This article describes current progress in the engineering of oilseed crops for the production of long-chain omega-3 fatty acids such as DHA. This example highlights the importance of algal genetic resources to the future of agricultural biotechnology.
Abstract
Background
Algae are becoming an increasingly important component of land plant metabolic engineering projects. Land plants and algae have similar enough genetics to allow relatively straightforward gene transfer and they also share enough metabolic similarities that algal enzymes often function in a plant cell environment. Understanding metabolic systems in algae can provide insights into homologous systems in land plants. As examples, algal models are currently being used by several groups to better understand starch and lipid metabolism and catabolism, fields which have relevance in land plants. Importantly, land plants and algae also have enough metabolic divergence that algal genes can often provide new metabolic traits to plants. Furthermore, many algal genomes have now been sequenced, with many more in progress, and this easy access to genome-wide information has revealed that algal genomes are often relatively simple when compared with plants.
Scope
One example of the importance of algal, and in particular microalgal, resources to land plant research is the metabolic engineering of long-chain polyunsaturated fatty acids into oilseed crops which typically uses microalgal genes to extend existing natural plant biosynthetic pathways. This review describes both recent progress and remaining challenges in this field.
Long-chain vs. short-chain polyunsaturated fatty acids
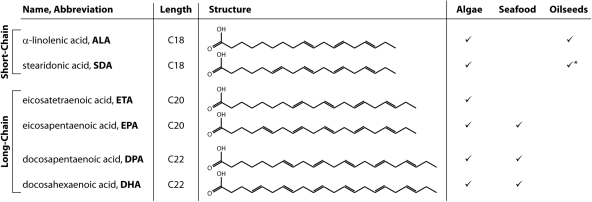
Long-chain polyunsaturated fatty acids (LC-PUFA) have a carbon backbone of at least 20 carbons in length and contain multiple double-bond desaturations. Long-chain polyunsaturated fatty acids can be grouped into either an omega-3 (ω3) or ω6 category based on the position of the first double bond from the methyl, or ω, fatty acid terminus. Fatty acid nomenclature can be used to succinctly refer to fatty acids and, as an example, 18:4Δ6,9,12,15 refers to the short-chain stearidonic acid which contains only 18 carbons and four double bonds, or desaturations, at the 6, 9, 12 and 15 carbon positions from the carboxyl, or Δ, terminus (Fig. 1). Long-chain polyunsaturated fatty acids have critical roles in human health and development, with studies indicating that deficiencies in these fatty acids can increase the risk or severity of cardiovascular disease (von Schacky 2006), inflammatory diseases and rheumatoid arthritis (Kremer et al. 1995; Simopoulos 2002; Nagel et al. 2003), hypertension (Ueshima et al. 2007) and neuropsychiatric disorders such as depression or dementia (Freeman et al. 2006; Parker et al. 2006; Schaefer et al. 2006). Recently, the human fatty acid receptor GPR120 was characterized as being an ω3-specific receptor with strong anti-inflammatory and insulin-sensitizing effects (Oh et al. 2010). Humans have limited ability to synthesize LC-PUFA, with infants in particular requiring the majority of these fatty acids through the diet. This is particularly the case with the ω3 LC-PUFA eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) and the ω6 LC-PUFA arachidonic acid (ARA), with studies indicating positive effects on infant cognitive development after ARA and DHA supplementation (Birch et al. 2000). As a result many infant formulae now contain LC-PUFA, although it is worth noting that ARA supplementation for adults is, in many cases, often considered to be of lesser value since the ω6 LC-PUFA tend to be pro-inflammatory in nature and counteract the strong anti-inflammatory effects of ω3 LC-PUFA.
Fig. 1.
ω3 long-chain (≥C20) polyunsaturated fatty acids and their predominant natural sources. *Some Boraginaceae such as Echium plantagineum contain SDA, although this species is not generally recognized as an oilseed crop species.
Just as there are differences in physiological effect between ω3 and ω6 LC-PUFA (i.e. anti- vs. pro-inflammatory, respectively), it is important to note that there are significant differences arising from intake of long-chain vs. short-chain (SC) ω3 PUFA. Alpha-linolenic acid (ALA) is a common ω3 SC-PUFA found in seed oils of flax/linseed, canola/rapeseed, soybean and walnut. Stearidonic acid (SDA), another ω3 SC-PUFA, is less common in plant oils, with the predominant natural plant dietary sources being echium and borage. Human studies along with livestock and aquafeeding trials have demonstrated that SDA in particular can be converted, in part, to the LC-PUFA EPA and, to a lesser extent, DPA (James et al. 2003; Tocher et al. 2006; Harris et al. 2008). Thus, although the direct health benefits of ω3 SC-PUFA are relatively limited when compared with LC-PUFA, they are viewed by some as a surrogate for dietary EPA supplementation (Whelan 2009) due to this in vivo conversion and transgenic SDA crops are under development.
