Abstract
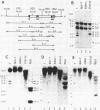
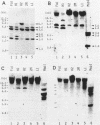
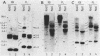
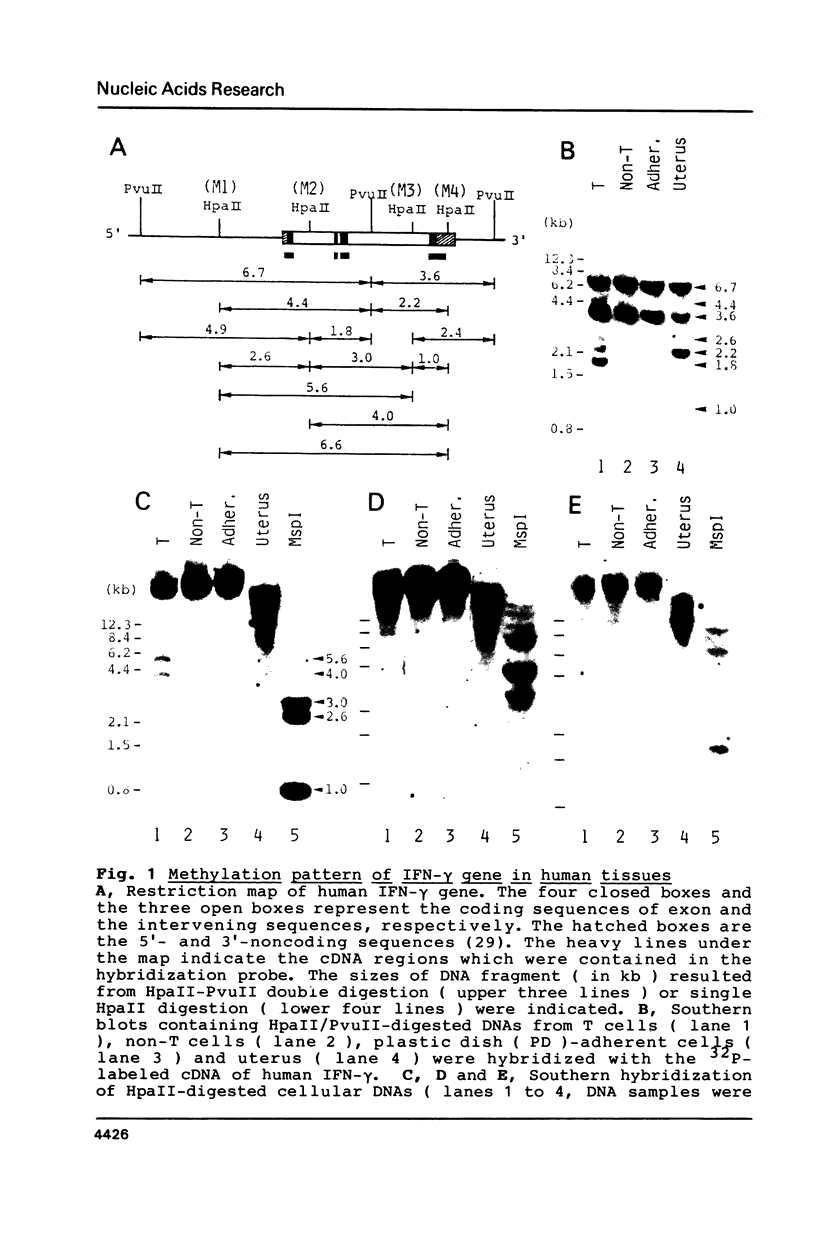
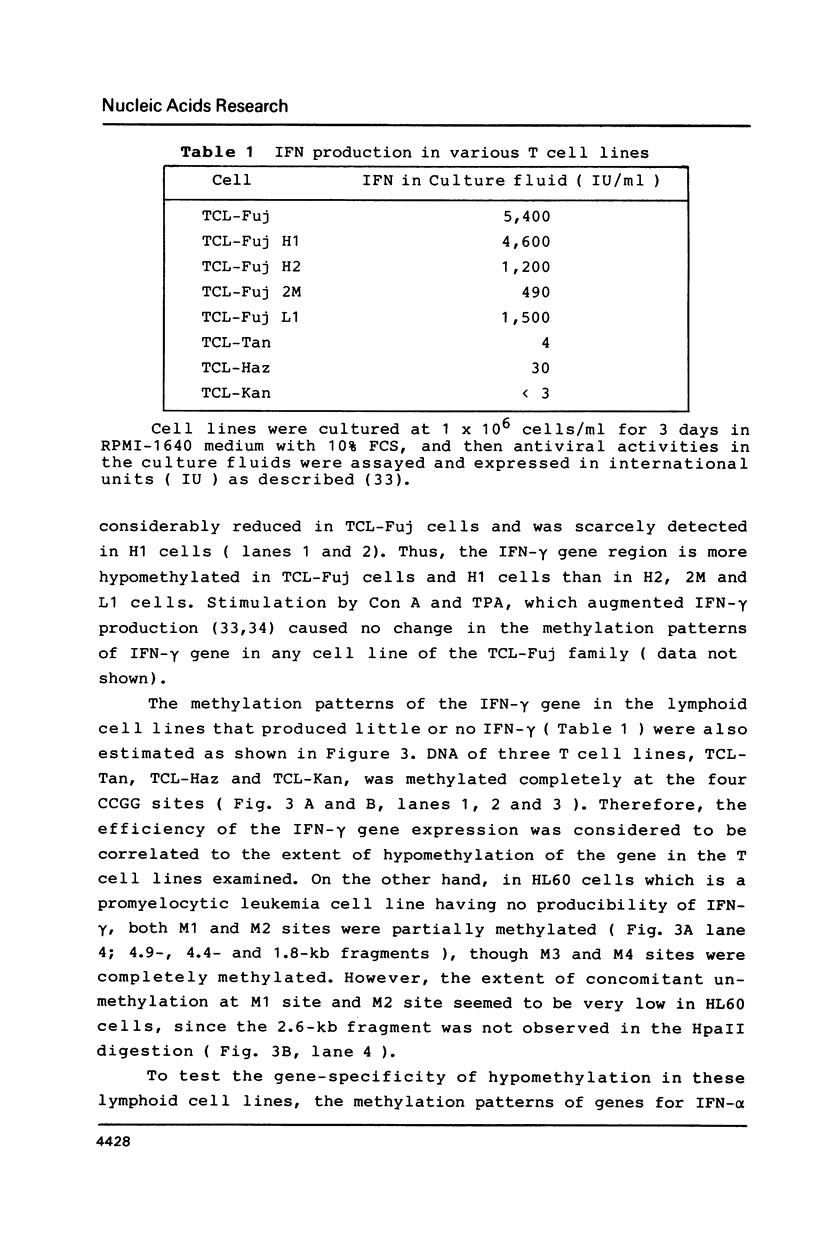
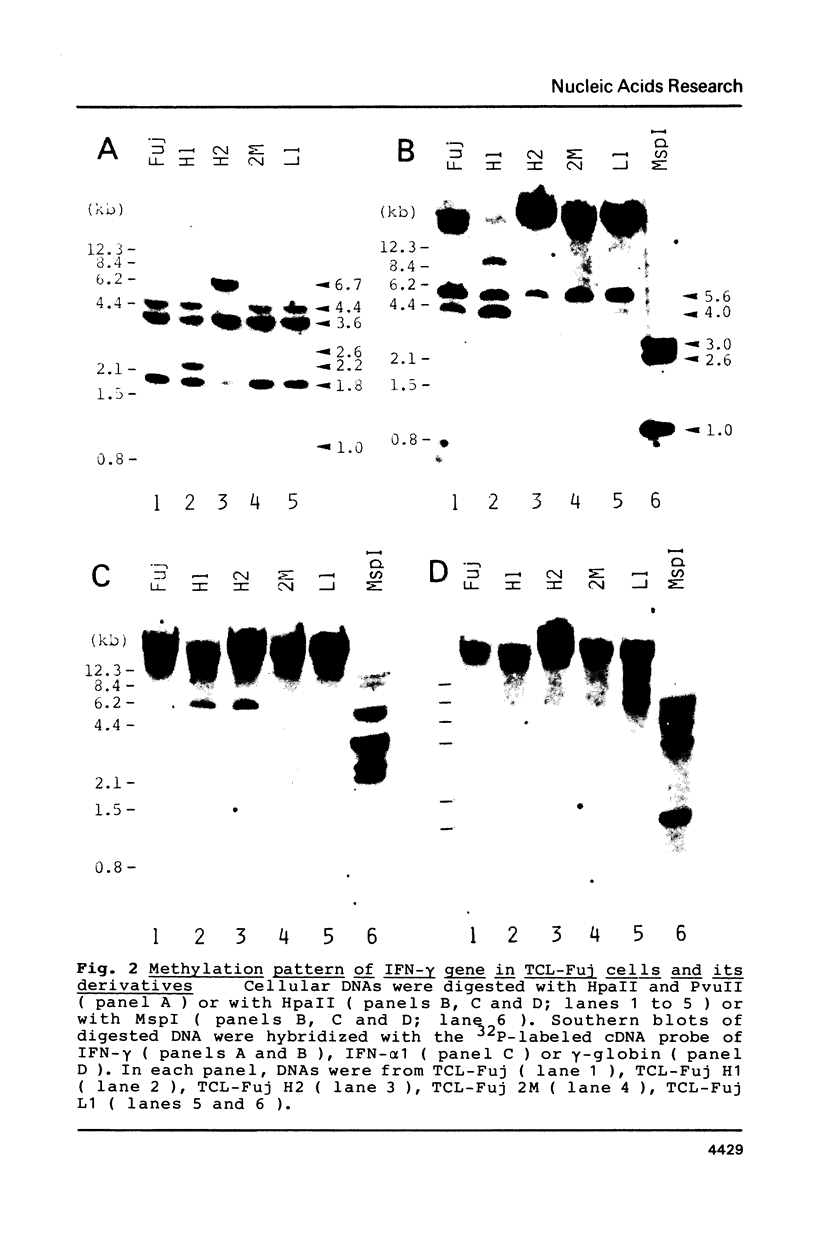
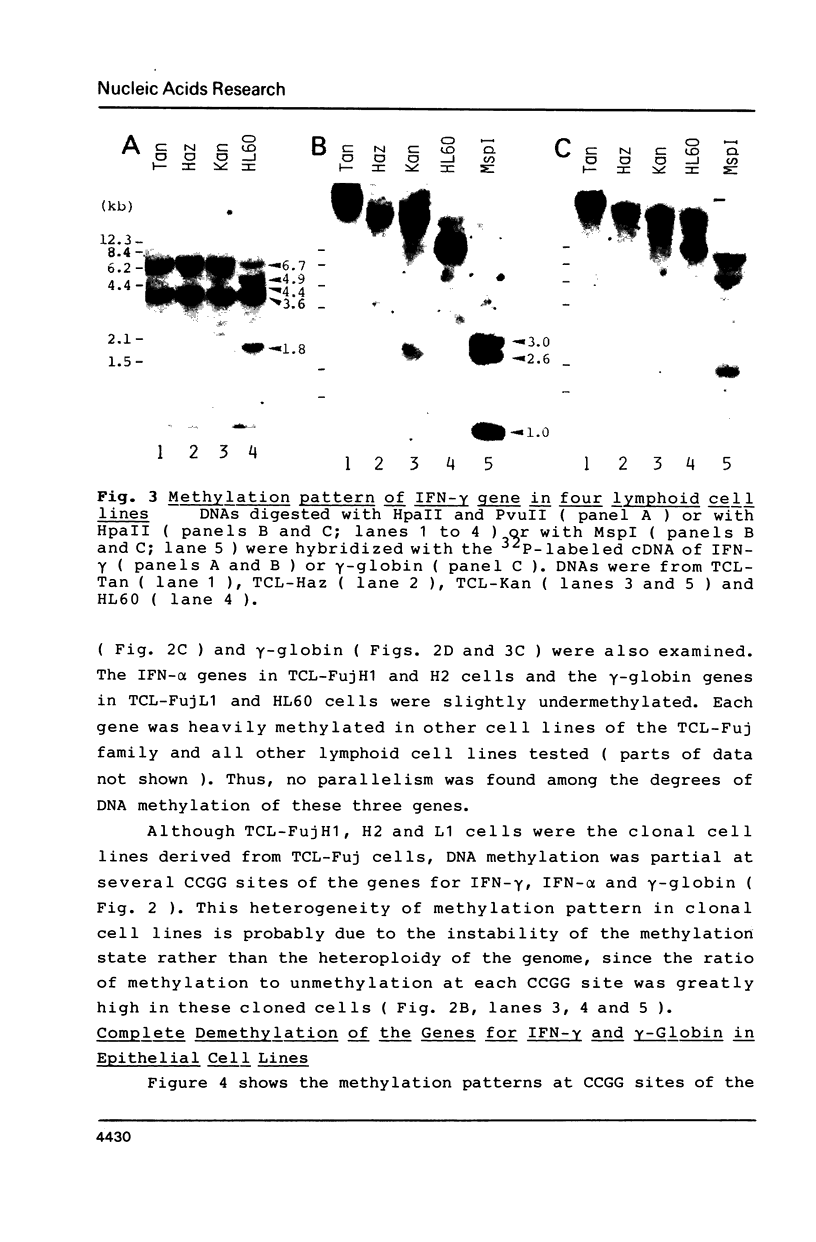
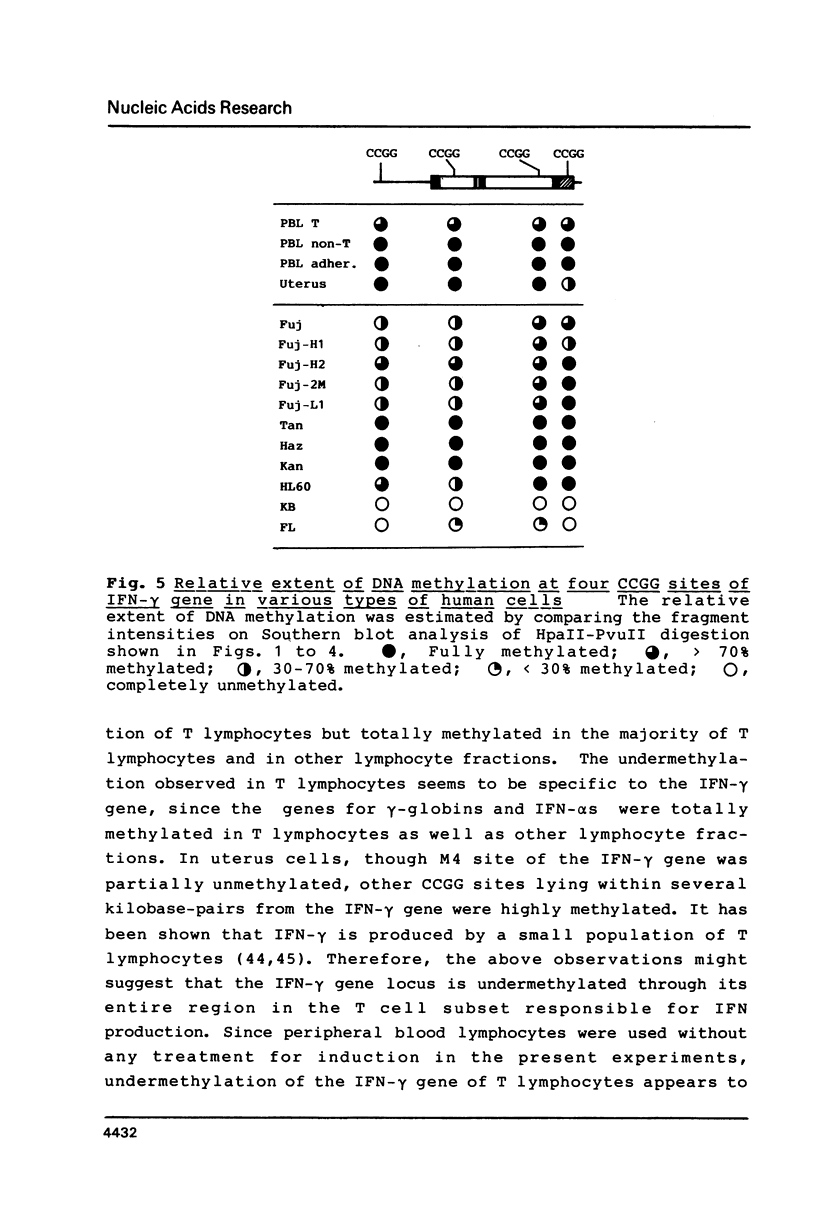
The relative levels of DNA methylation at CCGG sequences within and around the interferon-gamma (IFN-gamma) gene in normal human tissues and cell lines were examined by Southern blot analysis using isoschizomeric restriction enzymes, HpaII and MspI. On the test of normal tissues, the IFN-gamma gene was undermethylated only in a small population of T lymphocyte, whereas the gene was fully methylated in T cell-depleted lymphocytes and uterus cells. In TCL-Fuj cell line which is a T cell line producing a high level of IFN-gamma spontaneously, the IFN-gamma gene was undermethylated. Moreover, the extent of DNA methylation was inversely correlated to the level of expression of the IFN-gamma gene in several T cell lines including sublines derived from TCL-Fuj cells. However, partial or complete unmethylation at the CCGG sites of IFN-gamma gene was observed in a promyelocytic