Abstract
A controversial question in cognitive neuroscience is whether comprehension of words and sentences engages brain mechanisms specific for decoding linguistic meaning or whether language comprehension occurs through more domain-general sensorimotor processes. Accumulating behavioral and neuroimaging evidence suggests a role for cortical motor and premotor areas in passive action-related language tasks, regions that are known to be involved in action execution and observation. To examine the involvement of these brain regions in language and nonlanguage tasks, we used functional magnetic resonance imaging (fMRI) on a group of 21 healthy adults. During the fMRI session, all participants 1) watched short object-related action movies, 2) looked at pictures of man-made objects, and 3) listened to and produced short sentences describing object-related actions and man-made objects. Our results are among the first to reveal, in the human brain, a functional specialization within the ventral premotor cortex (PMv) for observing actions and for observing objects, and a different organization for processing sentences describing actions and objects. These findings argue against the strongest version of the simulation theory for the processing of action-related language.
Keywords: action observation, embodiment, mirror neurons, object observation, premotor cortex
Introduction
Since the mid-19th century, it has been the common understanding that auditory language comprehension is a relatively localized function of the left temporal and inferior parietal regions of the human brain. Yet language comprehension is now increasingly seen as a broadly distributed process involving cortical and subcortical regions extending far beyond these regions. Of particular interest are the numerous reports of brain activation in frontal motor and premotor regions during passive (nonmotor) language tasks, regions that are primarily known for their role in action execution and, more recently, also for their role in action observation (di Pellegrino et al. 1992; Gallese et al. 1996; Rizzolatti et al. 1996). In the macaque, there exist individual “mirror” neurons with this dual property in the ventral premotor cortex (area F5) and inferior parietal lobe. It has been suggested that the ability to recognize actions is based on the ability to map observed actions onto one's own motor representations through an action execution matching process that would rely on the mirror neurons. Several researchers have proposed that mirror neurons also exist in humans (e.g., Rizzolatti et al. 1996; Buccino et al. 2001, 2004) but see also Turella et al. (2009) for a recent review of the evidence for mirror neurons in humans. The human mirror neurons would be located frontally either in the ventral premotor cortex (in the precentral gyrus and sulcus) or in the adjacent pars opercularis of the inferior frontal gyrus.
According to advocates of “embodied semantics,” understanding the meaning of a sentence or a word describing an action requires activation of the motor circuits required to produce that action, and by analogy with the macaque, are thought to involve mechanisms akin to those involving mirror neurons (e.g., Buccino et al. 2001; Tettamanti et al. 2005; Aziz-Zadeh et al. 2006). A related hypothesis is that because action words are often spoken with the action that they denote, they become associated with activation in sensorimotor regions (Pulvermuller 1996, 2001) by virtue of Hebbian learning, whereby “any two cells or systems of cells that are repeatedly active at the same time will tend to become associated, so that activity in one facilitates activity in the other” (Hebb 1949). In line with these hypotheses, several behavioral studies have shown that processing linguistic stimuli can interfere with action execution and vice versa, suggesting a link between action and language (e.g., Gentilucci et al. 2000; Glenberg and Kaschak 2002; Glover and Dixon 2002; Chambers and Alexis 2004; Glover et al. 2004; Glenberg et al. 2008). Furthermore, several brain-imaging studies have shown activation in motor and premotor cortex (PM) during passive language tasks (e.g., Hauk et al. 2004, Tettamanti et al. 2005; Aziz-Zadeh et al. 2006). For example, it has been shown that passive reading of action words related to mouth actions (e.g., lick) is associated with activation in the inferior frontal gyrus, whereas reading arm-related words (e.g., peel) and leg-related words (e.g., walk) is associated with a somatotopically organized PM activation (Hauk et al. 2004). Single-pulse transcranial magnetic stimulation (TMS) experiments have also shown somatotopic modulation of primary motor cortex (but not PM) during the processing of sentences (Buccino et al. 2005; Glenberg et al. 2008) and words (Pulvermuller et al. 2005).
Despite this apparent convergence of findings, however, there are also several nonnegligible points of controversy (for a review of some these inconsistencies, see Fernandino and Iacoboni 2010). For example, Postle et al. (2008) found no evidence of a somatotopic organization for effector-related words using cytoarchitecturally and functionally defined maps of the primary and PM. Likewise, using a voxel-based lesion symptom mapping approach in patients with left hemisphere stroke, Arévalo et al. (2010) did not find evidence of a somatotopically organized distribution of effector-specific regions. Moreover, although primary motor and premotor regions are certainly active during both language comprehension and overt action, no overlap between these active regions has yet been clearly demonstrated. For instance, in Hauk et al. (2004), somatotopy in the precentral regions was found for action execution and for processing action words, but there was very little overlap between the 2, suggesting the possibility of some degree of segregated processing. Moreover, there are also some discrepancies among the TMS results. For instance, Pulvermüller et al. (2005) demonstrated faster reaction time during a lexical decision task when the presented words were congruent with the part of primary motor cortex being stimulated (hand word paired with stimulation of hand motor cortex; leg word paired stimulation of leg motor cortex) compared with when it was incongruent (hand word paired with stimulation of leg motor cortex; leg word paired with stimulation of hand motor cortex), that is, congruency was “facilitatory.” In contrast, Buccino et al. (2005) showed the opposite pattern, that is, slower responses when participants listened to action sentences that were congruent with the part of primary motor cortex being stimulated (hand, leg), hence here the affect of congruency was an “interference.”
In sum, there is support from behavioral brain imaging and TMS studies to the idea that frontal motor areas, primarily PM, are active during the processing of action-related words and sentences. Strictly speaking, however, there is still no strong evidence for a causal relationship between activation in these areas and language comprehension per se. Activation in PM has been reported primarily in the context of passive audio or audiovisual language tasks, most often consisting of single word presentations. Hence, it is possible that activation in PM during language tasks reflects some idiosyncratic processes associated with passive language tasks. It is also possible that activation in motor areas during language tasks is not critical for semantic analysis of the linguistic stimuli but instead has a secondary semantic role or even little interpretive role at all (see Mahon and Caramazza 2008, and Hickok 2009) for arguments against the causal role of sensorimotor systems in semantic interpretation). In the present study, we used functional magnetic resonance imaging (fMRI) to examine the generalizability of prior findings of PM activation in language processing and, more generally, to advance current understanding of the role of the motor system in language comprehension. In particular, we used fMRI to elaborate the integrated versus segregated nature of frontal motor and premotor activation during the processing of action- and object-related language and comparable nonlinguistic stimuli. To this end, we started by identifying brain regions activated during the visual observation of actions and objects. Then, we identified context-independent “core” brain regions involved in language processing by examining the intersection of brain activation from listening to sentences, listening and repeating sentences, and generating sentences from object pictures. Next, we examined the frontal motor and premotor areas that formed part of this core to determine the role of these regions in comprehension and their relationship to nonlinguistic processing of actions and objects. To examine the shared activation for the processing of action and language, we took 3 steps. First, we identified regions sensitive to sentence meaning by comparing brain activation for action- and object-related sentences. Second, we intersected the brain activity in these regions for all action sentences with activity during an action observation task during which participants watched short hand action clips. Finally, we intersected the activity from processing object-related sentences with that from an object observation task. We hypothesized that if PM is a critical part of a language comprehension system, it should survive the intersection of activity (conjunction analysis) from of all language tasks, and it should be modulated by the semantic content of the sentences. In addition, if PM is involved in processing language and observing actions, we should observe overlap in these regions for the intersection of language and nonlanguage tasks.
Materials and Methods
Participants
Twenty-one healthy right-handed native speakers of English (mean 25 ± 4.4; 10 males), with a mean of 15.4 years of education participated in the fMRI experiment. All participants had normal pure-tone thresholds and normal speech recognition scores (92.3% accuracy on the Northwestern University auditory test number 6). The Institutional Review Board for the Division of Biological Sciences at The University of Chicago approved the study.
Experimental Procedures
Participants underwent 5 different tasks while in the scanner 1) passive object picture observation (OBJECTobs), 2) passive sentence listening (LISTEN), 3) listening and repeating sentences (REPEAT), 4) generating sentences from object pictures (GENERATE), and 5) passive observation of short action movies (ACTIONobs). Each condition was acquired in separate runs within one session. In addition, each task was interleaved with “rest” trials during which the participants were simply asked to relax and clear their head. For each condition, the experimental trials were interleaved with rest trials; the order of the conditions and the optimal number of rest trials was determined by OPTseq2 (http://surfer.nmr.mgh.harvard.edu/optseq/). All stimuli were presented using Presentation Software (Neurobehavioral Systems).
During OBJECTobs, 40 simple black-and-white line drawings representing common man-made objects were presented for 1 s and interleaved with 37 rest trials (crosshair fixation). The pictures were selected from the International Picture Norming Project corpus from the Center for Research in Language at the University of California, San Diego based on an online picture norming study (SurveyGizmo, Widgix Software) that was conducted on 49 English speakers (13 males, mean 32.2 ± 8.7 years) using a set 108 object pictures. Based on the result of this study, 2 sets of 40 pictures (see Supplementary Table S1) were selected that had high naming agreement, high familiarity, and high manipulability. One set was used for OBJECTObs, and the other set was used for GENERATE. None of the pictures represented a leg or a mouth-related object to avoid a body part confound (e.g., no food-related pictures were used). During LISTEN, a set of 80 short sentences (0.9–1.3 s) was presented (see Supplementary Table S1). Half of these sentences described manual object-directed actions, and the other half described visual properties of the same set of objects. In order to ensure that there was no intrinsic difference between the action-related and object-related sentences, we conducted a behavioral experiment during which 12 healthy English speakers (7 females, mean 26.8 ± 4.6 years) heard and repeated a subset of 40 sentences. The results revealed that participants were equally fast and accurate in both conditions. During the fMRI experiment, the experimental trials were interleaved with 30 rest trials.