It is clear, however, that direct intake of ω3 LC-PUFA results in the greatest health benefits (James et al. 2003; Burdge and Calder 2005; Wang et al. 2006). Furthermore, the conversion of ω3 SC-PUFA including SDA to DHA has not been observed in either humans or aquaculture species to any significant degree (James et al. 2003; Tocher et al. 2006; Harris et al. 2008). Docosahexaenoic acid fills specific physiological (e.g. Mori et al. 1999) and structural roles (such as a neural and retinal component) that cannot be replaced by EPA or other LC-PUFA, and ensuring adequate dietary intake of DHA is therefore required. Consumer awareness of the health benefits of ω3 LC-PUFA is growing (due in part to the efforts of organizations such as the Omega-3 Centre (www.omega-3centre.com) and GOED (www.goedomega3.com)), and there is an increasing demand for these fatty acids which will be difficult for current sources to meet in a sustainable manner.
Current and future sources of ω3 LC-PUFA
The main source of ω3 LC-PUFA is wild-harvest marine fish stocks and these, unfortunately, are widely recognized to be in decline. One widely cited paper describes the amount of large predatory fish in the oceans as being at only 10 % of pre-industrial times (Myers and Worm 2003), although this figure has been disputed. One of the authors claimed in a later publication that the state of the oceans and the effect of this on fish stocks was such that ‘all commercial fish and seafood may collapse' by 2048 (Worm et al. 2006). This view is hardly unanimous either (see Hölker et al. (2007), Wilberg and Miller (2007) and Jaenike (2007) for comment), but efforts are now under way to rebuild fisheries and to improve their sustainability. Regardless, fisheries alone are unlikely to meet the growing demand for ω3 LC-PUFA and alternative sources of these fatty acids must be found. Aquaculture cannot meet the demand since fish themselves do not produce LC-PUFA but, like humans, accumulate LC-PUFA through their diet. In fact, the aquaculture industry itself contributes enormously to demand for LC-PUFA, with ∼90 % of global fish oil production being used in aquafeeds. Logically, the major producers of ω3 LC-PUFA, microalgae, would be a good target for increased production and, indeed, commercial microalgal sources of ω3 LC-PUFA are available. Microbial production tends to supply relatively niche applications such as infant formulae and nutraceuticals due to high production costs and is unlikely to scale up adequately for large-volume applications.
Oilseed crops, with their production capacity and relatively low cost, would be an excellent and sustainable source of ω3 LC-PUFA. For example, several calculations have recently been performed to examine global consumer needs for these oils and using a daily 500 mg requirement, as is recommended by national health bodies, a global population reaching 8 billion by 2025, and assuming fish contain 0.2–3.5 g of ω3 LC-PUFA per 100 g of oil, it was estimated that the current global fish harvest (93 Mt per annum) will fall well short of this requirement. On the other hand, ∼2.5 million hectares of an oilseed crop (about 2 % of total world acreage under cultivation to major oilseed crops) containing 10–15 % DHA and EPA in its oil could replace all the fish oil currently being used globally. Oilseed crop plants do not naturally synthesize these fatty acids, meaning that a metabolic engineering solution would be required before this potential source could become a reality. An oilseed crop with ω3 LC-PUFA production capability would provide an excellent alternative to high-volume, environmentally sensitive marine-based oils. Such an oil could be used in many applications: (i) as an ingredient for aquaculture feeds to provide aquaculture species with ω3 LC-PUFA both for their own developmental requirements and to meet consumer demands for ω3 LC-PUFA content in fish; (ii) as an animal feed to produce ω3 LC-PUFA-enriched meat, eggs and milk; (iii) as a food (e.g. bread or milk) supplement in an oxidation-protected form; (iv) directly, as a nutraceutical supplement similar to the way in which fish oil capsules are currently used. As an additional benefit, an oilseed source would be vegetarian and thus satisfy a requirement for an important sector of the global market.
