluorescence is the integrated signal over a rectangular box of in-plane dimensions 6 × 6 μm centered in bleached area (to achieve quasi-1-dimensional recovery kinetics) with the confocal measurement z plane at the center of the 20-μm-thick fluid layer. Fluorescence recovery was slowed with increasing solution viscosity, as expected, with complete recovery at long times. The blue circles overlying the recovery curve for the 20% sucrose solution were computed by numerical solution of the 1-layer diffusion problem in three dimensions (see Materials and Methods). The theoretical points agreed well with the measured fluorescence recovery kinetics. Figure 1C shows diffusion into the rectangular bleach volume at different times after bleaching, which for computational purposes is equivalent to an initial condition of zero particle concentration within the rectangular bleach area. For subsequent analysis of ASL photobleaching data, fluorescence recovery t1/2 (time at 50% fluorescence recovery) and t1/4 (time at 25% recovery) were deduced from recovery curves. As expected for a linear diffusive process, as applies here, t1/2 and t1/4 each increased linearly with solution viscosity (Fig. 1D), allowing determination of relative viscosity from t1/2 or t1/4 measured in ASL.
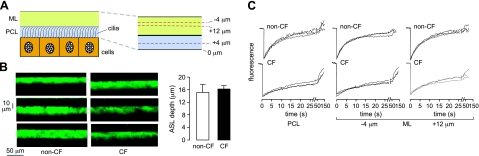
Diffusion measurements were made in unperturbed, well-differentiated primary cultures of human bronchial epithelial cells grown at an air-liquid interface. FITC-dextran was added (in powder form) 12 h before measurements to uniformly stain the ASL. For photobleaching measurements, cell cultures were mounted in a temperature-regulated (37°C) perfusion chamber in which the ASL was covered with a high-boiling-point perfluorocarbon having nearly the same refractive index as that of water to allow measurements using a water-immersion lens in a upright microscope configuration. Figure 2A shows a schematic of the epithelial cell layer, with the PCL and ML layers of ASL indicated (left panel), and the PCL and ML of thicknesses d1 and d2, respectively, and the z location for confocal detection of fluorescence recovery in the PCL at 4 μm above the cell surface and at 2 locations in the ML, at 4 μm below the ASL surface and at 12 μm above the cell surface (right panel). Two locations in the ML were chosen to ensure robust determination of ML viscosity because the thickness of the ML varied from culture to culture.
Figure 2.
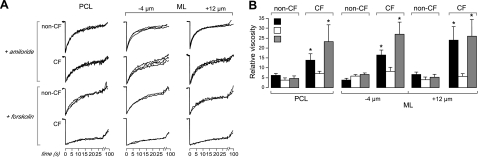
FITC-dextran diffusion in the ASL in unpertubed, well-differentiated primary cultures of human bronchial epithelial cells growth at an air-liquid interface. A) Schematic of PCL and ML overlying an epithelial cell monolayer (left panel), with z locations indicated for confocal detection of fluorescence recovery in the PCL and ML (right panel). B) ASL depth determined by z-scanning confocal microscopy: representative ASL fluorescence profiles for non-CF and CF cultures (left panel), with summary of averaged ASL depths (right panel; means ± se, n=10 cultures). C) Representative fluorescence recovery curves for 70-kDa FITC-dextran in non-CF and CF cell cultures made at indicated locations (3 curves from different cultures for each condition).
Figure 2B shows measurements of ASL depth in unperturbed non-CF and CF cell cultures. ASL depth was measured by z-scanning confocal microscopy. Representative fluorescence z-profiles are shown (Fig. 2B, left panel), and averaged ASL depths for 10 cultures are summarized (Fig. 2B, right panel). ASL depths for non-CF and CF cultures were comparable and greater than those of 5–8 μm for cultures that have been rinsed to remove the ML, as reported by our laboratory (5) and others (6). ASL depths measured here are in agreement with those measured on unperturbed cultures by surface laser reflectance microscopy (7). Figure 2C shows representative fluorescence recovery curves for FITC diffusion (3 curves from different cultures for each condition), with indicated z locations for the detection regions in the PCL or ML. Recovery rates were grossly slower in CF vs. non-CF cultures, in both PCL and ML. Fluorescence recovered nearly completely at long times in each case, as expected.