During REPEAT, participants heard a similar set of 80 sentences. Half of these sentence described manual object-directed actions, and the other half described visual properties of a similar set of objects. Stimulus presentation and responses occurred during a 4.5 s delay in time repetition (TR). At the beginning of the delay in TR, a Go cue was presented, instructing participants to start repeating the sentence. Participants’ responses were recorded and stored to disk for offline analysis. The sentence trials were interleaved with 30 rest trials (crosshair fixation).
In GENERATE, a set of 40 object pictures (similar to the one used in the OBJECTObs task) was presented, and participants were asked to generate short action and object sentences. The action and object trials were performed in 2 different runs to avoid a task-switching effect. The same pictures were viewed in the 2 conditions; the order of presentation of the conditions was counterbalanced across participants. Each run consisted of 40 experimental trials and 28 rest trials. In each experimental trial, a picture was presented for 1 s and was followed, after 500 ms, by the presentation of a Go cue, instructing participants to start generating the sentence. All speaking occurred during a 4.5 s delay in TR.
In ACTIONobs, we presented a set of twenty-nine 2-s video clips of an actor manipulating familiar objects with one hand. The head of the actor was not filmed in order to focus participants’ attention to the hand movements. A list of all videos is provided in Supplementary Table S1. The videos were interleaved with 97 rest trials. In order to avoid priming participants into verbalizing upon presentation of the pictures, OBJECTObs was always completed first. It was followed by LISTEN, REPEAT, GENERATE, and ACTIONObs. LISTEN preceded REPEAT to avoid priming participants into speaking or silently rehearsing the sentences. The ACTION observation task was completed last to prevent biasing participants into visualizing actions when hearing language or seeing pictures. This was critical to ensure that our findings reflected naturalistic sentence processing activation.
Image Acquisition and Analysis
The data were acquired on a 3 T General Electric Signa HDx imager with EXCITE. Subjects wore MR compatible headphones and goggles (NordicNeuroLab Audio/Visual system). Thirty-four axial slices (3.125 × 3.125 × 3.6 mm, no gap, field of view (FOV) = 256 × 256 mm2, matrix = 64 × 64) were acquired in 1.5 s using a multislice Echo-planar imaging sequence with parallel imaging (ASSET = 2; time echo = 26 ms; FOV = 20 cm; 64 × 64 matrix; flip angle: 73). To eliminate movement artifacts associated with speaking and to ensure that participants could hear the auditory stimuli, a sparse image acquisition technique was used during LISTEN, REPEAT, and GENERATE. A silent period (1.5 s for LISTEN and 4.5 s for REPEAT and GENERATE) was interleaved between each volume acquisition. Trials containing errors were excluded from the analysis of the behavioral and fMRI data. High-resolution T1-weighted volumes were acquired for anatomical localization.
Images were spatially registered, motion-corrected, mean-normalized, and despiked using AFNI (Cox 1996). There were separate regressors for each of the experimental conditions (OBJECTObs, Listen Action, Listen Object, Repeat Action, Repeat Object, Generate Action, Generate Object, and ACTIONObs). Additional regressors were the mean, linear, and quadratic trend components, as well as the 6 motion parameters (x, y, z, roll, pitch, yaw). A linear least squares model was used to establish a fit to each time point of the hemodynamic response function for each of these conditions. We modeled a 2-s period beginning at the start of the stimuli (whether sentence, picture or video).
We used FreeSurfer (Dale et al. 1999; Fischl et al. 1999) to create surface representations of each participant's anatomy. SUMA was used to import the surface representations and project the functional data onto the 2D surfaces. Data were smoothed on the surface with a Gaussian 6-mm full-width at half-maximum filter. The group analyses were performed using SUMA on the subjects’ beta values resulting from the first level analysis. We first examined the main effect of each condition compared against a resting baseline. A permutation approach (Nichols and Holmes 2002) was used to identify significant clusters of positively activated vertices, with an individual vertex threshold of P < 0.005, corrected for multiple comparisons to achieve a family-wise error rate of P < 0.05 (clusters ≥168 vertices). We also identified core (task-independent) brain areas involved in language by computing the intersection (or conjunction) (Nichols et al. 2005) of brain activity from the whole-brain contrasts, separately for the action and the object sentences. Five such conjunctions were computed: 1) all observation tasks: OBJECTObs ∩ ACTIONObs, 2) all action sentence tasks: LISTEN Action ∩ REPEAT Action ∩ GENERATE Action, 3) all object sentence tasks: LISTEN Object ∩ REPEAT Object ∩ GENERATE Object, 4) all action tasks: LISTEN Action ∩ REPEAT Action ∩ GENERATE Action ∩ ACTIONObs, and, finally, 5) all object tasks: LISTEN Object ∩ REPEAT Object ∩ GENERATE Object ∩ OBJECTObs. Conjunction analyses complement standard subtraction approaches by revealing the brain regions that are commonly activated across 2 or more distinct tasks (Nichols et al. 2005).
An analysis of anatomical regions of interest (ROIs) was also performed on a set of 9 sensorimotor regions selected a priori. Each ROI was identified on each individual's cortical surface representation using an automated parcellation scheme as implemented in FreeSurfer (Fischl et al. 2002, 2004; Desikan et al. 2006). This procedure uses a probabilistic labeling algorithm that incorporates the anatomical conventions of Duvernoy (1991) and thus is based on macroanatomical landmarks not on cytoarchitectonic maps, and therefore represents only an approximation to the actual motor and premotor areas. These ROIs were PMv and PMd, ventral and dorsal M1, the ventral and dorsal primary somatosensory areas, pars opercularis and triangularis of the Inferior frontal gyrus (IFG) and pre-supplementary motor area (pre-SMA). These ROIs were defined as follows: 1) IFG pars opercularis: Unedited FreeSurfer ROI, defined as the gyrus immediately anterior to the precentral gyrus. Pars opercularis is bounded caudally by the precentral sulcus and rostrally by pars triangularis. 2) IFG pars triangularis: Unedited FreeSurfer ROI, defined as the gyrus immediately anterior to the pars opercularis. Pars triangularis is bounded caudally by pars opercularis and rostrally by pars orbitalis and does not include the inferior frontal sulcus. 3) PM: For PM, we edited the FreeSurfer precentral sulcus and gyrus regions, by subdividing them into ventral (PMv) and dorsal (PMd) segments at the level of the junction of the inferior frontal sulcus and the precentral sulcus. The resulting PMv is bounded rostrally by the IFG pars opercularis, caudally by the central sulcus, and dorsally by PMd, and it includes the precentral sulcus. PMd, which is bounded rostrally by the superior frontal sulcus and gyrus and caudally by the central sulcus. 4) M1: For M1, we edited the FreeSurfer central sulcus region by subdividing it into a ventral (M1v) and a dorsal (M1d) segment at the level of the junction of the inferior frontal sulcus and the precentral sulcus. M1 is bounded rostrally by the precentral gyrus and caudally by the postcentral gyrus. 5) S1: For S1, we edited the FreeSurfer postcentral gyrus region by subdividing it into a ventral (S1v) and a dorsal (S1d) segment at the level of the junction of the inferior frontal sulcus and the precentral sulcus. S1 is bounded rostrally by the central sulcus and caudally by the postcentral sulcus. 6) Pre-SMA: For the pre-SMA, we edited the FreeSurfer superior frontal gyrus (SFG) region to keep only the medial aspect of SFG. Pre-SMA is bounded rostrally by a virtual line passing through the genu of the corpus callosum caudally by a virtual line passing through the anterior commissure (VAC line) and ventrally by the cingulate sulcus. The mean percentage of blood oxygen level–dependent signal change was extracted for each ROI and entered in a 3-way analysis of variance with repeated measurement on the Task (LISTEN, REPEAT, GENERATE), Semantic content (Object, Action), and Hemisphere. In addition, we compared OBJECTObs and ACTIONObs using 2-tailed paired sample t-tests.
Behavioral Data Analyses
Participants’ responses were recorded online using Labview (National Instruments) and stored to disk for offline analysis. The responses for 2 participants could not be analyzed due to technical difficulty. A research assistant naive to the purpose of the study transcribed the responses for the 19 remaining participants. For each sentence, we verified accuracy (whether or not it conformed to task instructions) and grammaticality (whether the sentence was correctly formed). In addition, we calculated the number of syllables and words per sentence. These analyses were necessary to determine whether the sentences produced during the sentence generation tasks were comparable with sentences produced in the sentence repetition task. Trials containing errors were removed from the analysis of the behavioral and fMRI data.
Results
Behavioral Data
The percentage of accurate responses during the fMRI tasks was high (92.3 ± 4.6%). In the sentence repetition task, accuracy reached 98.8% for the action sentences and 97.5% for the object sentence. This difference was not significant (T1,18 = 1.32, P = 0.25). Likewise, in the sentence generation task, accuracy reached 85.66 ± 9.5% for action sentences and 86.71 ± 9.61% for the object sentences. This difference was not significant (T1,18 = 1.05, P = 0.71). The number of words per sentence was 4.48 ± 0.18 on average and it did not vary as a function of task or semantic condition (LISTEN action: 4.4 words, LISTEN object: 4.57, REPEAT action: 4.41, REPEAT object: 4.57, GENERATE action: 4.73; GENERATE object: 4.22 words).