ω3 LC-PUFA biosynthesis
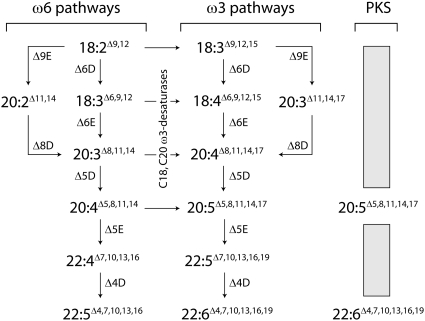
Biosynthesis of LC-PUFA occurs by either aerobic or anaerobic pathways in nature, with the latter found predominantly in some marine bacteria (e.g. Vibrio and Shewanella) and protists (e.g. Schizochytrium and Thraustochytrium). Anaerobic synthesis of LC-PUFA is performed by polyketide synthase (PKS) pathways which are cyclical in nature, with multi-enzyme complexes iteratively performing a series of desaturations and 2-carbon elongations using malonyl-CoA and acetyl-CoA as the source of the carbon units. The complex nature of the PKS pathway and the requirement for associate genes has to date resulted in this being a challenging pathway to introduce into plants. The aerobic pathway, on the other hand, consists of a series of distinct desaturation and elongation steps with the genes coding for each of the enzymes having been isolated and characterized. This review focuses on metabolic engineering of plants by the aerobic pathway, although it is worth noting that considerable progress has been made in understanding and engineering the PKS pathways (Metz et al. 2001, 2006, 2009; Lippmeier et al. 2009).
Aerobic production of LC-PUFA (Fig. 2) can be thought, in the context of plant metabolic engineering, to commence with the native plant fatty acid oleic acid. This fatty acid is first Δ12-desaturated to produce linolenic acid (LA), which is in turn ω3-desaturated to produce ALA. Linolenic acid and ALA are the respective substrates for the ω6 and ω3 LC-PUFA pathways, with the first committed step in both pathways being a Δ6-desaturation to produce either γ-linoleic acid (GLA) (ω6) or SDA (ω3). All the fatty acids up to this point can be found naturally in angiosperms, although GLA and SDA are relatively rare and efforts have been made to engineer these SC-PUFA into oilseed species (see below). The C18 fatty acids GLA and SDA are then Δ6-elongated to the long-chain (≥C20) fatty acids di-homo-γ-linoleic acid (DGLA) and eicosatetraenoic acid (ETA), respectively. This elongation (and the others described in this review) consists of four consecutive enzymatic steps (condensation, ketoreduction, dehydration and enoyl reduction), although the transgenic introduction of the condensing enzyme or ‘elongase’ is sufficient to confer specificity to the entire elongation with the other elongation reaction components being supplied by the host organism. The products of the Δ6-elongation, DGLA and ETA, are then Δ5-desaturated to ARA and EPA, respectively. Arachidonic acid marks the end of the traditionally represented ω6 LC-PUFA pathway, although ARA can be Δ5-elongated to docosatetraenoic acid which can finally be Δ4-desaturated to DPAω6. Similarly, EPA can be Δ5-elongated to DPA (DPAω3) which is then Δ4-desaturated to produce DHA. This ‘Δ6’ pathway is the most commonly found aerobic pathway, although the alternative ‘Δ8’ pathway also exists. In this pathway, LA and ALA are Δ9-elongated to eicosadienoic acid (EDA) and eicosatrienoic acid (ETRA), respectively, which are then Δ8-desaturated to DGLA and ETA (Fig. 2). At this point, the Δ6 and Δ8 pathways merge and subsequent desaturations and elongations continue as described above.
Fig. 2.
Long-chain polyunsaturated fatty acid biosynthesis pathways in lower plants, including algae (ω6 and ω3), marine bacteria and protists (PKS). Enzymes are referred to as either ‘E’ for elongase (e.g. Δ9E is Δ9-elongase) or ‘D’ for desaturase (e.g. Δ6D is Δ6-desaturase) and belong to the aerobic pathway for LC-PUFA synthesis. A generalized scheme for the processive synthesis of LC-PUFA by the anaerobic PKS pathway is also shown. In this system acetyl-CoA undergoes several rounds of sequential reactions (keto-synthase, keto-reductase, dehydratase and enoyl reductase) that result in repeated elongations by two carbons per cycle of a fatty acyl chain esterified to an acyl carrier protein. Names and abbreviations for the ω3 fatty acids are provided in Fig. 1 and ω6 fatty acids are 18:2Δ9,12, LA; 18:3Δ6,9,12, GLA; 20:3Δ8,11,14, DGLA; 20:4Δ5,8,11,14, ARA. The Δ9-elongated fatty acids are 20:2Δ11,14, EDA and 20:3Δ11,14,17, ETRA.