Finite-element computations of diffusion in the 2-layer geometry were done in order to deduce diffusion coefficients from fluorescence recovery data. Figure 3A shows the rectangular bleach geometry, which was used as the initial condition in solving the 2-layer diffusion problem. Figure 3B shows examples of fluorophore diffusion into the bleach region. Computations were done at constant D1 with different D2 values (Fig. 3B, left panel), and constant D2 with different D1 values (Fig. 3B, right panel). The issue addressed by these computations is the effect of rapid diffusion in the layer opposite to that being measured (“trans” layer). For example, very rapid diffusion in the PCL could accelerate fluorescence recovery measured in the ML, and hence reduce apparent relative viscosity, because diffusion occurs in 3 dimensions. As shown in Figs. 3C, D, as expected, increased recovery was found as a consequence of rapid trans-layer diffusion; however, the effect was minor, particularly at early time points, as expected, with t1/4 virtually insensitive to a 20-fold increase in trans-layer diffusion. We conclude that correction for trans-layer diffusion is not needed for the measurements here.
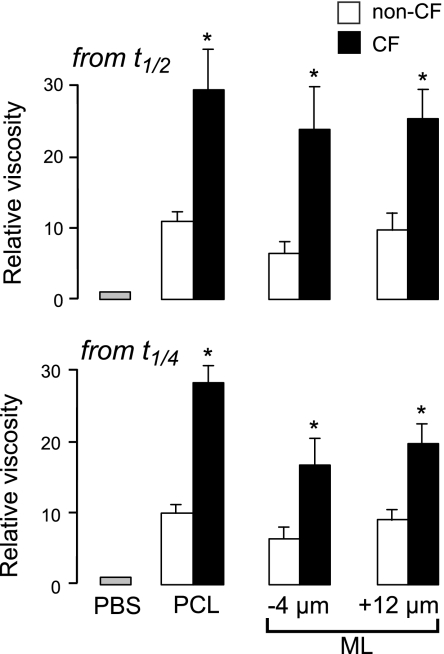
Figure 4 summarizes relative viscosities in the ASL deduced from analysis of t1/2 and t1/4 values. ASL viscosities were substantially greater than unity (the relative viscosity of PBS) and much greater in CF than in non-CF cultures for both ML and PCL. Relative viscosities were comparable as deduced from analysis of t1/2 or t1/4 values, supporting the robustness of the conclusions.
Figure 4.
ASL viscosity in the PCL and ML of non-CF and CF bronchial cell cultures. Summary of relative ASL viscosities at indicated z locations from data as in Fig. 2C, as deduced from fitted t1/2 (top panel) and t1/4 (bottom panel) values (means ± se; n=6–10 cultures/condition from 4 different non-CF and 3 different CF subjects). *P < 0.01 vs. non-CF.
Additional measurements of ASL relative viscosity were done following ENaC inhibition by amiloride and CFTR activation by forskolin. Figure 5A shows representative data for each condition. Comparing with recovery curves for control conditions (no compound added) in Fig. 2C, amiloride remarkably accelerated fluorescence recovery in the CF cultures in ML and PCL, whereas forskolin had little effect. The data summary in Fig. 5B shows the greatly reduced relative viscosity of ML and PCL following amiloride but no significant effect of forskolin.
Figure 5.
Effects of ENaC inhibition and CFTR activation on ASL viscosity. A) Representative fluorescence recovery curves (3 cultures/condition) for amiloride-treated (top panel) and forskolin-treated (bottom panel) non-CF and CF cultures, at indicated z-detection locations. B) Summary of relative ASL viscosities at indicated z locations as deduced from fitted t1/4 values (means ± se; n=4–6 cultures/condition from 2–3 different non-CF and CF subjects). *P < 0.01 vs. corresponding non-CF.
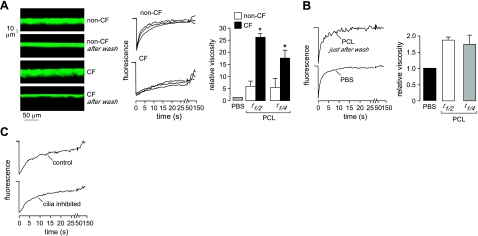
The experiments above were done in unperturbed, native cultures in which a relatively thick ASL formed over several weeks in culture. For comparison, we measured ASL relative viscosity in cultures in which the ASL was rinsed with saline 12 h prior to measurements, allowing reformation of a relatively thin ASL (Fig. 6A, left panel). Average depths in the rinsed cultures were 8.3 ± 0.5 and 6.2 ± 0.3 μm in the non-CF and CF cultures (means ± se, n=6), respectively, in agreement with prior studies (5). Photobleaching measurements made in the PCL (4 μm above the cell surface) showed greatly slowed fluorescence recovery in the CF cultures (Fig. 6A, middle panel) and corresponding increased relative viscosity (Fig. 6A, right panel). These data are similar to relative viscosities measured in unperturbed cultures, suggesting little influence of the mucus layer on relative viscosity in the PCL.
