Abstract
Psoriasis is a chronic condition of the skin characterised by distinctive scaly plaques. The immune system is now thought to play a major role in the development and pathogenesis of psoriasis with immune cells and cytokines influencing keratinocyte function. Keratinocytes in turn, can activate and recruit immune cells leading to a positive feedback loop in disease. Natural Killer (NK) cells are lymphocytes that are best known for killing virally infected and cancer cells. However, evidence is emerging to support a role for NK cells in psoriasis. NK cells are found in the inflammatory infiltrate in psoriatic skin lesions. They can produce a range of inflammatory cytokines, many of which are important in the pathogenesis of psoriasis. Recent genetic studies have identified a range of potential molecules relating to NK cell biology that are known to be important in psoriasis. This paper will discuss the evidence, both cellular and genetic, for NK cell involvement in psoriasis.
1. Psoriasis as an Inflammatory Disease
Psoriasis is chronic inflammatory condition of the skin with significant morbidity, affecting approximately 2% of the Caucasian population. The most common form of the disease, responsible for up to 90% of cases, is psoriasis vulgaris [1] and this paper will primarily deal with this form. It is characterised by demarcated, red, raised, scaly plaques that typically manifest on the elbows, knees, and scalp [1, 2]. Psoriasis guttate occurs in about 10% of patients [3] and displays small, scattered plaques [2, 4]. This form may develop into psoriasis vulgaris [4]. Pustular psoriasis is an uncommon form of the disease consisting of raised pus-filled bumps and large areas of reddened skin [4]. A proportion of psoriasis patients will develop psoriatic arthritis (PsA), a debilitating joint disease [2–4].
Psoriatic skin is marked by increased proliferation of keratinocytes, the major cell of the outermost layer of skin, resulting in a thickening of the epidermis. Altered differentiation and rapid maturation of keratinocytes is observed, as is parakeratosis, a process whereby keratinocytes retain their nuclei as they rise into the stratum corneum. The granular layer of the epidermis is reduced or absent and downward projections of the epidermis, known as rete, become elongated. There is marked angiogenesis and infiltration of immune cells into the skin [1, 2].
The cause of psoriasis is still unknown although it is clear that there is a strong genetic component to the disease. Several immune genes have been associated with psoriasis with the major histocompatibility complex on chromosome 6 being strongly implicated [5, 6]. Outbreaks of psoriasis can occur at sites of physical trauma and streptococcal infections have been particularly linked to psoriasis guttate, perhaps indicating a role for molecular mimicry [4]. There is some evidence that psoriasis may be an autoimmune disease; it shares many characteristics with multiple sclerosis and diabetes mellitus type 1 [7, 8], but as yet no autoantigens or self-reactive T-cells have been identified [6, 9].
There are a host of treatments for psoriasis ranging from topical creams to systematic drugs and phototherapy. Many effective treatments act on the immune system with TNF-α and T-cells being the common targets [2]. As our understanding of the disease immunopathogenesis expands, new therapeutic strategies targeting the immune system are being developed. Recent drugs targeting the IL-12/IL-23 family of cytokines has indicated this as a promising new treatment pathway for psoriasis [1, 10] and illuminates the effectiveness of targeting the immune system for treatment of this disease.
For much of its history psoriasis was believed to be solely a disorder of the skin characterised by aberrant keratinocyte activity. However, with increased understanding of the disease, a fundamental role for the immune system in its pathogenesis and maintenance has been established. Evidence for immune involvement in the course of psoriasis arose from several sources. The presence of a large number of immune cells in psoriatic skin suggested that they play a part in the disease and the discovery that therapies targeting the immune system were effective for the treatment of psoriasis further highlighted its importance. The curing of psoriasis following bone marrow transplantation from a healthy donor to a psoriatic host, and the inverse observation of the development of psoriasis after transplantation of bone marrow from a psoriatic donor to a healthy host both indicated the strong part played by the immune system in this disease [1].
Although the field has moved away from the idea of psoriasis being a disease only involving the skin, it is clear that resident skin cells do play a substantial role. Keratinocytes are believed to play an important part in the recruitment and activation of immune cells. Keratinocytes are themselves capable of producing proinflammatory cytokines such as IL-1, IL-6, and TNF-α, as well as antimicrobial peptides and chemokines that can stimulate immune cell migration to the skin [11, 12]. Keratinocytes are also highly responsive to cytokines secreted from immune cells. These may induce the development of psoriatic features [2], trigger the expression of adhesion molecules for immune cells or drive further production of inflammatory cytokines from keratinocytes [5], thus contributing to maintenance of the disease state. Vascular endothelial cells in psoriatic skin also possess adhesion molecules such as ICAM-1, VCAM-1, and E-selectin [2, 5]. These molecules are usually found in the lymph nodes and allow the adherence of immune cells. Immune cells resident in the skin may also play an important part in the disease with some authors suggesting the local immune response may be sufficient for development of lesions [13].
The role of the innate immune system in psoriasis is increasingly seen as important. Neutrophils are found in the stratum corneum of psoriatic skin [2]. As these cells are shortlived (approx 3 days), their sustained presence suggests that they are continually recruited. Dendritic cells (DCs) are increased in psoriatic lesions and are believed to contribute to shaping the T-cell response [1, 2]. DC subsets not usually found in the skin are also observed. Plasmacytoid DCs are potent producers of IFN-α, which is thought to be a key cytokine in triggering lesion development, and myeloid DCs, with the ability to secrete TNF-α and inducible nitric oxide synthase, have been also been observed in psoriatic skin [2, 5, 13]. There are increased numbers of mature and activated DCs in psoriatic lesions [5] implying that these cells may be stimulating other aspects of the immune response.
Natural killer T (NKTs) cells are a subset of lymphocytes that bear the T-cell receptor along with classical NK cell molecules including CD56, CD16, CD161, and CD94 [14, 15]. They have a limited T-cell receptor repertoire and are activated following recognition of glycolipid presented by the MHC class-1-like molecule CD1d [11, 14]. Interestingly, keratinocytes express CD1d and its expression is upregulated in psoriatic skin [16, 17]. It has been shown that NKT cells cultured with keratinocytes were activated to produce large amounts of IFN-γ [16, 18]. While additional studies are required to clarify possible changes in NKT populations in psoriasis, there is evidence of their increased expression within psoriatic lesions [14]. Previous data on circulating NKT cells in psoriasis is conflicting, with some authors observing no difference [7], while others finding a reduction in their numbers in patients relative to controls with this number increasing following treatment [15]. Evidence from animal models of psoriasis also implies a role for NKT cells with some studies reporting the ability of these cells to induce psoriasis in xenografted mice [17–19].