Neuroimaging Data
Nonlanguage Tasks (Object and Action Observation)
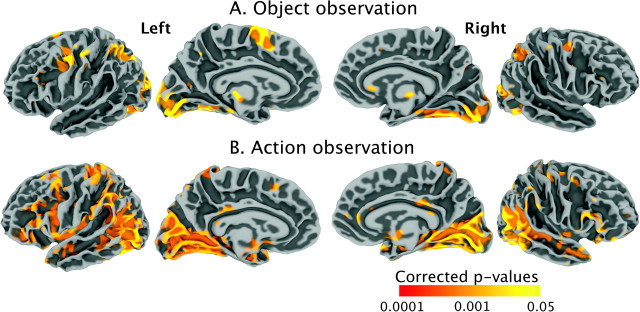
Whole-brain analyses. As shown in Figure 1 (top row), compared with a resting baseline, observation of objects (OBJECTObs) was associated with activation in the occipital lobe bilaterally, including parts of the lingual gyrus, cuneus, and calcarine sulcus. There was also activation in the dorsal intraparietal sulcus (IPS) bilaterally and in the left superior parietal lobule. In the frontal lobe, there was activation in the dorsal portion of the left PMv, in the dorsal portion of the left central sulcus representing the sensorimotor area (hand knob) for the right hand, and in the left pre-SMA. During action observation (ACTIONObs), activation was stronger and more widespread than in OBJECTObs and included additional clusters of activation in the right hand sensorimotor area, in the IFG bilaterally, in the ventral portion of the left PMv, in the posterior superior temporal sulcus (STS), middle temporal gyrus (MTG), in the ventral anterior sector of the IPS, and in the inferior parietal lobule (including the supramarginal gyrus) bilaterally. These findings are illustrated in Figure 1 (bottom row) and listed in Table 1.
Figure 1.
Group-level activation for (A) object observation compared against a resting baseline and (B) action observation compared against a resting baseline. The results are presented on average right and left lateral and medial brain surfaces.
Table 1.
Whole-brain analyses, nonlanguage tasks
| Description | Hemis | Coord | t | Nodes |
| a. Action observation | ||||
| Inferior temporal gyrus, lateral occipito-temporal sulcus, fusiform gyrus, middle occipital gyrus, extending dorsally and anteriorly into the posterior MTG, STS and STG supramarginal gyrus and IPS. The cluster also covers the medial aspect of the posterior hemisphere, including the parahippocampal gyrus, the entire calcarine sulcus, cuneus and lingual gyrus, and the parieto-occipital fissure. | Left | −41 −43 −18 | 11.017 | 30 758 |
| Precentral gyrus, central sulcus and postcentral gyrus, dorsally (hand motor area). | −29 −21 61 | 6.899 | 3675 | |
| Intraoccipital sulcus. | −32 −75 35 | 6.165 | 1837 | |
| Insula | −35 −10 −5 | 6.288 | 1386 | |
| Pars opercularis of the inferior frontal gyrus, precentral sulcus. | −46 13 21 | 5.384 | 1044 | |
| Middle frontal gyrus. | −33 9 57 | 5.458 | 1181 | |
| Dorsal central sulcus, extending into the precentral gyrus. | −11 −34 65 | 4.262 | 772 | |
| MTG and posterior STS. | −60 −38 −9 | 4.743 | 756 | |
| Pars orbitalis of the inferior frontal gyrus. | −45 38 −6 | 7.141 | 359 | |
| Middle frontal gyrus, extending into the inferior frontal sulcus. | −43 32 27 | 5.067 | 618 | |
| Anterior STS. | −50 −11 −10 | 4.229 | 369 | |
| Pars triangularis of the inferior frontal gyrus. | −47 34 9 | 5.247 | 341 | |
| Orbital gyrus. | −24 20 −22 | 4.505 | 260 | |
| Lateral superior frontal gyrus. | −18 19 53 | 5.16 | 361 | |
| Pars orbitalis of the inferior frontal gyrus. | −39 35 −6 | 4.425 | 269 | |
| Anterior inferior temporal gyrus. | −42 16 −30 | 3.902 | 238 | |
| Ventral central sulcus. | −46 −13 32 | 3.861 | 207 | |
| Collateral sulcus, fusiform gyrus and inferior occipital gyrus, extending into the medial aspect of the hemisphere, into the parahippocampal gyrus, the entire calcarine sulcus, cuneus and lingual gyrus, and dorsally into the parieto-occipital fissure. The cluster also covers the inferior temporal gyrus and sulcus, MTG and STS. | Right | 10 −74 15 | 10.216 | 28 361 |
| Intraoccipital sulcus. | 34 −72 38 | 7.366 | 1696 | |
| Anterior STG and STS. | 51 13 −18 | 5.442 | 805 | |
| IPS. | 33 −38 39 | 5.008 | 1224 | |
| Dorsal central sulcus and precentral gyrus (hand sensorimotor area). | 31 −20 60 | 5.208 | 547 | |
| Lateral occipito-temporal sulcus. | 39 −13 −27 | 5.834 | 290 | |
| Orbital gyrus. | 32 30 −18 | 5.854 | 300 | |
| Pars triangularis of the inferior frontal gyrus. | 55 27 14 | 4.311 | 394 | |
| Pars orbitalis of the inferior frontal gyrus. | 43 42 −15 | 5.962 | 191 | |
| Supramarginal gyrus. | 60 −37 33 | 5.174 | 267 | |
| Dorsal central sulcus and postcentral gyrus (hand sensorimotor area). | 31 −28 57 | 4.705 | 197 | |
| Dorsal postcentral gyrus and sulcus. | 15 −33 73 | 5.398 | 222 | |
| Pars triangularis of the inferior frontal gyrus. | 54 32 −2 | 5.25 | 196 | |
| Dorsal central sulcus. | 10 −31 76 | 4.475 | 180 | |
| b. Object observation | ||||
| Middle occipital gyrus, inferior IPS and superior parietal lobule, extending medially into the lingual gyrus, cuneus, posterior calcarine sulcus, and fusiform gyrus. | Left | −8 −93 −5 | 11.77 | 14 089 |
| Precentral gyrus and middle frontal gyrus. | −46 −1 49 | 6.05 | 1632 | |
| Medial frontal gyrus (pre-SMA). | −5 13 60 | 5.12 | 1238 | |
| Precentral sulcus, extending anteriorly into the superior frontal sulcus. | −24 −1 46 | 4.80 | 767 | |
| Dorsal central sulcus (hand sensorimotor area). | −33 −23 52 | 4.09 | 415 | |
| Middle frontal gyrus, extending into the superior frontal sulcus. | −29 6 55 | 4.50 | 204 | |
| Middle occipital gyrus, intraoccipital sulcus and IPS, superior parietal lobule, extending medially into the lingual gyrus, cuneus, posterior calcarine sulcus, and fusiform gyrus. | Right | 28 −77 −7 | 12.70 | 12 737 |
| Middle frontal gyrus and precentral sulcus. | 31 5 51 | 4.62 | 1136 | |
Note: List of all significant clusters for the contrast of object observation compared against a resting baseline (A) and action observation compared against a resting baseline (B). Legend: Hemis, Hemisphere; Coord, Talairach coordinates (x y z); t = t-value of the local maximum, corrected for multiple comparison using permutation; Nodes, number of nodes in cluster.
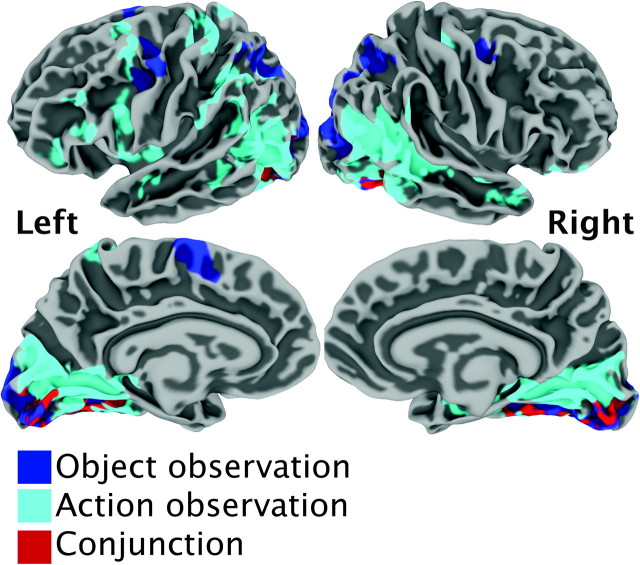
Conjunction. This analysis identified brain areas sensitive to observation by computing the conjunction of activation for OBJECTObs ∩ ACTIONObs, each significantly active above a resting baseline. As detailed in Figure 2, the results revealed activation in striate and extrastriate cortex bilaterally, in the left superior parietal, and in the IPS. Activations for these contrasts are listed in Table 2A.
Figure 2.
Group-level activation for object observation (turquoise), action observation (blue), and the conjunction of the 2 (red) presented on average left and right back, lateral and medial brain surfaces.
Table 2.