leukemia cell line and two epithelial cell lines that fail to produce IFN-gamma irrespective of induction. These results suggest that undermethylation of IFN-gamma gene is necessary but not sufficient for its efficient expression.
Full text
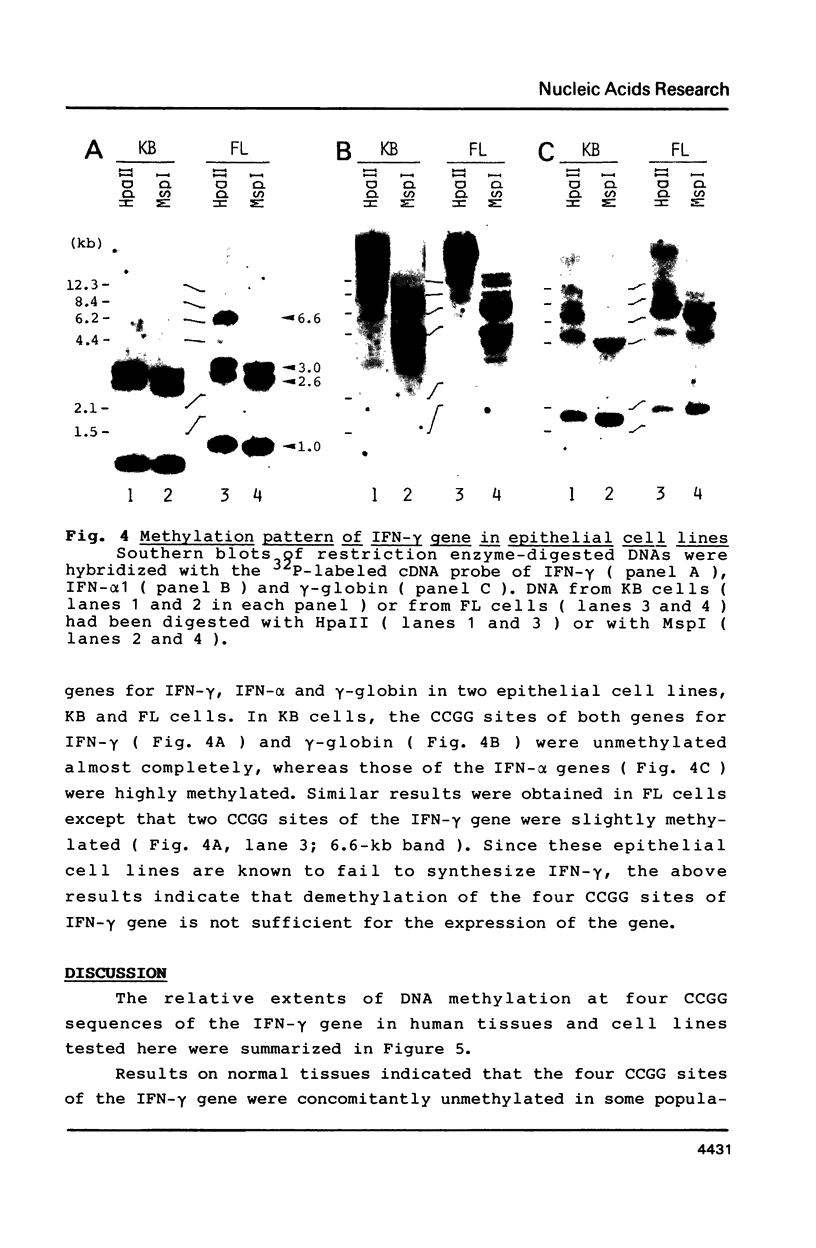
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P. DNA methylation--how important in gene control? Nature. 1984 Feb 9;307(5951):503–504. doi: 10.1038/307503a0. [DOI] [PubMed] [Google Scholar]
- Bower D. J., Errington L. H., Cooper D. N., Morris S., Clayton R. M. Chicken lens delta-crystallin gene expression and methylation in several non-lens tissues. Nucleic Acids Res. 1983 May 11;11(9):2513–2527. doi: 10.1093/nar/11.9.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Testa D., Kung P. C., Perry L., Dreskin H. J., Goldstein G. Cellular origin and interactions involved in gamma-interferon production induced by OKt3 monoclonal antibody. J Immunol. 1982 Feb;128(2):585–589. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Devos R., Cheroutre H., Taya Y., Degrave W., Van Heuverswyn H., Fiers W. Molecular cloning of human immune interferon cDNA and its expression in eukaryotic cells. Nucleic Acids Res. 1982 Apr 24;10(8):2487–2501. doi: 10.1093/nar/10.8.