Long-chain polyunsaturated fatty acid metabolic engineering
It is surprising to some people that fish and other marine species, the predominant source of ω3 LC-PUFA oils in the human diet, are not the source of the genes used in LC-PUFA metabolic engineering applications. Rather, the genes come from microalgae (that fish and other marine species consume), fungi and protists. As already mentioned, genes coding for each step in the LC-PUFA pathways have been isolated and characterized, and several groups have been expressing these in land plants to modify the fatty acid profiles of the plant oil (Fig. 3, Table 1). There are several technical or scientific challenges that must be met before oilseed crops can be engineered to accumulate adequate levels of LC-PUFA. These challenges are mainly focused on increasing the conversion of the native plant fatty acid substrates through to the LC-PUFA of interest with as few intermediate fatty acids as possible. In theory, this simply requires the use of transgenic enzymes which have high conversion efficiencies. In practice, the conversion efficiencies of LC-PUFA biosynthesis enzymes are not only affected by actual enzyme activity but also by factors such as substrate dichotomy, the requirement of some enzymes to use substrates from certain metabolic pools.
Fig. 3.
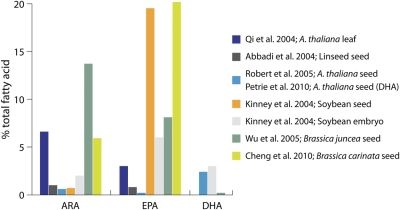
Some notable LC-PUFA engineering results discussed in this review. See Table 1 for details of genes used in these studies. Format adapted from Venegas-Calerón et al. (2010).
Table 1.
The genes used in the studies described in Fig. 3. *Denotes an algal gene source.
| Publication | Pathway | Host species | Genes |
|---|---|---|---|
| Qi et al. (2004) | Δ9 | Arabidopsis thaliana leaf | Isochrysis galbana Δ9-elongase* |
| Euglena gracilis Δ8-desaturase* | |||
| Mortierella alpina Δ5-desaturase | |||
| Abbadi et al. (2004) | Δ6 | Linum usitatissimum seed | Phaeodactylum tricornutum Δ6-desaturase* |
| Physcomitrella patens Δ6-elongase | |||
| Phaeodactylum tricornutum Δ5-desaturase* | |||
| Robert et al. (2005) | Δ6 | Arabidopsis thaliana seed | Danio rerio Δ5/6-desaturase |
| Caenorhabditis elegans Δ6-elongase | |||
| Pavlova salina Δ5-elongase* | |||
| Pavlova salina Δ4-desaturase* | |||
| Kinney et al. (2004) | Δ6 | Glycine max seed | Arabidopsis thaliana Δ15-desaturase |
| Saprolegnia diclina Δ17-desaturase | |||
| Mortierella alpina Δ6-desaturase | |||
| Mortierella alpina Δ6-elongase | |||
| Mortierella alpina Δ5-desaturase | |||
| Kinney et al. (2004) | Δ6 | Glycine max somatic embryo | As above plus: |
| Pavlova sp. Δ5-elongase* | |||
| Schizochytrium Δ4-desaturase* | |||
| Wu et al. (2005) | Δ6 | Brassica juncea seed | Calendula officinalis Δ12-desatuase |
| Phytophthora infestans ω3-desaturase | |||
| Thraustochytrium sp. LPCAT* | |||
| Pythium irregulare Δ6-desaturase | |||
| Thraustochytrium sp. C18 elongase* | |||
| Thraustochytrium sp. Δ5-desaturase* | |||
| Oncorhynchus mykiss C18/C20 elongase | |||
| Thraustochytrium sp. Δ4-desaturase* | |||
| Cheng et al. (2010) | Δ6 | Brassica carinata seed | Pythium irregulare Δ6-desaturase |
| Thraustochytrium sp. C18 elongase* | |||
| Thraustochytrium sp. Δ5-desaturase* | |||
| Calendula officinalis Δ12-desatuase | |||
| Phytophthora infestans ω3-desaturase | |||
| Petrie et al. (2010a) | Δ6 | Arabidopsis thaliana seed | Micromonas pusilla Δ6-desaturase* |
| Pyramimonas cordata Δ6-elongase* | |||
| Pavlova salina Δ5-desaturase* | |||
| Pyramimonas cordata Δ5-elongase* | |||
| Pavlova salina Δ4-desaturase* | |||
| Petrie et al. (2010b) | Δ9 | Nicotiana benthamiana leaf | Pavlova salina: |
| Δ9-elongase* | |||
| Δ8-desaturase* | |||
| Δ5-desaturase* | |||
| Δ5-elongase* | |||
| Δ4-desaturase* |
The biosynthesis of ω3 LC-PUFA in land plants was first reported in 2004 with publications describing the introduction of both the Δ8 and Δ6 pathways. Qi et al. (2004) demonstrated the production of 3 % EPA and 6.6 % ARA in Arabidopsis thaliana leaf tissue by a Δ8 pathway consisting of the Isochrysis galbana Δ9-elongase, Euglena gracilis Δ8-desaturase and Mortierella alpina Δ5-desaturase with constitutive 35S promoters. This study demonstrated that production of LC-PUFA in land plants was possible, albeit in leaf tissue. Shortly after, Abbadi et al. (2004) published the production of LC-PUFA in the seeds of tobacco and linseed by the Δ6 pathway. In this pathway, SDA (11.4 %) and GLA (16.8 %) were produced in linseed, although these fatty acids were not effectively Δ6-elongated with only 0.8 % EPA and 1.0 % ARA being produced. These studies were important proofs of concept that the production of ω3 LC-PUFA in land plants was possible.