Much of the work investigating the immunopathogenesis of psoriasis focuses on T lymphocytes and their importance in the disease is widely accepted. Both CD4+ and CD8+ T-cells have been found in lesional skin with CD4 cells primarily in the dermis and CD8 cells in the epidermis [4, 5, 9]. While initially it was believed that psoriasis was mediated by IFN-γ-producing type 1 T-cells, it is now thought that the recently characterised Th17 subset also has a significant role [1, 6]. This subset is noted for the production of IL-17 and IL-22, two cytokines which are present in psoriatic lesions, in response to IL-23 [20, 21]. The expression of activation markers by both CD4+ and CD8+ cells has been observed and most of the T-cells in psoriatic lesions also express the memory cell antigen, CD45RO [5]. Interestingly, data from variable T-cell receptor genes indicates the presence of clonal T-cell populations within psoriatic lesions. This implies the selective expansion of T-cells bearing receptors of the same antigen specificity. However, as yet no antigen has been identified as the trigger for psoriasis. It is postulated that the observed clonality of T-cells may be due to the presence of unidentified pathogen antigens, of a bacterial superantigen, or of host autoantigens possibly coming into play due to molecular mimicry [4, 11].
2. A Role of NK Cells in Psoriasis?
NK cells are lymphocytes that are generally considered to be part of the innate immune system. They are best known for their ability to kill virally infected and cancer cells; however, they also produce a range of cytokines including IFN-γ, TNF-α, and TGF-β. They can be defined phenotypically by the presence of particular surface antigens: either NKp46 or CD56+CD3−cells. While the role of NK cells in psoriasis still remains relatively unstudied, there is mounting evidence that these cells may contribute to disease. Ottaviani et al. [22] found that the inflammatory infiltrate into psoriatic skin consisted of 5–8% cells that expressed the NK cell phenotype of CD56+CD3−. Most of these were of the CD56bright subset of NK cells. Thought to represent more immature cells, CD56bright cells are less cytotoxic and more proficient at cytokine secretion compared to CD56dim NK cells. The cells present in the infiltrate found by Ottaviani et al. also expressed the activation antigen, CD69, and produced large quantities of IFN-γ in vitro in response to IL-2 stimulation. Supernatants from these IL2-stimulated NK cells induced activation of keratinocytes causing upregulation of MHC class I molecules and induction of the expression of ICAM1 and HLA-DR receptors. The keratinocytes were also observed to secrete chemokines that are known to attract NK cells (CXCL10, CCL5, and CCL20) thereby providing a mechanism of NK cell recruitment to the skin. Indeed, receptors for these chemokines were identified on NK cells found in psoriatic skin, with high levels of CXCR3 and CCR5 (receptors for CXCL10 and CCL5, resp) and moderate levels of CCR6 (CCL20 receptor) expressed [22]. In this study, there were ten patients examined; thus, the results need validation in other cohorts. In addition, the study examined cells in lesions but did not compare the findings to either uninvolved skin or normal healthy skin. Therefore, it is unclear as to whether the data represent the phenotype expected in normal skin or if it represents an altered phenotype associated with psoriasis. The three chemokine receptors noted in this study, CXCR3, CCR6, and CCR5 have also been recently identified as mediators of NK cell recruitment to the skin in allergic contact dermatitis [23]. In terms of NK chemotaxis to psoriatic skin, evidence has recently emerged for a role for chemerin which is found in psoriatic lesions and is known to attract cells expressing the receptor CMKLR1 [24]. The CD56dim subset has been noted to express this receptor [25] and migration of these cells in response to chemerin has been shown [24]. The relevance of this finding regarding NK cells in psoriasis remains to be explored.
In another study, Cameron et al. found increased frequencies of cells expressing CD16 or CD57 antigens in psoriatic skin lesions compared to either uninvolved skin or skin from normal healthy individuals [7]. These changes were found in both epidermal and papillary dermal skin layers. Neither of these markers is ideal for detecting NK cells that are more accurately defined by either NKp46 or a combination of CD56+CD3−. The authors also looked at cells expressing the NK cell receptors, CD94, and 2DL1, and found the frequency of cells expressing these elevated in the papillary dermis but not in other skin layers [7]. In this study, samples numbers were low (n = 10 patients and n = 4 normal controls) and would thus benefit from further investigations.
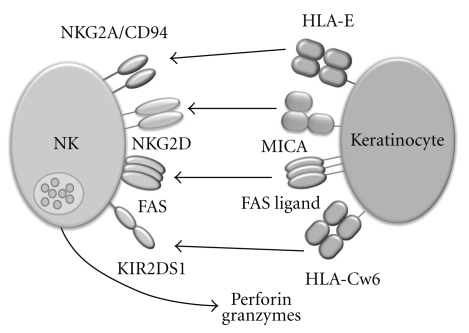
In addition to directly studying receptors expressed by NK cells, changes in NK cell ligands in psoriasis can provide evidence supporting a role for NK cells in the disease as alterations in ligand expression can directly affect NK cell function (Figure 1). HLA-G, a nonclassical class I molecule, is expressed in psoriatic skin lesions but not in healthy skin [26, 27]. HLA-G is recognised by 2DL4, a member the killer cell immunoglobulin-like receptors (KIRs) expressed by all NK cells, and LILRB1, which belongs to a related family of immunoglobulin-like receptors and also expressed by human NK cells. The leader sequence of the HLA-G protein may also be presented by the HLA-E molecule allowing recognition by another set of receptors found on NK cells, the CD94/NKG2 dimers [28]. LILRB1 has been found on T-cells in psoriatic skin lesions [26]; however, whether NK cells receptors for HLA-G are modulated in psoriasis has yet to be determined.
Figure 1.
NK cells can interact with keratinocytes through a range of cell surface receptors. NK cells express cell surface receptors that regulate their interactions with other cell types including keratinocytes. Among these receptors is the NKG2A/CD94 inhibitory receptor that recognises and binds to HLA-E on target cells. NK cells also express a number of activating receptors including NKG2D which recognises MICA/B stress antigen and the Fas receptor which can activate cytokine secretion by NK cells. The activating KIR receptor 2DS1 (and its inhibitory counterpart, 2DL1) binds the HLA-Cw6 molecule and HLA-Cw6 is the strongest genetic association known in psoriasis. There is evidence in the literature to suggest that these receptors play a role in psoriasis. Activated NK cells are triggered to release their cytotoxic granule contents which contain perforin and granzymes.
Although psoriasis is a skin condition, changes in peripheral blood NK cells have been reported. A decrease in circulating NK cells was found in patients with chronic psoriasis with lower frequency of cells expressing common NK cell markers CD56, CD16, CD94 and 2DL1 (CD158a) present. The extent of the decrease in circulating NK cell numbers did not correlate to the clinical severity of disease [8]. However, this decrease in peripheral blood NK cell frequency was not observed in a recent study in patients with new-onset psoriasis, with levels of 2B4, CD48, NKG2D, CD16, and CD56 unchanged between patients and healthy controls [29] or in a study focusing on NK cell chemotaxis to chemerin where percentages of CD56dimCD16+ and of CD56brightCD16− in the peripheral blood showed no difference between patients and controls [24]. Further to this, a study primarily investigating a role for NKT cells in psoriasis utilising multicolour CD56 and CD3 staining, reported no difference in the percentage of circulating NK cells in psoriasis compared to healthy controls [15]. Finally, preliminary data from our own lab also using multicolour staining to accurately define NK cells does not indicate a difference in NK cell numbers in the blood of patients compared to healthy controls (S. Dunphy, data not shown).