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Faith R. E., Luster M. I., Moore J. A. Chemical separation of helper cell function and delayed hypersensitivity responses. Cell Immunol. 1978 Oct;40(2):275–284. doi: 10.1016/0008-8749(78)90335-0. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Ruscetti F. W., Young H. A. 5-Azacytidine treatment of a murine cytotoxic T cell line alters interferon-gamma gene induction by interleukin 2. J Immunol. 1985 Sep;135(3):1551–1554. [PubMed] [Google Scholar]
- Felsenfeld G., McGhee J. Methylation and gene control. Nature. 1982 Apr 15;296(5858):602–603. doi: 10.1038/296602a0. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Wilson M. C. Delayed de novo methylation in teratocarcinoma suggests additional tissue-specific mechanisms for controlling gene expression. Nature. 1983 Jan 6;301(5895):32–37. doi: 10.1038/301032a0. [DOI] [PubMed] [Google Scholar]
- Gerber-Huber S., May F. E., Westley B. R., Felber B. K., Hosbach H. A., Andres A. C., Ryffel G. U. In contrast to other Xenopus genes the estrogen-inducible vitellogenin genes are expressed when totally methylated. Cell. 1983 May;33(1):43–51. doi: 10.1016/0092-8674(83)90333-1. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Structure of the human immune interferon gene. Nature. 1982 Aug 26;298(5877):859–863. doi: 10.1038/298859a0. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Jaenisch R., Schnieke A., Harbers K. Treatment of mice with 5-azacytidine efficiently activates silent retroviral genomes in different tissues. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1451–1455. doi: 10.1073/pnas.82.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Stewart C. L., Harbers K., Löhler J., Simon I., Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- Keshet I., Yisraeli J., Cedar H. Effect of regional DNA methylation on gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2560–2564. doi: 10.1073/pnas.82.9.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama M., Sugamura K., Hinuma Y. Constitutive production and characterization of interferon-gamma in a human T-lymphoblastoid cell line transformed by a human retrovirus. Microbiol Immunol. 1984;28(12):1333–1343. doi: 10.1111/j.1348-0421.1984.