ω6 vs. ω3 biosynthesis
These two 2004 publications also showed the complication of parallel ω6 and ω3 pathways in which an individual gene generally functions in both branches (Fig. 2). Using the Δ6 pathway as an example, the Δ6-desaturase is the first committed step of both ω6 and ω3 LC-PUFA production and can desaturate both LA and ALA. The Δ6-desaturation of LA to produce GLA, however, effectively reduces the production of ALA since LA is also the substrate for Δ15-desaturase activity, which produces ALA. One seemingly obvious solution is to increase the production of ALA by introducing more efficient Δ15-desaturase activity, but the success of this approach can be limited by the fact that competition for LA remains between the Δ15- and Δ6-desaturases. This can be overcome by introducing a more broadly functioning ω3-desaturase that not only converts LA to ALA by Δ15-desaturation but can also convert the ω6 fatty acids GLA, DGLA and ARA to their ω3 counterparts. Another approach is to reduce or remove the competition for LA experienced by the Δ15-desaturase by using a Δ6-desaturase that favours ALA as a substrate. A patent describing LC-PUFA synthesis in soybean somatic embryos (Kinney et al. 2004) contained an example of the first approach of including an ω3-desaturase gene that could convert ω6 products to their ω3 counterparts. In this work, a Δ6 pathway coupled to a strong Δ17-desaturase resulted in the production of nearly 20 % EPA with very little ARA accumulation due to the ω3-desaturase activity of the Δ17-desaturase. Wu et al. (2005) also included a Δ17-desaturase in their Δ6 pathway and this, combined with a lysophosphatidyl acyltransferase (LPAT) which was included as a way of increasing incorporation of novel fatty acids, resulted in the accumulation of up to 15 % EPA in Brassica juncea seeds. Similar results were obtained by Cheng et al. (2010) who engineered Brassica carinata with a Δ6 pathway including an ω3-desaturase and accumulated in excess of 20 % EPA in seed.
The alternative method of reducing ω6 products, namely to use a Δ6-desaturase with preference for the ω3 substrate ALA, has also been demonstrated. Several examples have been reported of genes encoding such Δ6-desaturases (Sayanova et al. 2003; García-Maroto et al. 2006; Hoffmann et al. 2008; Petrie et al. 2010a), although the strength of the preference varies. For example, the primula Δ6-desaturase isolated by Sayanova et al. (2003) essentially has ω3 specificity with high activity on ALA but very low ω6 activity (Ruiz-López et al. 2009). The ω3 preference observed by Hoffmann et al. (2008) and Petrie et al. (2010a) in their Δ6-desaturases was not as strong as that seen in the primula enzyme, although the Δ6-desaturases were from microalgal sources and seemingly able to access acyl-CoA substrate pools, as will be discussed below. Other microalgal acyl-CoA desaturases such as the Ostreococcus tauri Δ6-desaturase (Domergue et al. 2005) did not have ω3 preference.