Phenotypic differences have been noted between NK cells in the blood of psoriasis patients compared to healthy controls. It was found that psoriatic NK cells had increased expression of the apoptosis-associated Fas receptor, and lower expression of CD94 and NKG2A. Cell surface expressions of 2B4, CD48, CD16, NKG2D, and CD56 were unchanged between patients and healthy controls [29]. Previous work has also indicated that Fas may have an important role in the pathogenesis of psoriasis. While it is best known for its ability to induce apoptosis following engagement of Fas ligand (FasL), this receptor is also able to cause the production of proinflammatory cytokines including TNF-α, a key cytokine in psoriasis. Further evidence for a role for Fas was provided by Gilhar et al. who showed that SCID mice that receive grafts of uninvolved psoriatic skin from patients combined with injection of IL2-stimulated NK cells developed characteristics of psoriasis, and that the blocking of the Fas/FasL interaction could prevent these changes [30].
In addition to cell surface receptors, molecules important in NK cell functions, for example, cytotoxicity, have been implicated in psoriasis although the data are conflicting. In one study, perforin, a pore-forming protein found in the cytotoxic granules of NK cells and a key mediator of cytotoxicity, was expressed at higher levels in the lesional psoriatic skin relative to uninvolved psoriatic or healthy control skin [31]. Psoriasis patients also had higher levels of perforin in their peripheral blood lymphocytes compared to healthy controls. However, the source of this perforin does not seem to be circulating NK cells, as no difference between the number of CD56+Perforin+ or CD16+Perforin+ cells was observed in psoriasis patients compared to controls [32]; it seems likely that CTLs are responsible for the elevated perforin levels. Another study has given evidence for a role of NK cell-produced perforin in psoriasis, with a significantly higher percentage of CD56+Perforin+ found in the peripheral blood of patients with severe disease versus those with mild psoriasis, but interestingly this difference was not observed comparing individuals with severe psoriasis to healthy controls. It was also noted that the vast majority of blood cells expressing CD16 were also positive for perforin in those with severe disease while a significantly lower frequency of CD16 positive cells coexpressing perforin was found in patients with mild psoriasis [33]. Cells expressing granzyme B, a serine protease that is released from NK cell granules and that triggers DNA degradation in target cells, have also been found in significantly higher numbers in involved psoriatic skin compared to uninvolved psoriatic and healthy skin [31].
3. NK-Cell-Associated Cytokines Play Important Roles in Psoriasis
Cytokines are small soluble proteins that are secreted from cells and influence many aspects of cell biology including proliferation, differentiation, and activation. They are key immunological regulators and altered cytokine profiles have been implicated in a host of diseases. Many studies have conclusively demonstrated that cytokines play an important role in the pathogenesis of psoriasis. Several of these cytokines are important in NK cell biology.
IL-12 is a key cytokine in the proliferation and activation of both NK and T-cells. It triggers the production of the proinflammatory cytokines TNF-α and IFN-γ from these cells and promotes the development of Th1 type effector T-cells [12]. IL-12 is a heterodimeric cytokine composed of a p35 subunit and a p40 subunit which come together to form a 70 kDa protein [34]. This p70 protein has been found in increased levels in psoriatic lesions. The p40 subunit has also been implicated in psoriasis but current evidence suggests that this may be due to its presence as part of IL-23, a related cytokine. No differences have yet been observed concerning the p35 subunit. There are conflicting reports regarding the levels of IL-12 in the serum of psoriasis patients, with some authors finding decreased levels while others report no change compared to healthy controls [12].
IL-23 is a proinflammatory cytokine with emerging links to autoimmunity [10]. It is a member of the IL-12 cytokine family and consists of a unique p19 subunit, and a p40 subunit that it shares with IL-12 [10, 34]. It is produced by activated macrophages and dendritic cells [34] and is involved in the development of Th17 cells [35]. The Th17 subset of CD4+ T-cells have been identified as important cells in autoimmune diseases and IL-23 is thought to enhance secretion of both IL-22 and IL-17 from these cells [20]. IL-23 is also key in stimulating secretion of IL-22 from NK22 cells (discussed later in review). Memory T-cells are also stimulated to secrete IFN-γ by IL-23 [12, 36]. Genetic associations strongly support a role for IL-23 in psoriasis (see section below). In addition, IL-23 is highly expressed in lesional psoriatic skin and the expression of both subunits is significantly higher in lesions compared to normal skin [12]. It has been demonstrated that keratinocytes are capable of IL-23 production and that IL-23 expression is enhanced in keratinocytes of psoriatic patients [36]. Furthermore, therapies that target blocking of IL-23 have been shown to be effective in the treatment of psoriasis [10, 12].
Another key cytokine is IL-15 which is important for NK cell development, survival, and activation. It also acts to sustain inflammatory responses, promote angiogenesis, and suppress apoptosis [12]. It has been shown to trigger the production of IFN-γ, TNF-α, and IL-17 [37]. Keratinocytes have been shown to express IL-15 and its unique receptor subunit, the IL-15R α-chain, on their cell surface. It has been demonstrated that IL-15 is capable of inhibiting keratinocyte apoptosis; this may be relevant in psoriasis where abnormally low levels of apoptosis are displayed. IL-15 is highly expressed in psoriatic lesions compared to normal skin, as are binding sites for this cytokine [38]. Further evidence of a role for IL-15 in psoriasis is supplied by the observation that an antibody which interferes with IL-15R binding alleviated psoriasis in a mouse model of the disease [37].
Also important is IL-18 cytokine. It is produced in the immune response to pathogens and works together with IL-12 to drive IFN-γ production from NK and T-cells. It also stimulates the production of IL-13 and IL-4 from cells of the innate and adaptive immune response including NK cells, T-cells, basophils, and mast cells. IL-18 is thought to help drive inflammation in psoriasis. Lesional psoriatic skin contains higher levels of IL-18 mRNA compared to nonlesional or healthy skin, and increased expression of IL-18 is found in both involved and uninvolved psoriatic skin compared to healthy controls. Increased IL-18 has also been reported in plasma from psoriatic patients compared to healthy controls [12].
IL-22 is a member of the IL-10 family of cytokines [20, 39, 40]. It is produced by Th1 and Th17 cells [21, 39]. Recently, a novel population of NK cells, named NK22 cells, have been shown to secrete IL-22 [41]. IL-22 has a protective role at mucosal sites but has also been seen to be detrimental in autoimmune diseases by promoting inflammation [40]. The IL-22 receptor is expressed on keratinocytes [21, 39] and this cytokine has been shown to trigger keratinocyte production of several antimicrobial proteins, such as β-defensin 2, which have heightened levels in psoriasis [21]. Elevated levels of IL-22 mRNA have been reported in psoriatic lesions. Levels of IL-22 cytokine in the blood have been shown to be higher in psoriasis patients and cytokine level correlated with the disease severity [39]. Ex vivo treatment of keratinocytes with IL-22 results in the development of psoriatic features including hyperplasia, parakeratosis, downward epidermal projections, and acanthosis [21]. Importantly, IL-22 causes psoriasis-like skin changes in IL-22 transgenic mice; this effect was enhanced by TNF-α, a key cytokine in psoriasis [39].