Conjunction analyses
| Description | Hemis | Coord | Nodes |
| A. Intersection of the nonlanguage tasks | |||
| Fusiform gyrus, lingual gyrus, caudal calcarine sulcus, cuneus, lateral occipito-temporal sulcus and collateral sulcus. | Left | −29 −74 −12 | 2827 |
| Intraoccipital sulcus extending into the intraoccipital sulcus. | −28 −65 36 | 823 | |
| Lingual gyrus, caudal calcarine sulcus and cuneus. | −6 −85 0 | 633 | |
| Middle and inferior occipital gyri. | −35 −83 10 | 368 | |
| Fusiform gyrus, lateral occipito-temporal sulcus and collateral sulcus. | Right | 40 −56 −14 | 2047 |
| Lingual gyrus, caudal calcarine sulcus and cuneus. | 10 −85 −1 | 670 | |
| Intra-occipital sulcus. | 32 -69 29 | 575 | |
| B. Intersection of all object-related language tasks | |||
| Transverse temporal gyrus, extending posteriorly into the planum temporale, supramarginal gyrus, and parietal operculum, and medially into the posterior insula. | Left | −51 −31 24 | 3552 |
| Medial frontal gyrus (pre-SMA). | −7 8 45 | 1097 | |
| Ventral postcentral gyrus/parietal operculum. | −42 −16 18 | 670 | |
| Ventral precentral gyrus (PMAv). | −48 −7 39 | 525 | |
| Cingulate sulcus. | −8 2 37 | 462 | |
| Postcentral gyrus. | −57 −11 38 | 401 | |
| Inferior frontal gyrus, pars triangularis. | −48 34 2 | 260 | |
| Calcarine fissure | −25 −63 7 | 188 | |
| Anterior STS | −48 −4 −17 | 180 | |
| Frontal operculum | -46 4 4 | 200 | |
| Posterior STS | −61 −35 6 | 165 | |
| Middle STS | −57 −22 −2 | 163 | |
| Transverse temporal gyrus, extending posteriorly into the planum temporale, and medially into the posterior insula. | Right | 58 −20 7 | 2721 |
| Posterior STS | 62 −31 5 | 1517 | |
| Parietal operculum. | 63 −7 10 | 598 | |
| Anterior cingulate sulcus. | 10 13 34 | 295 | |
| Postcentral gyrus. | 55 −11 43 | 187 | |
| C. Intersection of all action-related language tasks | |||
| Transverse temporal gyrus, extending posteriorly into the planum temporale, supramarginal gyrus, and parietal operculum, and medially into the posterior insula. | Left | −44 −39 26 | 3853 |
| Middle STS | −60 −30 2 | 329 | |
| Medial frontal gyrus (pre-SMA). | −8 1 59 | 186 | |
| Ventral precentral gyrus (PMAv). | −48 −6 43 | 242 | |
| Posterior STS | −53 −52 6 | 269 | |
| Transverse temporal gyrus, extending posteriorly into the planum temporale, and medially into the posterior insula. | Right | 59 −20 6 | 2940 |
| STS | 62 −31 5 | 1781 | |
| Parietal operculum | 64 −11 16 | 326 | |
Note: List of all significant clusters for the conjunction of the object sentences (A), the conjunction of the action language (B), and for the conjunction of OBJECTObs and ACTIONObs (C). Legend: Hemis, Hemisphere; Coord, Talairach coordinates (x y z); Nodes, number of nodes in cluster.
ROI analyses. The dorsal postcentral gyrus bilaterally was sensitive to the Object/Action contrast (object < action). PMd in both hemispheres was also sensitive to this contrast but exhibited the opposite activation pattern (object > action). The results of the ROI analysis are reported in Table 3A.
Table 3.
ROI analysis
| ANOVA results |
|||||
| Area | Hemisphere | Content | Content by Hemi | ||
| A. Observation tasks (ObserveAction vs. ObserveObject) by hemisphere | |||||
| Pre-SMA | 0.06 | 0.08 | 0.38 | ||
| Dorsal precentral sulcus (dorsal PMA) | 0.82 | 0.003* | 0.38 | ||
| Ventral precentral sulcus (ventral PMA) | 0.0008* (A) | 0.30 | 0.38 | ||
| Dorsal precentral gyrus (dorsal M1) | 0.88 | 0.53 | 0.17 | ||
| Ventral precentral gyrus (ventral M1) | 0.61 | 0.53 | 0.61 | ||
| Dorsal postcentral gyrus (dorsal S1) | 0.44 | 0.0006* (B) | 0.38 | ||
| Ventral postcentral gyrus (ventral S1) | 0.57 | 0.66 | 0.38 | ||
| IFG opercularis | 0.57 | 0.13 | 0.38 | ||
| IFG triangularis | 0.57 | 0.13 | 0.38 | ||
| Area | Hemisphere | Task | Content | Content by Task | Content by Hemi |
| B. Language task (LISTEN, REPEAT, GENERATE) by content (Action, Object) by hemisphere | |||||
| Pre-SMA | 0.033* (A) | 0.0000045* (C) | 0.273 | 0.273 | 0.910 |
| Dorsal precentral sulcus (dorsal PMA) | 0.0045* (A) | 0.0001* (B) | 0.645 | 0.645 | 0.910 |
| Ventral precentral sulcus (ventral PMA) | 0.0009* (A) | 0.0001* (B) | 0.018* (H) | 0.018 | 0.910 |
| Dorsal precentral gyrus (dorsal M1) | 0.980 | 0.01* (C) | 0.968 | 0.968 | 0.910 |
| Ventral precentral gyrus (ventral M1) | 0.180 | 0.0001* (C) | 0.518 | 0.518 | 0.910 |
| Dorsal postcentral gyrus (dorsal S1) | 0.797 | 0.0000045* (C) | 0.720 | 0.720 | 0.910 |
| Ventral postcentral gyrus (ventral S1) | 0.195 | 0.0001125* (C) | 0.970 | 0.970 | 0.910 |
| IFG opercularis | 0.045 | 0.0001125* (C) | 0.645 | 0.645 | 0.910 |
| IFG triangularis | 0.934 | 0.0001* (C) | 0.273 | 0.273 | 0.910 |
Note: Results of the ROI analyses. (A) Results of the ROI analyses for the nonlanguage analysis of variance (ANOVA) conducted on the ROIs, with repeated measurements on the semantic content (Object, Action) and hemisphere (left, right). The statistics are FDR corrected P values (q values). Asterisks indicate significance at FDR level of 0.05. Legend: A: Left hemisphere > right hemisphere; B: Object Observation < Action Observation. (B) Results of the language ANOVA conducted on the ROIs, with repeated measurements on the task (LISTEN, REPEAT, GENERATE), semantic content (Object, Action) and hemisphere (left, right). The statistics are FDR corrected P values (q values). Asterisks indicate significance at FDR level of 0.05. Legend: A: Left hemisphere > right hemisphere. B: LISTEN < REPEAT < GENERATE; C: LISTEN < REPEAT = GENERATE; D: Object > Action.
*The statistics are FDR corrected P values (q values). Asterisks indicate significance at FDR level 0.05
Language Tasks
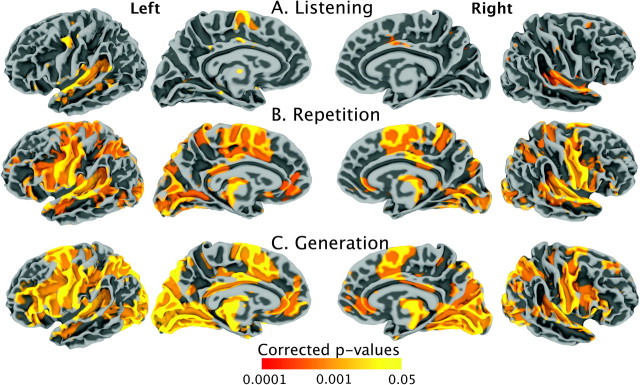
Whole-brain analyses. First we explored the pattern of task-related activation across the cortical surface for each language task (LISTEN, REPEAT, GENERATE) compared with the resting baseline. As shown in Figure 3, the result revealed activation for all tasks in the transverse temporal gyrus bilaterally, in the left superior PMv, and in the left pre-SMA. The speaking tasks (REPEAT, GENERATE) were associated with additional clusters of activation in PMv and the primary motor area (M1), bilaterally, in the pars triangularis and pars opercularis of IFG, and several parts of the parietal lobe bilaterally. No task was associated with active voxels in the hand sensorimotor area (the hand knob). Activations for these contrasts are listed in Table 4.
Figure 3.
Group-level activation for (A) sentence listening compared against a resting baseline, (B) sentence repetition compared against a resting baseline and (C) sentence generation compared against a resting baseline. The results are presented on average right and left lateral and medial brain surfaces.
Table 4.