Figure 6.
Effects of the mucus layer and cilia on relative viscosity measured in the PCL. A) Left panel: ASL depth profiles before and 12 h after washing to remove the ML. Middle panel: representative fluorescence recovery curves (3 curves/condition) for washed non-CF and CF cultures. Right panel: summary of ASL relative viscosities in the PCL as deduced from fitted t1/2 and t1/4 values (means ± se; n=6 cultures from 2 different non-CF and CF subjects). *P < 0.01 vs. non-CF. B) Recovery curves (left panel) and deduced relative viscosities (right panel) for measurements made in the PCL just after washing in saline containing 5 mM DTT and 2 mg/ml FITC-dextran. C) Recovery curves in non-CF cultures under control conditions and after inhibition of ciliary beating by 10 min treatment with ouabain (100 μM) and reduced temperature (15°C).
Last, control studies were done to investigate whether the presence of cilia could increase PCL relative viscosity by crowding effects, and whether ciliary beating could reduce relative viscosity. Photobleaching measurements were done just after washing the ASL with saline containing 5 mM DTT to remove most soluble and bound macromolecules, including mucins. Figure 6B shows photobleaching measurements made in the PCL of washed cultures, giving a relative viscosity of <2. We conclude that the presence of cilia has little effect on diffusion measurements, in agreement with theoretical considerations about crowding effects on molecular diffusion (20). Finally, we found that inhibition of ciliary beating by reduced temperature and/or ouabain had little effect on fluorescence recovery kinetics (Fig. 6C), indicating little effect of ciliary beating on the measurements here.
DISCUSSION
Our data provide the first direct measurements of ASL viscosity. ASL viscosity was substantially greater than that of water or saline, which is largely due to the presence of macromolecules, such as mucins, secreted by the epithelium (21, 22). Remarkably, both ML and PCL viscosities were much greater than those of water or saline, and were increased in CF vs. non-CF bronchial cell cultures. This finding challenges the original view of the PCL as a watery, nonviscous fluid layer in contact with a highly viscous ML. The ability of CF airway surface epithelial cells alone to generate a hyperviscous ASL, without submucosal gland secretions, provides a potential explanation for the early and substantial CF lung disease in the submucosal gland-free distal airways.
Our approach to determine ASL viscosity in situ was the measurement of fluorescent dye diffusion by fluorescence recovery after photobleaching, with z-depth resolution conferred by confocal detection. The technical challenge in these measurements was the selection of fluorescent dye, bleach geometry, bleach time, and detection parameters for quantitative determination of dye diffusion in the ML and PCL. We chose an elongated, rectangular bleach geometry in order to slow fluorescence recovery kinetics, such that the width of the in-plane bleach region is smaller than ASL depth. Finite-element computations of 2-layer diffusion simulating the experimental conditions validated the quantitative determination of relative viscosity. Utilizing a confocal detection volume in the PCL and ML allowed determination of diffusion in the PCL and ML with minimal effect of the viscous properties of the ML and PCL. The potential alternative approach for z-depth-resolved measurement of dye diffusion is 2-photon photobleaching; however, appropriate dyes for 2-photon photobleaching in the ASL have not been identified, nor it is clear whether the power of the femtosecond pulsed excitation source would be adequate for efficient photobleaching using relatively low magnification perfluorocarbon immersion optics. FITC-dextran, as used here, has been used extensively in photobleaching studies in cells and extracellular fluids, including airway gland mucus, because it is noninteractive, easily bleached, and does not undergo significant triplet-state conversion under experimental conditions here (14, 23). The diffusion of FITC-dextran, which is inversely related to viscosity, provides a quantitative measure of zero-order (nondynamic) viscosity. Measurement of dynamic (kinematic) viscous properties, such as thixotropy, spinnability and adhesivity, in the ASL layers in situ would be very challenging.