IFN-γ is a cytokine that plays a large number of roles in the immune response. It is secreted by NK cells and T-cells [42] and stimulates the release of numerous proinflammatory cytokines from various cell types. It has roles in the activation, growth, and differentiation of lymphocytes and stimulates the cytotoxic activities of NK cells, CTLs, and macrophages. Studies on serum levels of IFN-γ in psoriasis have yielded conflicting results, with some authors noting no change relative to healthy controls. However, there have also been reports of highly elevated levels of IFN-γ in the serum and blister fluid of patients; levels correlated with disease severity and decreased after treatment. Studies investigating IFN-γ levels in the skin also vary with some reports observing stronger expression of IFN-γ in both involved and uninvolved psoriatic skin than in the skin of healthy controls [12].
TNF-α is produced by a variety of cells including NK cells [43]. TNF-α has amassed substantial interest in psoriasis where it appears to be a key mediator of the proinflammatory cascade. TNF-α appears to be locally produced in psoriatic lesions and its synthesis has been reported to be increased in involved psoriatic skin compared to uninvolved or healthy skin. Expression of the TNF receptor 1 is altered in lesional skin and soluble receptor is also found in higher levels in the blood of psoriatic patients. Several studies have found increased levels of TNF-α in the serum of psoriasis patients with a positive correlation to disease severity noted. Peripheral blood mononuclear cells isolated from patients showed increased capacity for TNF-α production in vitro. TNF-α is the most widely studied and utilized target for anticytokine psoriasis therapies and several antibodies that interfere with its functions including etanercept, infliximab and adalimumab are all highly effective in the treatment of psoriasis [12].
It is clear that many cytokines known to be important in psoriasis have strong links with NK biology and are either produced by NK cells (IFN-γ, TNF-α, and IL-22) or are important in their activation (IL-15, IL-18, IL-12, and IL-23). The nature of the relationship between these NK cell associated cytokines, NK cells, and psoriasis remains to be explored.
4. Recent Advances in Genetic Analysis of Psoriasis
Analysis of psoriasis among twins supports that there is a strong genetic basis for the development of psoriasis. In Northern Europeans, the rate of concordance is approximately 72% among monozygotic twins compared to approximately 15–23% of dizygotic twins. In other populations, the trend is similar although the percentages differ [44–46]. However, identification of the genetic loci involved has not been straightforward. It is clear that no one genetic locus causes psoriasis but rather several loci may contribute to the phenotype of psoriasis. Thus, psoriasis is a complex genetic disease. Early studies examined a role for MHC genes (HLA in human) and identified HLA-Cw6 as a locus associated with psoriasis [47]. There have been approximately ten genomewide linkage scans performed and over 20 possible regions linked to psoriasis identified; however, many of these associations identified have not been replicated in other studies [48]. More recently, several genomewide association scans (GWASs) have been performed and they have identified many genes important in the development and pathogenesis of psoriasis. A GWAS is a modern genetic tool that scans the entire genome at defined single nucleotide polymorphisms (SNPs). These are compared between control and disease groups, and genetic markers that have an altered frequency in disease can be identified. Sometimes the SNPs are located within known genes and thus a putative functional role for that gene in disease can be predicted. However, some of the SNPs reside outside genes and thus act as a marker for genes in that area. Identification of a SNP does not necessarily identify a gene of interest but rather it identifies an area of a chromosome that contains a gene that contributes to the phenotype.
Studies in both Asian and European populations have been performed and several loci have been confirmed as important in psoriasis. The most significant association has been with the MHC class I region of chromosome 6 that includes HLA-A, -B, -C, and HLA-E genes [6, 49]. Data suggest that the SNPs associated with psoriasis in this region are closest to HLA-C gene and further analysis at the allele level suggested that associated SNP alleles strongly correlate with HLA-Cw6, confirming the original candidate gene genetic studies [50]. There are three other genes in this HLA class I region that have well-defined roles in NK cell biology: HLA-B, HLA-E, and MICA. HLA-B genes that encode for a Bw4 epitope provide ligands for a KIR receptor called KIR3DL1. Indeed, a haplotype containing HLA-B*57 (HLA-Bw4) has previously been implicated with psoriasis [51]. HLA-E is a nonclassical class I molecule that provides a ligand for NKG2A/CD94, and NKG2C/CD94 receptors expressed by NK cells, and MICA encodes an antigen expressed in response to stress or particular pathological situations that activates NK cells through ligation of NKG2D [52]. An allele of MICA that possibly encodes for a soluble receptor, termed MICA A5.1, is associated with an increased risk of Psoriasis Vulgaris in a Chinese patient cohort [53]. Although there has been some controversy in the literature [6, 54], it seems likely that HLA-Cw6 constitutes the actual risk variant rather than acting as a marker for a nearby gene that is responsible for the effects seen. HLA-C is particularly interesting from an NK cell perspective as all HLA-C alleles encode ligands for KIR expressed by NK cells [55].
Several loci outside of the MHC region have also been implicated in psoriasis. Some of these encode for molecules involved in skin and tissue functions, for example, within the epidermal differentiation complex, but many of them have known functions within the immune system. Among these are cytokines, defence molecules, and immune signalling components and regulators. These have been described in detail elsewhere [6, 35, 56] and this paper will focus only on those that relate to NK cells. The IL-12B gene that encodes the p40 subunit of both IL-12 and IL-23 has been identified by GWAS and replicates a previous candidate gene association study in the Japanese [57, 58]. The IL-23A gene encoding for the p19 subunit of IL-23 is also associated with psoriasis [59]. These p19 and p40 subunits combine to generate IL-23 cytokine that is known to promote differentiation of specialised T-cell (Th17) and NK cell (NK22) subsets [40]. Given that the IL-23R gene (receptor for IL-23) also associates with psoriasis, it seems that IL-12 or more likely, IL-23, plays a role in psoriasis. The role of IL-12 in NK cell biology is well established. It is known to activate NK cell functions, for example, cytotoxicity and promote secretion of IFN-γ [60]. A role for IL-23 has recently been shown in the generation of the novel NK cell subset, NK22 cells, that appear to have specialised functions in mucosal sites within the body [40, 61]. Two other loci (TNFAIP3 and TNIP1) identified by GWAS influence immune signalling [59]. It is thought that these gene products combine to inhibit NFκB signalling that is an important component in keratinocyte proliferation and differentiation in psoriasis. These molecules inhibit both TNF and Toll-like receptor signalling pathways.