Whole-brain analyses, language tasks
| Description | Hemis | Coord | t | Nodes |
| A. LISTEN | ||||
| Transverse gyrus, extending anteriorly into the anterior STG, and caudally into the planum temporale, posterior STG, dorsally into the parietal operculum, and medially into the insula. | Left | −52 −13 3 | 7.58 | 5649 |
| Ventral precentral gyrus, extending caudally into the central sulcus and postcentral gyrus. | −55 −7 12 | 5.62 | 1201 | |
| Posterior STS | −57 −32 2 | 5.65 | 954 | |
| Medial frontal gyrus (pre-SMA) | −6 6 66 | 4.43 | 774 | |
| Ventral Precentral gyrus. | −43 −8 46 | 4.60 | 433 | |
| Posterior MTG and STS. | −62 −48 3 | 4.21 | 370 | |
| Transverse gyrus, extending anteriorly into the anterior STG, and caudally into the planum temporale, posterior STG, and medially into the insula. | Right | 57 −10 0 | 8.28 | 3599 |
| Posterior STS. | 56 −31 3 | 7.30 | 1843 | |
| Ventral Precentral gyrus. | 51 −4 8 | 4.65 | 504 | |
| B. REPEAT | ||||
| Transverse gyrus, extending anteriorly into the anterior STG, and caudally into the planum temporale and posterior STG and STS. | Left | −38 −29 12 | 11.58 | 12 000 |
| Ventral precentral gyrus, extending caudally into the central sulcus and postcentral gyrus, and anteriorly into the precentral sulcus, inferior frontal gyrus, including both pars opercularis and triangularis. | −45 −8 38 | 12.36 | 10 088 | |
| Lingual gyrus, cuneus, middle occipital gyrus, inferior occipital gyrus, and fusiform gyrus. | −11 −98 −7 | 9.01 | 4857 | |
| Medial frontal gyrus (pre-SMA, SMA), and anterior cingulate gyrus. | −8 16 40 | 10.39 | 4640 | |
| Dorsal Precentral gyrus, extending into the central sulcus and the postcentral gyrus. | −17 −24 59 | 9.81 | 2836 | |
| Lateral occipito-temporal sulcus, extending medially into the fusiform gyrus. | −43 −48 −11 | 7.27 | 1273 | |
| Superior parietal lobule, IPS, precuneus. | −17 −67 45 | 7.06 | 2000 | |
| Body of the calcarine sulcus, extending dorsally into the cuneus. | −17 −73 11 | 5.42 | 1064 | |
| Inferior frontal gyrus, pars orbitalis | −49 34 3 | 10.25 | 1804 | |
| Midbrain | −1 −20 7 | 8.27 | 820 | |
| Posterior cingulate gyrus and sulcus. | −4 −6 39 | 6.90 | 638 | |
| Inferior frontal gyrus, pars orbitalis. | −42 33 −9 | 6.36 | 498 | |
| Posterior cingulate gyrus. | −5 −37 25 | 5.22 | 547 | |
| Paracentral lobule. | −8 −38 60 | 6.44 | 529 | |
| Anterior calcarine sulcus. | −19 −51 3 | 6.66 | 336 | |
| Precuneus, extending anteriorly into the occipitoparietal sulcus. | −9 −87 37 | 5.78 | 363 | |
| Cingulate sulcus/Medial frontal gyrus (SMA). | −8 −18 48 | 5.35 | 172 | |
| Ventral precentral gyrus, extending caudally into the central sulcus and postcentral gyrus, and anteriorly into the inferior frontal gyrus, pars opercularis, and triangularis. The cluster also extends ventrally into the insula, and posterior STG, transverse temporal gyrus and runs anteriorly along the STG and STS. | Right | 60 −7 23 | 12.56 | 23 556 |
| Posterior collateral sulcus, occipital gyrus, extending caudally into the fusiform gyrus, laterally into the lateral occipitoparietal sulcus, and anteriorly and dorsally into the inferior and medial occipital gyri and occipital pole. | 22 −88 −7 | 10.31 | 6407 | |
| Medial frontal gyrus (SMA and pre-SMA), extending ventrally into the cingulate sulcus and gyrus. | 6 6 63 | 12.40 | 6445 | |
| Dorsal postcentral gyrus, extending anteriorly into the dorsal central sulcus and gyrus. | 22 −28 66 | 12.29 | 4500 | |
| Calcarine sulcus (body and anterior), and cuneus. | 13 −67 10 | 6.74 | 1352 | |
| Superior and inferior segment of the IPS, and superior parietal lobule. | 28 −63 49 | 6.84 | 1198 | |
| Posterior thalamus. | 6 −27 1 | 6.73 | 705 | |
| Parieto-occipital sulcus, extending dorsally into the superior occipital gyrus. | 16 −78 34 | 6.17 | 918 | |
| Intraoccipital sulcus and superior occipital gyrus. | 35 −77 25 | 5.78 | 256 | |
| Lingual gyrus, extending into the body of the calcarine sulcus. | 5 −80 3 | 5.63 | 181 | |
| Middle frontal gyrus and superior frontal sulcus. | 37 35 30 | 6.85 | 214 | |
| Inferior frontal gyrus, pars triangularis. | 50 29 1 | 5.55 | 321 | |
| C. GENERATE | ||||
| Lingual gyrus, cuneus, middle occipital gyrus, fusiform gyrus, lateral occipito-temporal sulcus and inferior temporal gyrus, extending anteriorly into the supramarginal gyrus, and more dorsally into the IPS and superior parietal lobule. | Left | −2 −79 1 | 12.78 | 36 469 |
| Ventral central sulcus, extending caudally into the postcentral gyrus, and dorsally and medially into the medial frontal gyrus (SMA, pre-SMA) and anterior cingulate, and anteriorly into the Precentral gyrus and inferior frontal gyrus, including both pars opercularis and triangularis. | −43 −17 36 | 12.63 | 24 100 | |
| Transverse gyrus, extending anteriorly into the anterior STG, and caudally into the planum temporale | −49 −20 5 | 13.47 | 4387 | |
| Posterior midbrain. | −6 −26 −5 | 9.83 | 1992 | |
| Posterior STG and sulcus. | −54 −39 −6 | 7.95 | 1545 | |
| Dorsal Precentral gyrus and central sulcus. | −16 −25 59 | 8.53 | 913 | |
| Posterior cingulate gyrus. | −4 −31 29 | 6.55 | 1162 | |
| Posterior cingulate sulcus. | −10 −22 36 | 5.89 | 673 | |
| Postcentral gyrus. | −21 −32 62 | 6.48 | 555 | |
| Entorhinal cortex, extending laterally into the anterior inferior temporal gyrus. | −30 −1 −38 | 6.06 | 273 | |
| Insula. | −32 −7 15 | 5.28 | 238 | |
| Inferior occipital sulcus, fusiform gyrus, collateral sulcus, extending dorsally into the lingual gyrus, calcarine fissure, cuneus, parieto-occipital sulcus and lateral occipito-temporal sulcus. The cluster also extends onto the lateral surface including the intraoccipital fissure, middle and superior occipital gyri and superior parietal lobe. | Right | 45 −61 −10 | 14.59 | 34 464 |
| Anterior cingulate sulcus and gyrus, extending dorsally into the medial frontal gyrus (Pre-SMA and SMA), and laterally onto the dorsal superior frontal gyrus and sulcus, precentral sulcus, ventral precentral gyrus, central sulcus and postcentral sulcus, and inferior frontal gyrus, pars opercularis. | 10 15 33 | 13.90 | 13 881 | |
| Transverse temporal gyrus, planum temporale, posterior STG and STS. | 55 −10 1 | 8.90 | 7524 | |
| Anterior insula, inferior frontal gyrus, pars orbitalis, and orbital gyrus. | 31 17 4 | 8.62 | 1890 | |
| Posterior thalamus. | 10 −26 8 | 11.18 | 1425 | |
| Dorsal postcentral gyrus, central sulcus and precentral gyrus. | 20 −29 63 | 6.78 | 965 | |
| Posterior cingulate gyrus. | 6 −31 29 | 6.39 | 680 | |
| Inferior frontal gyrus, pars triangularis. | 47 13 1 | 7.02 | 464 | |
| Posterior cingulate sulcus. | 11 −13 38 | 5.46 | 443 | |
| Insula. | 34 −4 14 | 7.36 | 209 | |
| Paracentral lobule. | 10 −36 61 | 5.15 | 206 | |
| Parahippocampal gyrus. | 26 −3 −36 | 4.64 | 197 | |
Note: List of all significant clusters for sentence listening compared against a resting baseline (A), sentence repetition compared against a resting baseline (B) and sentence generation generate compared against a resting baseline (C). Legend: Hemis, Hemisphere; Coord, Talairach coordinates (x y z); t = t-value of the local maximum, corrected for multiple comparison using permutation; Nodes, number of nodes in cluster.
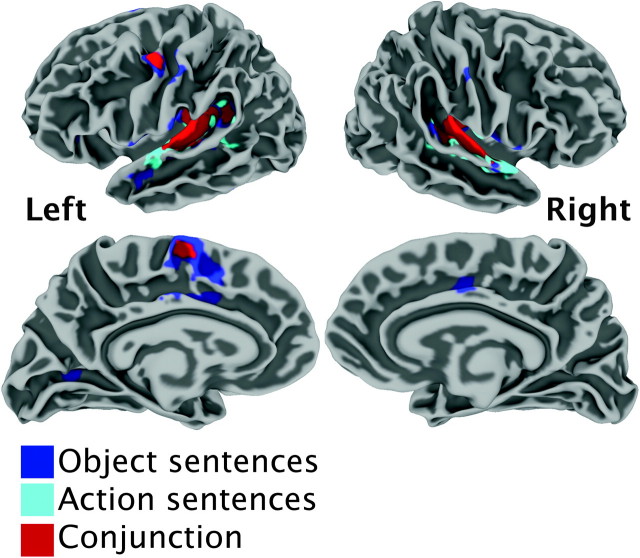
Conjunctions. This analysis examined the conjunction of activation for all language tasks, each significantly active above a resting baseline, separately for the action and object sentences, yielding 2 task-independent conjunction maps representing “action sentences” and “object sentences,” respectively. As shown in Figure 4 and Table 2B and 2C, both of these maps revealed activation along the bilateral transverse temporal gyrus and posterior superior temporal gyrus (STG), in the posterior STS and MTG bilaterally, as well as in the left superior PMv and left pre-SMA. In addition, for the object sentences, there was activation in the left anterior STS, right pre-SMA, and left calcarine sulcus. The conjunction of all the language tasks across semantic content shows activation in the left superior PMv, bilateral transverse temporal gyrus, and left pre-SMA.