Mucin glycoproteins (mucins) are probably in large part responsible for the substantial viscosity of the PCL in non-CF and CF cultures and for the ASL hyperviscosity in CF. We found greatly reduced PCL viscosity following nominal mucin washout. Mucins are high-molecular-mass (2–20×105 Da) glycoproteins containing large tandem repeat regions rich in serine and threonine residues (mucin domains) in which large glycan chains are attached via N-acetylgalactosamine (GalNAc). These glycosylated regions confer features to specific mucins, such as resistance to protease digestion, binding of microorganisms, and their unique rheological properties (24). Mucins account for about half of the weight of dialyzed, lyophilized mucus from healthy individuals. Eighteen mucin genes have been described (25). The gel-forming mucins include MUC2, MUC5AC, MUC5B, MUC6, and MUC19, each of which, except MUC6, is detected in airway secretory cells (26, 27). Airway surface goblet cells secrete MUC5AC, MUC5B, and, to a limited extent, MUC2; submucosal gland mucous cells secrete MUC5B and MUC19. Two nonpolymerizing, secreted mucins (MUC7 and MUC8) are also expressed in respiratory tissues. Submucosal gland serous cells secrete MUC7, which exhibits innate immune function but does not contribute rheological properties to the mucus gel (24).
MUC5AC and MUC5B account for ∼90% of the mucin content of sputum and may largely account for ML hyperviscosity in CF (28). The remaining mucins in human sputum consist of 2 membrane-tethered mucins, MUC1, and MUC4, along with MUC16. Functions attributed to MUC1 and MUC4 include hydration, lubrication, protection from proteases, pathogen defense, and cell signaling (29). MUC1 and MUC4 are largely adherent to the apical surface of airway epithelial cells within the PCL and thus might account for PCL hyperviscosity in CF along with MUC16, a very large secreted monomeric mucin (30) that also localizes in the PCL. Our results thus support the emerging view that the PCL contains mucins tethered to the cilia that form massive “brushes” and that mutual repulsion between negative charges on the tethered mucins creates a nearly frictionless interaction among cilia (29, 31).
Our results have implications regarding CF lung pathogenesis mechanisms and the evaluation of “corrector” drug therapies that are designed to normalize chloride and sodium transport in CF airway epithelium. An important and unanticipated finding was that the CF airway surface epithelium, even without hyperviscous secretions from submucosal glands, produces a hyperviscous ASL. Ion channel-targeted therapy for CF should thus correct both the airway surface epithelium and glandular epithelial cells. Finally, our results support the utility of ASL viscosity measurement in bronchial cell cultures as a new surrogate in vitro assay of the efficacy of CF drug therapy.
Acknowledgments
This work was supported by grants HL73856, DK72517, DK86125, DK35124, EB00415, and EY13574 from the U.S. National Institutes of Health, and a Research Program Development grant from the Cystic Fibrosis Foundation. N.D. was supported in part by Cystic Fibrosis Research Inc.
REFERENCES
- 1. Blouquit-Laye S., Chinet T. (2007) Ion and liquid transport across the bronchiolar epithelium. Respir. Physiol. Neurobiol. 159, 278–282 [DOI] [PubMed] [Google Scholar]
- 2. Boucher R. C. (2007) Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 58, 157–170 [DOI] [PubMed] [Google Scholar]
- 3. Verkman A. S., Song Y., Thiagarajah J. R. (2003) Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am. J. Physiol. Cell Physiol. 284, C2–C15 [DOI] [PubMed] [Google Scholar]
- 4. Jayaraman S., Song Y., Vetrivel L., Shankar L., Verkman A. S. (2001) Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J. Clin. Invest. 107, 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song Y., Namkung W., Nielson D. W., Lee J. W., Finkbeiner W. E., Verkman A. S. (2009) Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: dependence on Na+ and Cl– channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L1131–L1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tarran R., Grubb B. R., Gatzy J. T., Davis C. W., Boucher R. C. (2001) The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J. Gen. Physiol. 118, 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thiagarajah J. R., Song Y., Derichs N., Verkman A. S. (2010) Airway surface liquid depth imaged by surface laser reflectance microscopy. J. Gen. Physiol. 136, 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsui H., Grubb B. R., Tarran R., Randell S. H., Gatzy J. T., Davis C. W., Boucher R. C. (1998) Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 9. Amaral M. D., Kunzelmann K. (2007) Molecular targeting of CFTR as a therapeutic approach to cystic fibrosis. Trends Pharmacol. Sci. 28, 334–341 [DOI] [PubMed] [Google Scholar]