5. Role of NK Cell Receptors in Psoriasis
The KIR genes are a family of genes found on human chromosome 19q13.4 [55]. They encode for membrane bound receptors that are expressed by NK cells and a subset of T-cells. These receptors interact with HLA class I antigen expressed on the surface of all nucleated cells. Some of the KIR receptors activate NK cells upon interactions with ligand and others inhibit NK cell functions. The activating receptors (termed 2DS or 3DS KIR) lack a functioning cytoplasmic tail but rather associate with adaptor molecules that transduce a positive signal to NK cells. In contrast, the inhibitory receptors (termed 2DL or 3DL KIR) have inherent inhibitory signalling capacity through Immuno Tyrosine-based Inhibitory Motifs (ITIMs) in their cytoplasmic tails. KIR haplotypes vary in terms of gene number, gene content, and allelic polymorphism such that there is huge genetic variation even within closely related ethnic groups [55, 62]. Significant progress has been made in terms of molecular typing to the extent that all genes can be identified by relatively straightforward PCR-SSP reactions [63]. However, studying the cellular expression and functions of these receptors is more difficult due to the fact that they are clonally expressed (i.e., individual NK cells will express different combinations of the KIR genes encoded for in their chromosomes) and that specific antibodies to individual KIR receptors have been difficult to generate given the high sequence similarities of receptors in their extracellular domains. Thus genetic analysis of KIR genes far supersedes our understanding of their biology [64].
HLA class I ligands for many of the KIR receptors have been described. Unlike the TCR that recognises individual alleles of HLA class I antigen (though the process of positive selection during development), NK cell receptors have evolved to recognise conserved epitopes of HLA class I receptors. Recognition of HLA-C by NK cells is particularly important as every HLA-C allele contains a motif recognised by a KIR receptor: HLA-C1 alleles have an NxxK motif at residues 77–80 of the α1 heavy chain that provides an epitope recognised by 2DL2, 2DL3, and 2DS2 receptors while HLA-C2 alleles have an SxxN motif at this same region that is recognised by 2DL1 and 2DS1 KIR receptors [55]. More recently this relatively straightforward delineation of specificities has blurred with the recognition that the 2DL2 receptor (that recognises HLA-C1) also weakly recognises HLA-C2 [65]. A subset of HLA-B genes encode a serological epitope termed Bw4 that provides a ligand for the 3DL1 and possibly the 3DS1 receptor [66]. Recognition of HLA-A gene products is different as it appears that a particular KIR receptor, 3DL2, has evolved to recognise individual HLA-A alleles (A3 and A11) and recognition is peptide dependent [67].
While HLA class I ligands for some activating KIR have been described as mentioned above, binding affinity of activating KIR for HLA class I is generally weaker than that of the inhibitory receptors. Recently, a systematic search of HLA class I allotypes for 2DS3, 2DS5 interactions failed to identify a ligand [68]. Together these facts have resulted in a growing consensus that perhaps HLA class I is not the primary ligand for activating KIR receptors. Possible ligands could include pathogen encoded molecules or endogenous molecules expressed under pathogenic conditions.
Molecular typing methods have provided tools for KIR gene association studies. KIR genes are good candidates for genetic associations with disease for a number of reasons: (1) genes of the immune system are often associated with the development or pathogenesis of disease; (2) KIR genes are polygenic and highly polymorphic; (3) KIR proteins interact with HLA class I gene products and HLA class I genes consistently provide the strongest associations with particular disease. KIR genes have been associated with the development or progression of disease in a number of clinical situations including infections, for example, HIV [69] and HCV infections [70] and noninfectious conditions, for example, recurrent miscarriage [71, 72]. It has become clear that the effects of KIR genes on disease may be subtle and may only manifest in the presence of cognate HLA class I ligand. As KIR genes and HLA class I genes segregate on different chromosomes, their presence together is relatively random. Thus disease association studies often need to investigate both KIR genes and HLA class I genes for interactions to become apparent.
Several studies have looked for KIR gene associations with the development of psoriasis and these have mainly focussed on Psoriasis Vulgaris. However, the data emerging do not provide a clear consensus with some studies reporting a significant association of 2DS1 with the development of disease while others find no KIR gene association. For the studies that did find a KIR gene association, 2DS1 has consistently been identified as an important locus. In brief, no KIR gene associations have been found in either Chinese [73] or US Caucasians [74]. Our preliminary analyses support these studies with no individual KIR gene associated with the development of psoriasis in an Irish cohort (S. Dunphy, data not shown). 2DS1 has been associated with the development of psoriasis is Swedish [75], Polish [76] and Japanese patients [77]. In the Japanese study, 2DL5 was also associated with the development of psoriasis. As the populations studied to date come from a variety of ethnic backgrounds, this is unlikely to account for the differences observed. Another factor to be considered is the size of the cohorts examined in terms of statistical power to demonstrate a result. The studies cited range from 96 patients and 50 controls at the lower end (Japanese cohort) to 237 patient and 372 controls at the higher end (Swedish cohort) and thus sample size may also contribute to differences seen. The frequency of 2DS1 within the control populations varies and this will also impact the ability to identify a role for 2DS1 is psoriasis. Therefore, additional highly powered studies are needed to resolve the role of 2DS1 in the development of psoriasis. However, it is interesting that the three studies that found a KIR gene association with psoriasis all identified 2DS1 as a locus of interest. 2DS1 is particularly attractive in this context as it is the only activating KIR receptor that has been shown to interact with HLA-Cw6 [68], which as previously mentioned, is the strongest genetic susceptibility locus identified to date in the development of psoriasis. A functional interaction between 2DS1 and HLA-Cw6 receptors may provide a biological rationale for NK cell involvement in psoriasis and thus, confirmation of 2DS1 as a susceptibility locus, is an important research question for NK cell biologists. It is worth noting that even if 2DS1 is confirmed other studies, the nature of KIR gene haplotype structure means that 2DS1 may be functioning as a marker for another nearby locus that is mediating the biological effect.
Psoriatic arthritis is a form of severe arthritis found in a subset of patients with psoriasis. The first study published on KIR genes in PsA identified two activating KIR genes, 2DS1 and 2DS2 associated with the development of psoriatic arthritis but only in the absence of their cognate HLA-C ligands in a US cohort [78]. The revised model proposed by the authors suggested that increasing susceptibility to psoriatic arthritis was determined by presence of more activating KIR genotypes (i.e., presence of activating KIR or lack of inhibitory KIR, in presence of ligand) [79]. A Swedish cohort found a trend for 2DS1 involvement in psoriatic arthritis but it did not reach statistical significance [75]. Finally, a second US cohort identified 2DS1 as a risk factor for the development of psoriatic arthritis among patients with psoriasis; however, numbers of patients in this analysis were low [74]. A single study looking at KIR genes in guttate psoriasis found no significant gene associations with development of disease [75].