Several Δ9-elongases have now been isolated (Qi et al. 2002; Damude et al. 2008), although none has a strong ω3 preference. Use of these genes in high-level ω3 LC-PUFA production would therefore likely require the addition of an ω3-desaturase. This is exemplified by the isolation and transgenic plant expression of the entire Δ8 DHA pathway from a single algal species, Pavlova salina (Petrie et al. 2010b). In this study, a high proportion of the newly produced LC-PUFA were ω6 despite these being almost absent in the native algal fatty acid profile, in which large amounts of ω3 LC-PUFA are present (Zhou et al. 2007). Since the P. salina Δ9-elongase did not have ω3 preference, it is likely that this alga either has a strong ω3-desaturase or a method of selectively accumulating ω3 LC-PUFA.
Acyl-CoA pathways
Alternative approaches to building ω3 LC-PUFA pathways have been taken with several groups focusing on the construction of acyl-CoA pathways to reduce the ‘pool shuffling’ that can occur in transgenic LC-PUFA pathways. The LC-PUFA elongation condensing reactions occur using acyl-CoA thioester substrates (the ‘acyl-CoA pool’). In contrast, the majority of desaturases now isolated are lipid-linked desaturases that generally use a phosphatidylcholine substrate (the ‘acyl-PC pool’). A desaturated fatty acid must therefore be transferred between the acyl-PC and acyl-CoA pools by acyltransferase enzymes before a subsequent elongation can occur. The synthesis and accumulation of triacylglycerol in seeds are likely more complex than previously thought (Bates et al. 2009) and the requirement for acyltransfer between pathway steps presents opportunities for the novel fatty acid to be lost into pools which are, relatively, metabolically inert. This leads to a build-up of intermediate fatty acids along the biosynthetic pathway, with the eventual result being that the terminal product (e.g. DHA) does not accumulate to adequate levels. One solution to this challenge is the addition of acyltransferase enzymes which are capable of rapidly converting the newly elongated or newly desaturated fatty acids to the appropriate pool (e.g. the approach taken by Wu et al. (2005) described above in which an LPAT was included in the pathway). Unfortunately, this approach requires the addition of at least one extra gene to an already sizeable transgenic pathway. Fortunately, an alternative solution exists in the form of acyl-CoA desaturases.
The majority of desaturases are lipid linked, although there are published examples of enzymes which are able to function on acyl-CoA thioesters (Kajikawa et al. 2004; Domergue et al. 2005; Hoffmann et al. 2008; Petrie et al. 2010a) and several of these have been used in DHA pathways. Using such acyl-CoA desaturases means that elongation and desaturation can occur in the same metabolic pool, and thus avoid the efficiency and accumulation losses associated with the ‘pool shuffling’ described above. Acyl-CoA LC-PUFA pathways can often be characterized by high elongation efficiency. Petrie et al. (2010a), for instance, described a large increase in Δ6-elongation efficiency when the Δ6-elongase substrate, SDA, was produced by an acyl-CoA Δ6-desaturase when compared with SDA produced by an acyl-PC Δ6-desaturase. Similarly, Hoffmann et al. (2008) were able to construct an acyl-CoA pathway with efficient elongation, although the amounts of LC-PUFA produced were very low.
Improving Δ5-elongation
A final area of recent focus has been the Δ5-elongation step. Although Wu et al. (2005) and Cheng et al. (2010) demonstrated significant levels of EPA production (15 and 25 %, respectively), there have, to date, been no reports of high levels of DHA production in transgenic plants. This is mostly due to low Δ5-elongation efficiency and it has been suggested that the inclusion of enzymes with suitable acyl-transfer function (such as the acyltransferases described above) may be required to achieve adequate efficiency. Indeed, the authors of this review have previously had similar difficulty in obtaining adequate Δ5-elongation efficiency (Robert et al. 2005), although the recent isolation of a highly efficient enzyme from the microalga Pyramimonas cordata is encouraging (Petrie et al. 2009). This enzyme functions extremely well in plants (Fig. 3) and may be an important component of a transgenic DHA biosynthesis pathway for oilseed crops. Figure 3 demonstrates the importance of careful enzyme selection when constructing transgenic pathways, with a clear increase in EPA elongation observed with the P. cordata Δ5-elongase. Furthermore, Fig. 3 also demonstrates the effectiveness of using a Δ6-desaturase with ω3 preference (i.e. extremely low ω6 fatty acid levels without using an ω3-desaturase) and an acyl-CoA-like nature (i.e. the subsequent Δ6-elongation is extremely efficient). The high conversion efficiency of the P. salina Δ5-desaturase used in this pathway, both in terms of desaturation and provision of the Δ5-desaturated products to the Δ5-elongase, indicates that this enzyme may also be able to access acyl-CoA substrates although this is not yet biochemically proven. If so, this demonstrates the importance of using a pathway in which most of the desaturases are acyl-CoA-dependent for DHA synthesis. It is also worth noting that all transgenes in this pathway are from algal sources.