- 10. Anderson P. (2010) Emerging therapies in cystic fibrosis. Ther. Adv. Respir. Dis. 4, 177–185 [DOI] [PubMed] [Google Scholar]
- 11. Jayaraman S., Joo N. S., Reitz B., Wine J. J., Verkman A. S. (2001) Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc. Natl. Acad. Sci. U. S. A. 98, 8119–8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang C., Finkbeiner W. E., Widdicombe J. H., Miller S. S. (1997) Fluid transport across cultures of human tracheal glands is altered in cystic fibrosis. J. Physiol. 501, 637–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joo N. S., Wine J. J., Cuthbert A. W. (2009) Lubiprostone stimulates secretion from tracheal submucosal glands of sheep, pigs, and humans. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L811–L824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salinas D., Haggie P. M., Thiagarajah J. R., Song Y., Rosbe K., Finkbeiner W. E., Nielson D. W., Verkman A. S. (2005) Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J. 19, 431–433 [DOI] [PubMed] [Google Scholar]
- 15. Song Y., Salinas D., Nielson D. W., Verkman A. S. (2006) Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am. J. Physiol. Cell Physiol. 290, C741–C749 [DOI] [PubMed] [Google Scholar]
- 16. Donaldson S. H., Corcoran T. E., Laube B. L., Bennett W. D. (2007) Mucociliary clearance as an outcome measure for cystic fibrosis clinical research. Proc. Am. Thorac. Soc. 4, 399–405 [DOI] [PubMed] [Google Scholar]
- 17. Mall M. A. (2008) Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J. Aerosol. Med. Pulm. Drug Deliv. 21, 13–24 [DOI] [PubMed] [Google Scholar]
- 18. Levin M. H., Sullivan S., Nielson D., Yang B., Finkbeiner W. E., Verkman A. S. (2006) Hypertonic saline therapy in cystic fibrosis: evidence against the proposed mechanism involving aquaporins. J. Biol. Chem. 281, 25803–25812 [DOI] [PubMed] [Google Scholar]
- 19. Tajima M., Crane J. M., Verkman A. S. (2010) Aquaporin-4 (AQP4) associations and array dynamics probed by photobleaching and single-molecule analysis of green fluorescent protein-AQP4 chimeras. J. Biol. Chem. 285, 8163–8170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dix J. A., Verkman A. S. (2008) Crowding effects on diffusion in solutions and cells. Annu. Rev. Biophys. 37, 247–263 [DOI] [PubMed] [Google Scholar]
- 21. Gray T., Coakley R., Hirsh A., Thornton D., Kirkham S., Koo J. S., Burch L., Boucher R., Nettesheim P. (2004) Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L320–L330 [DOI] [PubMed] [Google Scholar]
- 22. Tarran R., Trout L., Donaldson S. H., Boucher R. C. (2006) Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J. Gen. Physiol. 127, 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Periasamy N., Verkman A. S. (1998) Analysis of fluorophore diffusion by continuous distributions of diffusion coefficients: application to photobleaching measurements of multicomponent and anomalous diffusion. Biophys. J. 75, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thornton D. J., Rousseau K., McGuckin M. A. (2008) Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 70, 459–486 [DOI] [PubMed] [Google Scholar]
- 25. Evans C. M., Koo J. S. (2009) Airway mucus: the good, the bad, the sticky. Pharmacol. Ther. 121, 332–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y., Zhao Y. H., Kalaslavadi T. B., Hamati E., Nehrke K., Le A. D., Ann D. K., Wu R. (2004) Genome-wide search and identification of a novel gel-forming mucin MUC19 in glandular tissues. Am. J. Respir. Cell Mol. Biol. 30, 155–165 [DOI] [PubMed] [Google Scholar]
- 27. Rose M. C., Brown C. F., Jacoby J. Z., Lynn W. S., Kaufman B. (1987) Biochemical properties of tracheobronchial mucins from cystic fibrosis and non-cystic fibrosis individuals. Pediatr. Res. 22, 545–551 [DOI] [PubMed] [Google Scholar]
- 28. Sheehan J. K., Kesimer M., Pickles R. (2006) Innate immunity and mucus structure and function. Novartis Found. Symp. 279, 155–166 [PubMed] [Google Scholar]
- 29. Hattrup C. L., Gendler S. J. (2008) Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 70, 431–457 [DOI] [PubMed] [Google Scholar]
- 30. Davies J. R., Kirkham S., Svitacheva N., Thornton D. J., Carlstedt I. (2007) MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int. J. Biochem. Cell Biol. 39, 1943–1954 [DOI] [PubMed] [Google Scholar]
- 31. Rubinstein M. (2010) Theoretical basis for the role of hydration in maintaining mucus transport. 24th North American CF Conference, S07-02 (symposium) [Google Scholar]