Although GWAS studies have successfully identified many loci involved in psoriasis, it is clear that much of the genetic contribution to psoriasis remains to be identified. KIR genes may be among the “missing” genetic risk loci as it is unlikely that they would be identified on a genomewide scan of SNPs, given their complex haplotype variability and polymorphic nature.
6. Future Areas of Research
While there is evidence emerging to support a role for NK cells in psoriasis, more work needs to be done before we have a clearer picture of what that actual role might be. One issue that complicates this question is the variety of clinical patients cohorts that have been examined in the context of NK cells. Some groups have studied new onset Psoriasis Vulgaris while others look at guttate psoriasis, PsA or the effect of treatment for psoriasis on NK cell function. While some of the KIR studies replicate an association of the 2DS1 gene with psoriasis, others have failed to do so. Thus, additional cohorts with adequate numbers and statistical power to address the relevance of this are required. In general, few of the studies in the literature have been replicated in other cohorts which makes it difficult to assess the overall role of NK cells in psoriasis. The field would greatly benefit from some basic NK cell studies in well-defined and characterised cohorts that have adequate numbers to confirm basic findings to date.
However, evidence from large GWAS studies and cell-based experimental systems have identified many potential molecules involved with NK cell biology that are associated with psoriasis including IL-12 and IL-23. Such analysis may lead to new exciting avenues of research, for example, a possible role for NK22 cells in psoriasis. From the GWAS studies, IL-23 is known to be important in psoriasis [57]. As previously described, it is the key cytokine responsible for the differentiation of the NK22 cell type that secretes IL-22 but not IL-17 cytokine (Figure 2) [40, 61]. NK22 cells are not found in peripheral blood but rather in mucosal/tissue sites within the body. It is tempting to speculate a role for NK22 cells particularly as IL-22 is known to be pathogenic in psoriasis. One could envision a dynamic interaction between NK cells that are localised to the skin by their chemokine receptor expression patterns and that differentiate towards NK22 cells in the presence of IL-23 cytokine that is secreted by keratinocytes. The NK22 cells could then secrete IL-22 which mediates its pathogenic effects. Alternatively, NK cells recruited to the skin may exhibit a more conventional NK cell phenotype and secrete IFN-γ and TNF-α that are known to play a role in psoriasis. If NK cells are involved, and much evidence supports that they are, it is as yet impossible to predict how these events are coordinated. Are NK cells normally resident in the skin activated to secrete factors that activate keratinocytes, or do activated keratinocytes recruit NK cells to the skin and activate them to secrete cytokines? Do both cell types participate in a positive feedback loop? In summary, evidence is emerging to support a role for NK cells in psoriasis but this field is in relative infancy compared to studies on other immune cells, for example, T-cells. More basic studies are required but early indications are that such studies will be fruitful and reveal new molecular mechanisms for NK cell involvement with initiation and progression of psoriasis.
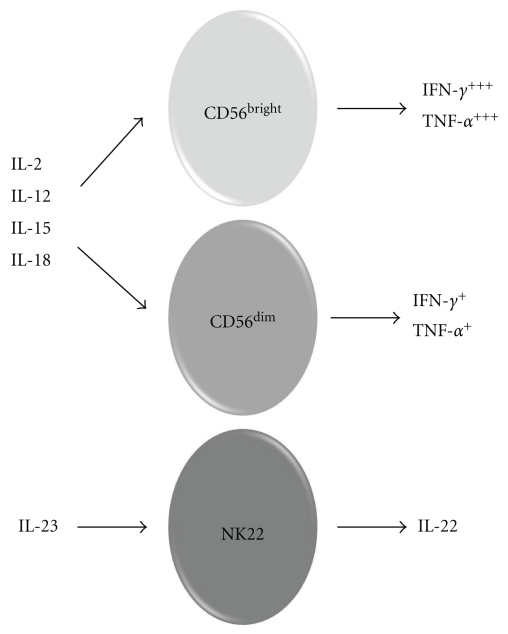
Figure 2.
Subsets of NK cells secrete distinct cytokine profiles. Conventional NK cells, consisting of CD56bright and CD56dim subsets, are found in the general blood circulation. They are responsive to cytokines, with IL-2, IL-12, IL-15, and IL-18 being viewed as most important. These cytokines, some of which may act alone while others are only effective in as part of a milieu, activate effector functions such as secretion of cytokines including IFN-γ and TNF-α and degranulation of cytotoxic granules. In contrast, NK22 cells are a recently described subset of NK cells that are primarily resident in mucosal sites within the body. They differentiate towards the NK22 phenotype in response to IL-23 stimulation. NK22 cells secrete IL-22 cytokine (but not IL-17). IL-22 is known to be pathogenic in psoriasis.
References
- 1.Nestle FO, Kaplan DH, Barker J. Mechanisms of disease: psoriasis. The The New England Journal of Medicine. 2009;361(5):444–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 3.Lebwohl M. Psoriasis. The Lancet. 2003;361(9364):1197–1204. doi: 10.1016/S0140-6736(03)12954-6. [DOI] [PubMed] [Google Scholar]
- 4.Schön MP, Boehncke WH. Psoriasis. The New England Journal of Medicine. 2005;352(18):1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes and Immunity. 2007;8(1):1–12. doi: 10.1038/sj.gene.6364351. [DOI] [PubMed] [Google Scholar]
- 6.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nature Reviews Immunology. 2005;5(9):699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 7.Cameron AL, Kirby B, Fei W, Griffiths C. Natural killer and natural killer-T cells in psoriasis. Archives of Dermatological Research. 2002;294(8):363–369. doi: 10.1007/s00403-002-0349-4. [DOI] [PubMed] [Google Scholar]
- 8.Cameron AL, Kirby B, Griffiths CEM. Circulating natural killer cells in psoriasis. British Journal of Dermatology. 2003;149(1):160–164. doi: 10.1046/j.1365-2133.2003.05319.x. [DOI] [PubMed] [Google Scholar]
- 9.Bachelez H. Immunopathogenesis of psoriasis: recent insights on the role of adaptive and innate immunity. Journal of Autoimmunity. 2005;25(supplement):69–73. doi: 10.1016/j.jaut.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Di Meglio P, Nestle FO. The role of IL-23 in the immunopathogenesis of psoriasis. F1000 Biology Reports. 2010;2(1) doi: 10.3410/B2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos JD, De Rie MA, Teunissen MBM, Piskin G. Psoriasis: dysregulation of innate immunity. British Journal of Dermatology. 2005;152(6):1098–1107. doi: 10.1111/j.1365-2133.2005.06645.x. [DOI] [PubMed] [Google Scholar]
- 12.Pietrzak AT, Zalewska A, Chodorowska G, et al. Cytokines and anticytokines in psoriasis. Clinica Chimica Acta. 2008;394(1-2):7–21. doi: 10.1016/j.cca.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO. The pathogenic role of tissue-resident immune cells in psoriasis. Trends in Immunology. 2007;28(2):51–57. doi: 10.1016/j.it.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Peternel S, Kaštelan M. Immunopathogenesis of psoriasis: focus on natural killer T cells. Journal of the European Academy of Dermatology and Venereology. 2009;23(10):1123–1127. doi: 10.1111/j.1468-3083.2009.03292.x. [DOI] [PubMed] [Google Scholar]