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Giampaolo A., Carè A., Migliaccio G., Calandrini M., Russo G., Pagliardi G. L., Mastroberardino G., Marinucci M., Peschle C. Molecular mechanisms of human hemoglobin switching: selective undermethylation and expression of globin genes in embryonic, fetal, and adult erythroblasts. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6907–6911. doi: 10.1073/pnas.80.22.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Nagata S., Taira H., Hall A., Johnsrud L., Streuli M., Ecsödi J., Boll W., Cantell K., Weissmann C. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980 Mar 27;284(5754):316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T. Alternative induction of IFN-alpha and IFN-gamma by Listeria monocytogenes in human peripheral blood mononuclear leukocyte cultures. J Immunol. 1981 Jun;126(6):2139–2142. [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- O'Malley J. A., Nussbaum-Blumenson A., Sheedy D., Grossmayer B. J., Ozer H. Identification of the T cell subset that produces human gamma interferon. J Immunol. 1982 Jun;128(6):2522–2526. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Cassio D., Levilliers J., Sala-Trepat J., Weiss M. C. Undermethylation at the 5' end of the albumin gene is necessary but not sufficient for albumin production by rat hepatoma cells in culture. Cell. 1982 Oct;30(3):825–833. doi: 10.1016/0092-8674(82)90287-2. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Razin A., Webb C., Szyf M., Yisraeli J., Rosenthal A., Naveh-Many T., Sciaky-Gallili N., Cedar H. Variations in DNA methylation during mouse cell differentiation in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2275–2279. doi: 10.1073/pnas.81.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Gruenbaum Y., Pollack Y., Razin A., Cedar H. Clonal inheritance of the pattern of DNA methylation in mouse cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):61–65. doi: 10.1073/pnas.79.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Razin A., Cedar H. In vitro methylation of the hamster adenine phosphoribosyltransferase gene inhibits its expression in mouse L cells. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3418–3422. doi: 10.1073/pnas.79.11.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K., Matsuyama M., Fujii M., Kannagi M., Hinuma Y. Establishment of human cell lines constitutively producing immune interferon: transformation of normal T cells by a human retrovirus. J Immunol. 1983 Oct;131(4):1611–1612. [PubMed] [Google Scholar]
- Tanaka K., Appella E., Jay G. Developmental activation of the H-2K gene is correlated with an increase in DNA methylation. Cell. 1983 Dec;35(2 Pt 1):457–465. doi: 10.1016/0092-8674(83)90179-4. [DOI] [PubMed] [Google Scholar]
- Vaquero C., Sanceau J., Sondermeyer P., Falcoff R. Kinetics of messenger accumulation coding for IFN gamma, related to modifications in the poly(A) RNA population of activated human lymphocytes. Nucleic Acids Res. 1984 Mar 26;12(6):2629–2640. doi: 10.1093/nar/12.6.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H. The inheritance of methylation patterns in vertebrates. Cell. 1981 May;24(2):285–286. doi: 10.1016/0092-8674(81)90317-2. [DOI] [PubMed] [Google Scholar]
- Wilks A., Seldran M., Jost J. P. An estrogen-dependent demethylation at the 5' end of the chicken vitellogenin gene is independent of DNA synthesis. Nucleic Acids Res. 1984 Jan 25;12(2):1163–1177. doi: 10.1093/nar/12.2.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T., Wilson L. B., deRiel J. K., Villa-komaroff L., Efstratiadis A., Forget B. G., Weissman S. M. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucleic Acids Res. 1978 Feb;5(2):563–581. doi: 10.1093/nar/5.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]