Figure 4.
Group-level activation for the object sentence conjunction (turquoise), action sentence conjunction (blue), and the conjunction of the 2 (red) presented on average left and right back, lateral and medial brain surfaces.
Anatomical ROI analyses. This analysis revealed that most of the ROIs were modulated by task (LISTEN, REPEAT, GENERATE) with stronger activation for speaking than for listening. The main objective of this analysis was to identify regions sensitive to the semantic content (Object vs. Action) of concrete sentences independent of the task. This pattern was found in only one region, PMv. The results of the ROI analysis are presented in Table 3B.
Language Versus Observation
To examine whether understanding the meaning of a sentence or a word describing an action requires activation of the motor circuits required to produce that action, we computed the conjunction of Action sentence and Action observation, which is shown in Figure 5A. Interestingly, although left PMv was present in both conjunction maps, different parts of this region were active and did not overlap at all. Processing action sentences activated the superior part of PMv, while observing actions activated a more ventral part of PMv. Another striking difference was the presence of activation in the hand sensorimotor area during ACTIONObs (bilaterally) but not in any of the language conditions. Areas of overlap were found in posterior STG and posterior STS. In addition to this analysis, we also computed the conjunction of the Object conditions, which is shown in Figure 5B. This analysis revealed overlap at the level of the left superior PMv and left pre-SMA. There was activation along the calcarine sulcus for language and nonlanguage but it did not overlap.
Figure 5.
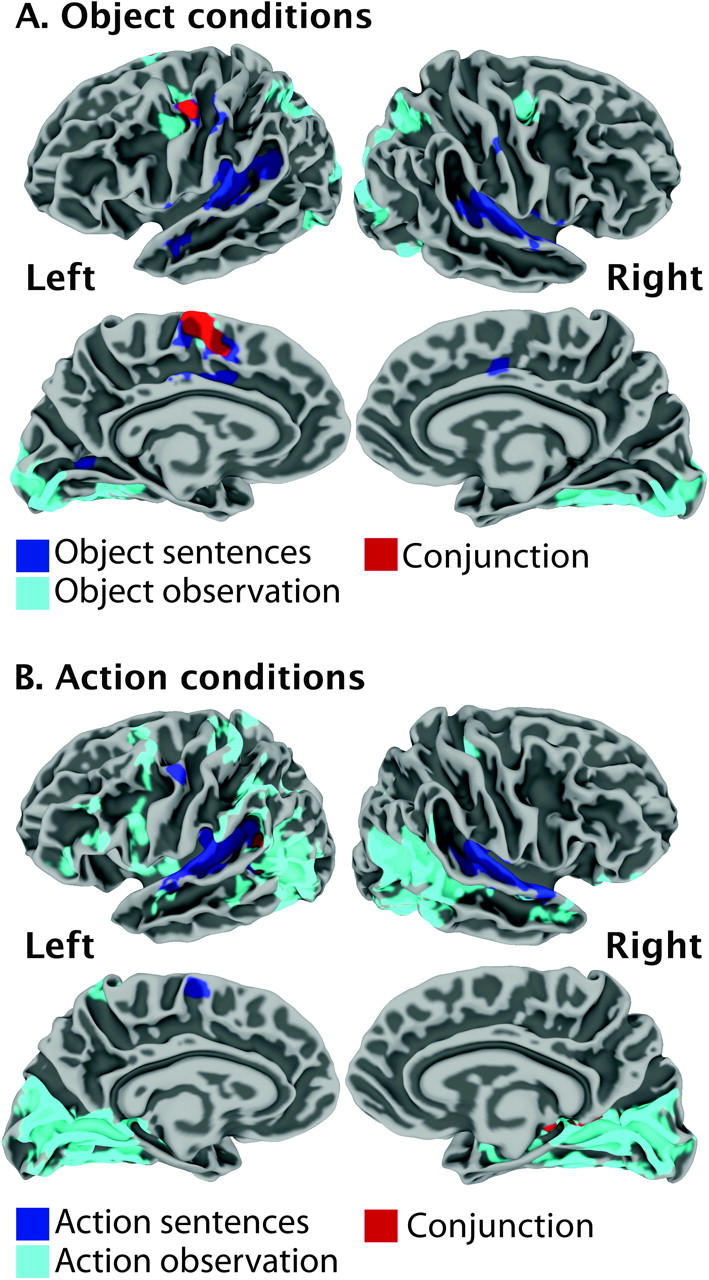
(A) Group-level activation for action sentence (blue), action observation (turquoise), and the conjunction of the 2 (red) presented on average right and left lateral and medial brain surfaces. (B) Group-level activation for object sentence (blue), object observation (turquoise), and the conjunction of the 2 (red) presented on average right and left lateral and medial brain surfaces.
Discussion
This study was designed to examine the pattern of activation in primary motor and premotor cortices during action and object observations and to characterize the role for these areas in language processing. While our results argue for a role for the motor system in language comprehension, they also argue against the notion that action simulation alone suffices to explain semantic interpretation during language comprehension. The lack of congruence in functional anatomy for observing actions and understanding action-related sentences makes it difficult to support a strong simulation account without postulating additional mechanisms. These findings and their implications are discussed in detail below.
Action and Object Observations
Consistent with previous imaging studies (Chao and Martin 2000; Buccino et al. 2001; Grezes et al. 2003; Handy et al. 2006), we found significant PMv activation during action and object observations. Interestingly, action and object observations activated different sectors of PMv, with a more inferior and anterior locus for action observation. Moreover, action and object observations were associated with dissociable activation patterns in the parietal lobe, with activation in the anterior IPS and supramarginal gyrus for action observation, and activation in a more dorsal and posterior part of IPS for object observation. In the monkey brain, ventral premotor area F5 contains, in addition to mirror neurons, a population of neurons called canonical neurons. Canonical neurons respond to the execution of actions and to the sight of objects that afford these actions but not to the sight of actions per se (Rizzolatti et al. 1988; Jeannerod et al. 1995). While mirror neurons are located primarily in the caudal sector of F5 in the cortical convexity of F5 (area F5c), canonical neurons are mostly located in a more rostral sector of F5, buried within the posterior bank of the inferior arcuate sulcus (area F5ab). Interestingly, areas F5c and F5ab have distinct connectivity patterns with the parietal lobe, with area F5c strongly connected with the rostral inferior parietal lobule (area PF) (Kurata 1991; Rizzolatti et al. 1998; Tanne-Gariepy et al. 2002), which corresponds roughly to the human supramarginal gyrus, and area F5ab strongly connected to the IPS (Luppino et al. 1999; Borra et al. 2008), with which it forms a circuit involved in visual transformation for grasping (Jeannerod et al. 1995; Rizzolatti et al. 1998). Hence, the current results suggest a similar organizational principle within the human PMv with a different topography, with a ventral PMv sector containing neurons with mirror properties, and a dorsal PMv sector containing neurons with canonical properties. One limitation of the current study is the absence of an action execution task, which limits our ability to determine whether the populations of neurons responsible for PMv activation actually have canonical and mirror-like properties. Nevertheless, the current findings suggest that the human PMv shares important organizational principles with macaque area F5.
Another highlight of the present study is the finding of activation in another premotor area, the left pre-SMA, for action and object observations. Activation in the pre-SMA during object observation has been shown in the monkey (Rizzolatti et al. 1990). It is noteworthy that activation in the left pre-SMA was more widespread for object than action observation, perhaps reflecting a greater demand on pre-SMA for selecting a motor response during object observation. In the present study, when participants looked at an object such as a book, multiple hand actions (motor programs) may have become coactivated, such as holding the book, flipping the pages of the book, closing the book, etc., resulting in strong and widespread activation of the left pre-SMA. In contrast, when participants watched a goal-directed action (e.g., an actor flipping the pages of a book), only one hand-related action became activated (i.e., flipping the pages of the book in this example), resulting in lower activation in the pre-SMA. This interpretation is consistent with previous results showing that when motor responses are selected from among several equally appropriate responses, activation in pre-SMA increases (Deiber et al. 1996; , Van Oostende et al. 1997; Sakai et al. 2000; Lau et al. 2004, 2006). The present results extend these previous studies by suggesting that the pre-SMA may be involved in response selection even in the absence of an overt behavior.