- 15.Koreck A, Surányi A, Szöny BJ, et al. CD3+CD56+ NK T cells are significantly decreased in the peripheral blood of patients with psoriasis. Clinical and Experimental Immunology. 2002;127(1):176–182. doi: 10.1046/j.1365-2249.2002.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonish B, Jullien D, Dutronc Y, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-γ production by NK-T cells. Journal of Immunology. 2000;165(7):4076–4085. doi: 10.4049/jimmunol.165.7.4076. [DOI] [PubMed] [Google Scholar]
- 17.Gilhar A, Ullmann Y, Kerner H, et al. Psoriasis is mediated by a cutaneous defect triggered by activated immunocytes: induction of psoriasis by cells with natural killer receptors. Journal of Investigative Dermatology. 2002;119(2):384–391. doi: 10.1046/j.1523-1747.2002.01812.x. [DOI] [PubMed] [Google Scholar]
- 18.Nickoloff BJ, Bonish B, Huang BB, Porcelli SA. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. Journal of Dermatological Science. 2000;24(3):212–225. doi: 10.1016/s0923-1811(00)00120-1. [DOI] [PubMed] [Google Scholar]
- 19.Nickoloff BJ, Wrone-Smith T, Bonish B, Porcelli SA. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes: detection of T cells expressing receptors typically present on natural killer cells, including CD94, CD158, and CD161. Archives of Dermatology. 1999;135(5):546–552. doi: 10.1001/archderm.135.5.546. [DOI] [PubMed] [Google Scholar]
- 20.Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Seminars in Cutaneous Medicine and Surgery. 2010;29(1):3–9. doi: 10.1016/j.sder.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. British Journal of Dermatology. 2008;159(5):1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ottaviani C, Nasorri F, Bedini C, de Pità O, Girolomoni G, Cavani A. CD56brightCD16(-) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. European Journal of Immunology. 2006;36(1):118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 23.Carbone T, Nasorri F, Pennino D, et al. CD56highCD16-CD62L- NK cells accumulate in allergic contact dermatitis and contribute to the expression of allergic responses. Journal of Immunology. 2010;184(2):1102–1110. doi: 10.4049/jimmunol.0902518. [DOI] [PubMed] [Google Scholar]
- 24.Skrzeczyńska-Moncznik J, Stefańska A, Zabel BA, Kapińska-Mrowiecka M, Butcher EC, Cichy J. Chemerin and the recruitment of NK cells to diseased skin. Acta Biochimica Polonica. 2009;56(2):355–360. [PMC free article] [PubMed] [Google Scholar]
- 25.Parolini S, Santoro A, Marcenaro E, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109(9):3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 26.Aractingi S, Briand N, Le Danff C, et al. HLA-G and NK receptor are expressed in psoriatic skin: a possible pathway for regulating infiltrating T cells? American Journal of Pathology. 2001;159(1):71–77. doi: 10.1016/S0002-9440(10)61675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardili RN, Alves TG, Freitas JCOC, et al. Expression of human leucocyte antigen-G primarily targets affected skin of patients with psoriasis. British Journal of Dermatology. 2010;163(4):769–775. doi: 10.1111/j.1365-2133.2010.09917.x. [DOI] [PubMed] [Google Scholar]
- 28.López-Botet M, Navarro F, Llano M. How do NK cells sense the expression of HLA-G class Ib molecules? Seminars in Cancer Biology. 1999;9(1):19–26. doi: 10.1006/scbi.1998.0107. [DOI] [PubMed] [Google Scholar]
- 29.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 30.Gilhar A, Yan R, IV, Assy B, Serafimovich S, Ullmann Y, Kalish RS. Fas pulls the trigger on psoriasis. American Journal of Pathology. 2006;168(1):170–175. doi: 10.2353/ajpath.2006.041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yawalkar N, Schmid S, Braathen LR, Pichler WJ. Perforin and granzyme B may contribute to skin inflammation in atopic dermatitis and psoriasis. British Journal of Dermatology. 2001;144(6):1133–1139. doi: 10.1046/j.1365-2133.2001.04222.x. [DOI] [PubMed] [Google Scholar]
- 32.Prpić L, Štrbo N, Sotošek V, Gruber F, Podack ER, Rukavina D. Assessment of perforin expression in peripheral blood lymphocytes in psoriatic patients during exacerbation of disease. Acta Dermato-Venereologica, Supplement. 2000;(211):14–16. doi: 10.1080/00015550050500059. [DOI] [PubMed] [Google Scholar]
- 33.Prpić Massari L, Kaštelan M, Laškarin G, Zamolo G, Massari D, Rukavina D. Analysis of perforin expression in peripheral blood and lesions in severe and mild psoriasis. Journal of Dermatological Science. 2007;47(1):29–36. doi: 10.1016/j.jdermsci.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Langrish CL, McKenzie BS, Wilson NJ, De Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunological Reviews. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 35.Nair RP, Ding J, Duffin KC, et al. Psoriasis bench to bedside: genetics meets immunology. Archives of Dermatology. 2009;145(4):462–464. doi: 10.1001/archdermatol.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piskin G, Sylva-Steenland RMR, Bos JD, Teunissen MBM. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. Journal of Immunology. 2006;176(3):1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 37.Villadsen LS, Schuurman J, Beurskens F, et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. Journal of Clinical Investigation. 2003;112(10):1571–1580. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruckert R, Asadullah K, Seifert M, et al. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? Journal of Immunology. 2000;165(4):2240–2250. doi: 10.4049/jimmunol.165.4.2240. [DOI] [PubMed] [Google Scholar]
- 39.Wolk K, Haugen HS, Xu W, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-γ are not. Journal of Molecular Medicine. 2009;87(5):523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 40.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31(1):15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 43.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends in Immunology. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 44.Brandrup F, Hauge M, Henningsen K, Eriksen B. Psoriasis in an unselected series of twins. Archives of Dermatology. 1978;114(6):874–878. [PubMed] [Google Scholar]
- 45.Duffy DL, Spelman LS, Martin NG. Psoriasis in Australian twins. Journal of the American Academy of Dermatology. 1993;29(3):428–434. doi: 10.1016/0190-9622(93)70206-9. [DOI] [PubMed] [Google Scholar]
- 46.Pisani M, Ruocco V. ’Twin’ psoriasis in monozygotic twins. Archives of Dermatology. 1984;120(11):1418–1419. [PubMed] [Google Scholar]
- 47.Tiilikainen A, Lassus A, Karvonen J. Psoriasis and HLA-Cw6. British Journal of Dermatology. 1980;102(2):179–184. doi: 10.1111/j.1365-2133.1980.tb05690.x. [DOI] [PubMed] [Google Scholar]
- 48.Bowcock AM. Psoriasis genetics: the way forward. Journal of Investigative Dermatology. 2004;122(6):xv–xvii. doi: 10.1111/j.0022-202X.2004.22627.x. [DOI] [PubMed] [Google Scholar]