Long-chain polyunsaturated fatty acid production and accumulation in oilseed crops
Figure 4 describes results from a rapid transient expression system in a model plant, Nicotiana benthamiana (Wood et al. 2009; Petrie et al. 2010c). This transient expression of a DHA pathway alongside the A. thaliana DGAT1 yielded an impressive level of EPA and DHA accumulation in the leaf triglycerides. Such an oil composition, if replicated in oilseeds, would yield oils whose fatty acid profile will compare very favourably to that of a fish. Other groups rely extensively on A. thaliana, cultured soybean embryos or similar, relatively rapid, systems to test hypotheses and constructs before transforming crop species. The transition from an interesting result in a model system to a good result in an oilseed crop species is not necessarily trivial. Whilst this transition is broadly applicable to most crop metabolic engineering projects, the introduction of LC-PUFA to crops presents some specific problems. LC-PUFA, including DHA, are unusual fatty acids in a land plant context and may not necessarily be metabolized by germinating seed of oilseed crop species. The physiological impact of this would likely vary, depending on both the amount of oil present and available in the seed during germination and the proportion of LC-PUFA accumulated in the seed oil. High-oil species such as rapeseed or flaxseed naturally have a greater energy store than lower oil crops which can then be accessed during germination since these species have been selectively bred to produce oil in excess of germination requirements. The effective removal of a fraction of these ‘surplus’ triacylglycerols would likely have a relatively small effect on germination and seedling vigour when compared with lower oil crop species such as soybean. Similarly, it could reasonably be expected that the production of high levels of LC-PUFA in lower oil crop species could dramatically affect germination rates and seedling vigour.
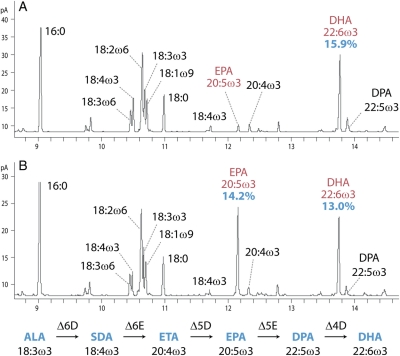
Fig. 4.
Docosahexaenoic acid production in plant leaf. Gas chromatography (GC) traces of fatty acid methyl esters (FAME) produced from triacylglycerol in N. benthamiana leaf tissue transiently expressing single-gene Cauliflower mosaic virus 35S promoter-driven binary constructs containing the P19 gene silencing suppressor, Micromonas pusilla Δ6-desaturase, P. cordata Δ6-elongase, P. salina Δ5-desaturase, P. cordata Δ5-elongase (A) or P. salina Δ5-elongase (B), and the P. salina Δ4-desaturase. The accumulation of EPA in the sample using the P. salina Δ5-elongase demonstrates the manner in which metabolic pathways can be tailored by careful selection of a single gene in the pathway.
It will also be interesting to observe the positional distribution of LC-PUFA in the triacylglycerols constituting high LC-PUFA crop oil, especially since studies have indicated that LC-PUFA located on the sn-2 position are more stable under storage and also more bioavailable to human infants, and possibly adults, than those on the sn-1,3 positions (Berry 2009).
Acceptance of crop-based ω3 LC-PUFA
As with many projects in the broader field of land plant metabolic engineering, the scientific and technical hurdles around the production of LC-PUFA are only part of the challenge. The real-world delivery of a crop-based source of ω3 LC-PUFA will inevitably require acceptance of the technology by the broader public. A genetically modified ω3 LC-PUFA crop will have tremendous environmental sustainability credentials due to the role it will play in relieving pressure on global fish stocks. Furthermore, such a crop will have direct health benefits for the consumers and, given the increasing awareness of the importance of ω3 LC-PUFA in the general public, these factors are likely to play an important part in increasing consumer acceptance.