Action and Object Observations Versus Language
PMv and M1
As discussed in the introduction, most studies demonstrating a role for the motor system in language comprehension have come to that conclusion by focusing exclusively on passive listening. In the present study, we aimed to understand the extent to which these findings are generalizable to a range of sentence-level language tasks. To this aim, we used a 2-step analysis that first identified brain regions involved in language processing by 1) examining the intersection of brain activation from 3 language tasks—listening to sentences, listening and repeating sentences, and generating sentences from object pictures—and then 2) identifying from this intersection those regions sensitive to sentence meaning by comparing brain activation for action- and object-related sentences. The results of this analysis demonstrate activation in a relatively circumscribed sector of the left superior PMv across all language tasks (LISTEN ∩ REPEAT ∩ GENERATE) which is sensitive to the semantic content of the sentences (object > action), thereby suggesting a role for this region in comprehending concrete sentences describing manual actions and manipulable objects. Certainly, regions that survive the conjunction analysis can be related to a variety of linguistic and nonlinguistic processes involved in sentence processing and not be critical to comprehension per se, such as working memory, attention, and phonological processing. For instance, it could be argued that activation in the left PMv is related to motor processes such as subvocal rehearsal or phonological processing, engaged not only in producing but also in perceiving language, consistent with previous results (Zatorre et al. 1992; Watkins et al. 2003; Watkins and Paus 2004; Meister et al. 2007). However, the finding that PMv is sensitive to the semantic content of the sentences argues against strict perceptual-motor, executive, or memory-related interpretations but instead suggests that PMv may be contributing sensorimotor information used to comprehend language. In support of this interpretation, the behavioral data acquired during the tasks indicate that accuracy for the action and object sentences was identical, suggesting that participants were paying attention to both types of sentences. Moreover, in the behavioral study conducted prior to the imaging sessions, action and object sentences were treated similarly, with identical repetition accuracy and reaction times. Finally, the sentences were carefully matched in syntactic complexity and thus should have made similar demands on working memory. In sum, the available evidence suggests a role for PMv in comprehending sentences describing manual actions and manipulable objects. The role of PMv in language comprehension may be limited to these specific types of contexts, and it is possible that comprehension does not require contribution of this region. In keeping with this hypothesis, a recent fMRI study by Tomasino et al. (2010) suggests that activation in M1 and PM decreases when participants process negative sentences such as “Don't grasp,” as compared with affirmative sentences such as “Do grasp.” The authors interpreted this finding to suggest that the contribution of sensorimotor regions is not a requirement for language comprehension. Indeed, participants in that study understood the negative sentences just as well as the affirmative sentences, yet the activation magnitude in these areas decreased when negative constructions were used. Unfortunately, brain-imaging studies, for all their advantages, cannot answer the question of whether PMv is critical or accessory to language comprehension. Additional studies using brain stimulation methods such as TMS are required to further characterize the importance of the contribution of PMv to concrete sentence processing.
An interesting finding of the present study is that PMv was more strongly active for object-related sentences than for action-related sentences. It could be argued that activation in PMv is stronger for object than action sentences because processing object sentences may coactivate a range of related motor programs reflecting the different ways that an object can be manipulated/used, while processing action sentences may activate only one motor program. An alternative interpretation is that activation in PMv during sentence processing reflects subjective processes by which objects and actions are perceived by imagining, or visualizing, how they may be used or manipulated. If imagery occurs at a motor level, it is conceivable that the stronger activation for object compared with action reflects the (internal) enactment of multiple related hand motor programs. Both accounts are consistent with previous studies showing little or no effect of low frequency repetitive TMS stimulation of PMv on participants’ ability to perceive speech (Sundara et al. 2001; Sato et al. 2009), suggesting that the contribution of this region may not be to process the speech sound signal but to contribute to language comprehension or imagery.
One of the most important findings of the present study is that activation in PMv associated with processing action sentences does not overlap with activation in PMv for observing actions. This challenges the hypothesis that localized observation execution matching, that is, an anatomically defined mirror mechanism, underlies all language comprehension. It may be that a putative human mirror neuron system (and the ventral PMv) is not a necessary component for language understanding generally, although this does not imply that it does not play a role in certain circumstances (although this remains to be demonstrated). Nevertheless, this interpretation is consistent with the results of a recent fMRI study showing a lack of congruency in the involvement of motor/premotor areas during action observation/execution and action word processing (Postle et al. 2008). It is also consistent with the argument from advocates of “disembodied cognition” (e.g., Mahon and Caramazza 2008), who are still awaiting lesion data demonstrating the necessity of mirror neurons for comprehension. It is clear that PMv is a core region that is part of a distributed network involved in processing language, as it survived the language conjunction analysis and was modulated by the semantic content of the sentences, but the precise nature of its role is less clear. It should be noted, however, that the present results do not necessarily speak to a contribution of the left PMv to language processing in general. It is possible that processing abstract sentences would not engage the left PMv. The possible role of PMv in language comprehension or motor imagery will need to be clarified through the examination of a potential causal relationship between language and PMv, for example, by evaluating patients with brain lesions or by using transcranial magnetic stimulation, which can help determine the importance of a region on a behavior or process by inducing a focal “virtual lesion.”
Another interesting finding of the present study is that while we observed activation in the hand sensorimotor areas bilaterally during the observation of actions, consistent with previous reports (Hari et al. 1998; Gazzola and Keysers 2009), there was no activation of this region for any of the language tasks. It is possible that observing actions, but not processing language, automatically triggers (kinetic) motor imagery, a process that has been associated with M1 activation (Grafton et al. 1996; Porro et al. 1996; Roth et al. 1996; Grezes and Decety 2002; Solodkin et al. 2004, see also Jeannerod (2001) for a review). Results of a recent study show that unless explicitly instructed to perform mental imagery, M1 is not activated during language processing (Tomasino et al. 2007), a finding which is consistent with the current results. Other studies also downplay a role for M1 in language (Papeo et al. 2009). Willems et al. (2010) compared brain responses to manual (e.g., throw) and nonmanual (e.g., kneel) action words during mental imagery and lexical decision tasks and found effector sensitive activation in primary motor and PM for both. There was, however, no overlap between the effector sensitive voxels in the imagery and lexical decision tasks, suggesting that different mechanisms are engaged during language processing and imagery. Together with previous findings, the current results support the notion that processing language does not automatically elicit imagery, although this does not mean that it never elicits it. If language processing does not naturally elicit imagery, then it follows that the activation that we have found in PMv during sentence processing is likely to reflect a contribution of this region in the processing of sentence meaning, not imagery, an interpretation that is further supported by the finding that activation level in PMv is modulated by sentence semantics (object > action), as discussed above. These findings also suggest that that imagery is not the primary mechanism of semantic interpretation.
Pre-SMA
In addition to activation in PMv, we also found activation in the left pre-SMA for language (object > action) and also for the nonlanguage tasks (object > action), as discussed in the previous section. Previous results show that when overt or covert words are selected from among several equally appropriate words, activation in pre-SMA is enhanced (Etard et al. 2000; Crosson et al. 2001; Persson et al. 2004; Alario et al. 2006; Tremblay and Gracco 2006; Tremblay and Gracco 2009). Here, we suggest that the pre-SMA is more strongly activated for object than goal-directed action stimuli (whether linguistic or not) because of the greater number of motor programs that are associated with manipulable man-made objects, as discussed in the previous section. Hearing sentences describing visual properties of objects such as “The pencil is red,” engages the pre-SMA more strongly than hearing a sentence like “I grasp the pen,” perhaps reflecting the coactivation of several competing motor programs for object-related sentences (grasping the pen, holding the pen, drawing, writing, etc.) compared with action-related (and goal-directed) sentences which are activating only one specific motor program. In the macaque, the pre-SMA is tightly connected with the prefrontal cortex, with anterior premotor areas (in particular F5), but it has no direct connection with M1, with the spinal cord, or with the cranial nerve motor nuclei (e.g., Dum and Strick 1991; Bates and Goldman-Rakic 1993; Luppino et al. 1993; Lu et al. 1994; Wang et al. 2005). This connectivity patterns suggests that the pre-SMA is involved with high-order aspects of movements such as response selection. Interestingly, as we have claimed elsewhere (Tremblay and Gracco 2009), the pre-SMA appears to be involved in response selection in a domain general manner. This interpretation is drawn from the finding of a very similar patter of activation in the present study for the language and nonlanguage tasks.
Inferior Frontal Gyrus
Interestingly, the present results demonstrate a lack of sensitivity in both pars opercularis and pars triangularis of the inferior frontal gyrus to the semantic content of the sentences (action, object), suggesting that these regions are not specifically involved in the comprehension of action-related words. It has been suggested that the pars opercularis (pIFG) is the human homologue of macaque area F5, which contains mirror neurons (e.g., Rizzolatti and Arbib 1998; Rizzolatti and Craighero 2004) but see Petrides et al. (2005). Consistent with the idea that pIFG is part of a human mirror neuron system, Hauk et al. (2004) have shown, using fMRI that, while hand and leg words activate the precentral gyrus, face words (e.g., lick), activate pIFG. Likewise, Tettamanti et al. (2005) demonstrated that passive listening to mouth-related sentences activates pIFG. More recently, however, de Zubicaray et al. (2010) showed that pIFG is not preferentially involved in the comprehension of action word meaning, by demonstrating the lack of a modulation in this region for the comparison of words and nonwords. Interestingly, there was activation in pIFG for observation and execution of action, which is consistent with our finding of activation in this area for ACTIONObs. Taken together, our results and prior studies converge to suggest that while the pIFG may is involved in observing/executing actions; it does not appear to contribute preferentially to the comprehension of action words.