- 49.Nair RP, Stuart P, Henseler T, et al. Localization of psoriasis-susceptibility locus PSORS1 to a 60-kb interval telomeric to HLA-C. American Journal of Human Genetics. 2000;66(6):1833–1844. doi: 10.1086/302932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair RP, Stuart PE, Nistor I, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. American Journal of Human Genetics. 2006;78(5):827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng BJ, Sun LD, Soltani-Arabshahi R, et al. Multiple loci within the major histocompatibility complex confer risk of psoriasis. PLoS Genetics. 2009;5(8) doi: 10.1371/journal.pgen.1000606. Article ID e1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cerwenka A, Lanier LL. NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens. 2003;61(5):335–343. doi: 10.1034/j.1399-0039.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 53.Cheng L, Zhang SZ, Xiao CY, et al. The A5.1 allele of the major histocompatibility complex class I chain-related gene A is associated with psoriasis vulgaris in Chinese. British Journal of Dermatology. 2000;143(2):324–329. doi: 10.1046/j.1365-2133.2000.03658.x. [DOI] [PubMed] [Google Scholar]
- 54.Jenisch S, Westphal E, Nair RP, et al. Linkage disequilibrium analysis of familial psoriasis: identification of multiple disease-associated MHC haplotypes. Tissue Antigens. 1999;53(2):135–146. doi: 10.1034/j.1399-0039.1999.530203.x. [DOI] [PubMed] [Google Scholar]
- 55.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annual Review of Immunology. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 56.Roberson EDO, Bowcock AM. Psoriasis genetics: breaking the barrier. Trends in Genetics. 2010;26(9):415–423. doi: 10.1016/j.tig.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. American Journal of Human Genetics. 2007;80(2):273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsunemi Y, Saeki H, Nakamura K, et al. Interleukin-12 p40 gene (IL12B) 3’-untranslated region polymorphism is associated with susceptibility to atopic dermatitis and psoriasis vulgaris. Journal of Dermatological Science. 2002;30(2):161–166. doi: 10.1016/s0923-1811(02)00072-5. [DOI] [PubMed] [Google Scholar]
- 59.Nair RP, Duffin KC, Helms C, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nature Genetics. 2009;41(2):199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. Journal of Immunology. 1996;156(3):1138–1142. [PubMed] [Google Scholar]
- 61.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nature Reviews Immunology. 2009;9(4):229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 62.Guinan KJ, Cunningham RT, Meenagh A, et al. Signatures of natural selection and coevolution between killer cell immunoglobulin-like receptors (KIR) and HLA class i genes. Genes and Immunity. 2010;11(6):467–478. doi: 10.1038/gene.2010.9. [DOI] [PubMed] [Google Scholar]
- 63.Vilches C, Castaño J, Gómez-Lozano N, Estefanía E. Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens. 2007;70(5):415–422. doi: 10.1111/j.1399-0039.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 64.Gardiner CM. Killer cell immunoglobulin-like receptors on NK cells: the how, where and why. International Journal of Immunogenetics. 2008;35(1):1–8. doi: 10.1111/j.1744-313X.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 65.Moesta AK, Norman PJ, Yawata M, Yawata N, Gleimer M, Parham P. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C Than KIR2DL3. Journal of Immunology. 2008;180(6):3969–3979. doi: 10.4049/jimmunol.180.6.3969. [DOI] [PubMed] [Google Scholar]
- 66.Gumperz JE, Barber LD, Valiante NM, et al. Conserved and variable residues within the Bw4 motif of HLA-B make separable contributions to recognition by the NKB1 killer cell-inhibitory receptor. Journal of Immunology. 1997;158(11):5237–5241. [PubMed] [Google Scholar]
- 67.Hansasuta P, Dong T, Thananchai H, et al. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. European Journal of Immunology. 2004;34(6):1673–1679. doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- 68.Moesta AK, Graef T, Abi-Rached L, Aguilar AMO, Guethlein LA, Parham P. Humans differ from other hominids in lacking an activating NK cell receptor that recognizes the C1 epitope of MHC class I. Journal of Immunology. 2010;185(7):4233–4237. doi: 10.4049/jimmunol.1001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin MP, Qi Y, Gao X, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nature Genetics. 2007;39(6):733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 71.Hiby SE, Apps R, Sharkey AM, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. Journal of Clinical Investigation. 2010;120(11):4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Human reproduction. 2008;23(4):972–976. doi: 10.1093/humrep/den011. [DOI] [PubMed] [Google Scholar]
- 73.Chang YT, Chou CT, Shiao YM, et al. The killer cell immunoglobulin-like receptor genes do not confer susceptibility to psoriasis vulgaris independently in Chinese. Journal of Investigative Dermatology. 2006;126(10):2335–2338. doi: 10.1038/sj.jid.5700415. [DOI] [PubMed] [Google Scholar]
- 74.Williams F, Meenagh A, Sleator C, et al. Activating killer cell immunoglobulin-like receptor gene KIR2DS1 is associated with psoriatic arthritis. Human Immunology. 2005;66(7):836–841. doi: 10.1016/j.humimm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Holm SJ, Sakuraba K, Mallbris L, Wolk K, Ståhle M, Sánchez FO. Distinct HLA-C/KIR genotype profile associates with guttate psoriasis. Journal of Investigative Dermatology. 2005;125(4):721–730. doi: 10.1111/j.0022-202X.2005.23879.x. [DOI] [PubMed] [Google Scholar]
- 76.Łuszczek W, Mańczak M, Cisło M, et al. Gene for the activating natural killer cell receptor, KIR2DS1, is associated with susceptibility to psoriasis vulgaris. Human Immunology. 2004;65(7):758–766. doi: 10.1016/j.humimm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki Y, Hamamoto Y, Ogasawara Y, et al. Genetic polymorphisms of killer cell immunoglobulin-like receptors are associated with susceptibility to psoriasis vulgaris. Journal of Investigative Dermatology. 2004;122(5):1133–1136. doi: 10.1111/j.0022-202X.2004.22517.x. [DOI] [PubMed] [Google Scholar]
- 78.Martin MP, Nelson G, Lee JH, et al. Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. Journal of Immunology. 2002;169(6):2818–2822. doi: 10.4049/jimmunol.169.6.2818. [DOI] [PubMed] [Google Scholar]
- 79.Nelson GW, Martin MP, Gladman D, Wade J, Trowsdale J, Carrington M. Cutting edge: heterozygote advantage in autoimmune disease: hierarchy of protection/susceptibility conferred by HLA and killer Ig-like receptor combinations in psoriatic arthritis. Journal of Immunology. 2004;173(7):4273–4276. doi: 10.4049/jimmunol.173.7.4273. [DOI] [PubMed] [Google Scholar]