Conclusions
To summarize, the present study highlights similarities and differences in the involvement of frontal motor/premotor areas during the processing of language and nonlanguage stimuli. By focusing on the conjunction of different language tasks involving the production and perception of sentences, we were able to identify task-independent motor/premotor areas for sentence processing (PMv, pre-SMA) and to examine the extent to which the action and language systems overlap. Our results emphasize significant differences in the neural basis of action observation and action-related language and argue against a strong action simulation explanation for the processing of action-related language, providing an alternative position that acknowledges the important role of the motor system in language but fails to support the strong linkage between action observation and sentence comprehension that is critical for the strong simulation argument.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institute on Deafness and other Communication Disorders (R01 DC003378 to S.L.S.); postdoctoral fellowship from Canadian Institutes of Health Research to P.T.
Supplementary Material
Acknowledgments
We thank T. Talavage, E. Mok, R. Siugzdaite, M. Flynn, B. Buchholz, J. Sheffield, and A. S. Dick for their help collecting and/or analyzing the data. We also thank H. C. Nusbaum for helpful suggestions on the experimental design. Their support is gratefully acknowledged. Conflict of Interest: None declared.
References
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076:129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Arévalo AL, Baldo JV, Dronkers NF. What do brain lesions tell us about theories of embodied semantics and the human mirror neuron system? Cortex. 2010 doi: 10.1016/j.cortex.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Wilson SM, Rizzolatti G, Iacoboni M. Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Curr Biol. 2006;16:1818–1823. doi: 10.1016/j.cub.2006.07.060. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex. 2008;18:1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Buccino G, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action-related sentences modulates the activity of the motor system: a combined TMS and behavioral study. Brain Res Cogn Brain Res. 2005;24:355–363. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Chambers C, Alexis O. Creating an inclusive environment for black and minority ethnic nurses. Br J Nurs. 2004;13:1355–1358. doi: 10.12968/bjon.2004.13.22.17276. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gökçay D, Mohr C, Auerbach EJ, Freeman AJ, Leonard CM, Briggs RW. Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. J Cogn Neurosci. 2001;13:272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Sadato N, Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol. 1996;75:233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- de Zubicaray G, Postle N, McMahon K, Meredith M, Ashton R. Mirror neurons, the representation of word meaning, and the foot of the third left frontal convolution. Brain Lang. 2010;112:77–84. doi: 10.1016/j.bandl.2008.09.011. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: structure, three-dimensional sectional anatomy and MRI. New York: Springer-Verlag; 1991. [Google Scholar]
- Etard O, Mellet E, Papathanassiou D, Benali K, Houde O, Mazoyer B, Tzourio-Mazoyer N. Picture naming without Broca's and Wernicke's area. Neuroreport. 2000;11:617–622. doi: 10.1097/00001756-200002280-00036. [DOI] [PubMed] [Google Scholar]
- Fernandino L, Iacoboni M. Are cortical motor maps based on body parts or coordinated actions? Implications for embodied semantics. Brain Lang. 2010;112:44–53. doi: 10.1016/j.bandl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilucci M, Benuzzi F, Bertolani L, Daprati E, Gangitano M. Language and motor control. Exp Brain Res. 2000;133:468–490. doi: 10.1007/s002210000431. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Kaschak MP. Grounding language in action. Psychon Bull Rev. 2002;9:558–565. doi: 10.3758/bf03196313. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Sato M, Cattaneo L, Riggio L, Palumbo D, Buccino G. Processing abstract language modulates motor system activity. Q J Exp Psychol (Colchester) 2008;61:905–919. doi: 10.1080/17470210701625550. [DOI] [PubMed] [Google Scholar]
- Glover S, Rosenbaum DA, Graham J, Dixon P. Grasping the meaning of words. Exp Brain Res. 2004;154:103–108. doi: 10.1007/s00221-003-1659-2. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Does visual perception of object afford action? Evidence from a neuroimaging study. Neuropsychologia. 2002;40:212–222. doi: 10.1016/s0028-3932(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Grezes J, Tucker M, Armony J, Ellis R, Passingham RE. Objects automatically potentiate action: an fMRI study of implicit processing. Eur J Neurosci. 2003;17:2735–2740. doi: 10.1046/j.1460-9568.2003.02695.x. [DOI] [PubMed] [Google Scholar]
- Handy TC, Tipper CM, Schaich Borg J, Grafton ST, Gazzaniga MS. Motor experience with graspable objects reduces their implicit analysis in visual—and motor-related cortex. Brain Res. 2006;1097:156–166. doi: 10.1016/j.brainres.2006.04.059. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci U S A. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: Wiley; 1949. [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J Cogn Neurosci. 2009;21:1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–320. [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Kurata K. Corticocortical inputs to the dorsal and ventral aspects of the premotor cortex of macaque monkeys. Neurosci Res. 1991;12:263–280. doi: 10.1016/0168-0102(91)90116-g. [DOI] [PubMed] [Google Scholar]
- Lau H, Rogers RD, Passingham RE. Dissociating response selection and conflict in the medial frontal surface. Neuroimage. 2006;29:446–451. doi: 10.1016/j.neuroimage.2005.07.050. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J Physiol Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Meister IG, Wilson SM, Deblieck C, Wu AD, Iacoboni M. The essential role of premotor cortex in speech perception. Curr Biol. 2007;17:1692–1696. doi: 10.1016/j.cub.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nichols T, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papeo L, Vallesi A, Isaja A, Rumiati RI. Effects of TMS on different stages of motor and non-motor verb processing in the primary motor cortex. PLoS ONE. 2009;4:e4508. doi: 10.1371/journal.pone.0004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Sylvester CY, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque monkey homologue of Broca's area. Nature. 2005;435:1235–1238. doi: 10.1038/nature03628. [DOI] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, di Prampero PE. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci. 1996;16:7688–7698. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle N, McMahon KL, Ashton R, Meredith M, de Zubicaray GI. Action word meaning representations in cytoarchitectonically defined primary and premotor cortices. Neuroimage. 2008;43:634–644. doi: 10.1016/j.neuroimage.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F. Hebb's concept of cell assemblies and the psychophysiology of word processing. Psychophysiology. 1996;33:317–333. doi: 10.1111/j.1469-8986.1996.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F. Brain reflections of words and their meaning. Trends Cogn Sci. 2001;5:517–524. doi: 10.1016/s1364-6613(00)01803-9. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. Eur J Neurosci. 2005;21:793–797. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Gentilucci M, Camarda RM, Gallese V, Luppino G, Matelli M, Fogassi L. Neurons related to reaching-grasping arm movements in the rostral part of area 6 (area 6a beta) Exp Brain Res. 1990;82:337–350. doi: 10.1007/BF00231253. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C, Morand S, Gemignani A, Decorps M, Jeannerod M. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport. 1996;7:1280–1284. doi: 10.1097/00001756-199605170-00012. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Takino R, Miyauchi S, Nielsen M, Tamada T. What and when: parallel and convergent processing in motor control. J Neurosci. 2000;20:2691–2700. doi: 10.1523/JNEUROSCI.20-07-02691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Tremblay P, Gracco VL. A mediating role of the premotor cortex in phoneme segmentation. Brain Lang. 2009;111:1–7. doi: 10.1016/j.bandl.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14:1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Sundara M, Namasivayam AK, Chen R. Observation-execution matching system for speech: a magnetic stimulation study. Neuroreport. 2001;12:1341–1344. doi: 10.1097/00001756-200105250-00010. [DOI] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa SF, Perani D. Listening to action-related sentences activates fronto-parietal motor circuits. J Cogn Neurosci. 2005;17:273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Weiss PH, Fink GR. To move or not to move: imperatives modulate action-related verb processing in the motor system. Neuroscience. 2010;169:246–258. doi: 10.1016/j.neuroscience.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Werner CJ, Weiss PH, Fink GR. Stimulus properties matter more than perspective: an fMRI study of mental imagery and silent reading of action phrases. Neuroimage. 2007;36(2 Suppl):T128–T141. doi: 10.1016/j.neuroimage.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. Contribution of the frontal lobe to externally and internally specified verbal responses: fMRI evidence. Neuroimage. 2006;33:947–957. doi: 10.1016/j.neuroimage.2006.07.041. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. On the selection of words and oral motor responses: evidence of a response-independent fronto-parietal network. Cortex. 2009;46:15–28. doi: 10.1016/j.cortex.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Turella L, Pierno AC, Tubaldi F, Castiello U. Mirror neurons in humans: consisting or confounding evidence? Brain Lang. 2009;108:10–21. doi: 10.1016/j.bandl.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Van Oostende S, Van Hecke P, Sunaert S, Nuttin B, Marchal G. FMRI studies of the supplementary motor area and the premotor cortex. Neuroimage. 1997;6:181–190. doi: 10.1006/nimg.1997.0287. [DOI] [PubMed] [Google Scholar]
- Wang Y, Isoda M, Matsuzaka Y, Shima K, Tanji J. Prefrontal cortical cells projecting to the supplementary eye field and presupplementary motor area in the monkey. Neurosci Res. 2005;53:1–7. doi: 10.1016/j.neures.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Watkins K, Paus T. Modulation of motor excitability during speech perception: the role of Broca's area. J Cogn Neurosci. 2004;16:978–987. doi: 10.1162/0898929041502616. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Strafella AP, Paus T. Seeing and hearing speech excites the motor system involved in speech production. Neuropsychologia. 2003;41:989–994. doi: 10.1016/s0028-3932(02)00316-0. [DOI] [PubMed] [Google Scholar]
- Willems RM, Toni I, Hagoort P, Casasanto D. Neural dissociations between action verb understanding and motor imagery. J Cogn Neurosci. 22:2387–2400. doi: 10.1162/jocn.2009.21386. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.