Some regions have a high level of aversion to transgenic technologies due to perceived risks (Hansen et al. 2003; Food and Agriculture Organization 2004; Cox et al. 2008a, b), although this does not appear to be the case in other countries. One study, for example, has found that 99 % of consumers in the USA were more concerned with hygiene, sanitation and food-borne illnesses than with biotechnology (IFIC 2005). Regardless, it is useful to review consumers' attitudes to transgenic ω3 LC-PUFA in particular: Cox et al. (2008a, b, 2011) have investigated both US and Australian consumer attitudes toward ω3 LC-PUFA and also ω3 LC-PUFA produced from transgenic oilseeds. They found that whilst a minority had concerns about the use of transgenic technology, the majority of study participants, generally, were either ambivalent or positive about the use of transgenic ω3 LC-PUFA oilseeds. This agrees with earlier data which also suggested that consumers are likely to accept transgenic technology where there is a direct benefit to the consumer (Hossain et al. 2003; Hossain and Onyango 2004). Interestingly, consumers tended to prefer a transgenic ω3 LC-PUFA oilseed as the preferred source of oil to be incorporated into bread, milk and supplements above oils sourced from fish and algae (Cox et al. 2011).
Conclusions and forward look
Algal genes have contributed significantly to the LC-PUFA metabolic engineering efforts described in this short review. Similarly, research towards the in planta production of carotenoids, and in particular astaxanthin, continues to require the use of genes from algal species that naturally synthesize these compounds (see Misawa (2009) for a review). In a broader context, algal model species such as Chlamydomonas reinhardtii are now being used to better understand metabolism and catabolism in plants and plant-like species (Wijffels and Barbosa 2010). Recent focus on finding or engineering sustainable sources of biofuels has seen increased use of algal models to investigate mechanisms of lipid accumulation and starch conversion. Wang et al. (2009) and Moellering and Benning (2010) have described the formation of lipid droplets in C. reinhardtii after nitrogen starvation, with Miller et al. (2010) then describing changes in transcript abundance in these systems. It is likely that findings such as these will have relevance in land plants due to the high degree of similarity between the organisms (Hicks et al. 2001). There has been considerable effort under way by the US Department of Energy-funded Joint Genome Institute to sequence the genomes of a number of algal species. This data set is publically available at http://genome.jgi-psf.org/. Detailed analyses of algal genomes not only provide important information for research into functional genomics, but also increase our understanding of the physiology, environmental adaptation mechanisms, morphogenesis and evolution of organisms (especially the origin of photosynthetic organisms) and their evolution into land plants.
Conflicts of interest statement
None declared.
Acknowledgements
All our work described in this article was performed by the CSIRO Food Futures Flagship Omega-3 team based in the Plant Industry, Marine and Atmospheric Research, Food & Nutritional Sciences and Livestock Industry Divisions of CSIRO Australia and supported by the Grains Research & Development Corporation.
References
- Abbadi A, Domergue F, Bauer J, Napier J, Welti R, Zähringer U, Cirpus P, Heinz E. Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. The Plant Cell. 2004;16:2734–2748. doi: 10.1105/tpc.104.026070. doi:10.1105/tpc.104.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P, Durrett T, Ohlrogge J, Pollard M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiology. 2009;150:55–72. doi: 10.1104/pp.109.137737. doi:10.1104/pp.109.137737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: an overview and implications for cardiovascular disease. Nutrition Research Reviews. 2009;22:3–17. doi: 10.1017/S0954422409369267. doi:10.1017/S0954422409369267. [DOI] [PubMed] [Google Scholar]
- Birch E, Garfield S, Hoffman D, Uauy R, Birch D. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Developmental Medicine and Child Neurology. 2000;42:174–181. doi: 10.1017/s0012162200000311. doi:10.1017/S0012162200000311. [DOI] [PubMed] [Google Scholar]
- Burdge G, Calder P. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduction Nutrition Development. 2005;45:581–597. doi: 10.1051/rnd:2005047. doi:10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- Cheng B, Wu G, Vrinten P, Falk K, Bauer J, Qiu X. Towards the production of high levels of eicosapentaenoic acid in transgenic plants: the effects of different host species, genes and promoters. Transgenic Research. 2010;19:221–229. doi: 10.1007/s11248-009-9302-z. doi:10.1007/s11248-009-9302-z. [DOI] [PubMed] [Google Scholar]
- Cox D, Evans G, Lease H. Australian consumers’ preferences for conventional and novel sources of long chain omega-3 fatty acids: a conjoint study. Food Quality and Preference. 2008a;19:306–314. [Google Scholar]
- Cox D, Evans G, Lease H. Predictors of Australian consumers’ intentions to consume conventional and novel sources of long-chain omega-3 fatty acids. Public Health Nutrition. 2008b;11:8–16. doi: 10.1017/S136898000700016X. [DOI] [PubMed] [Google Scholar]
- Cox D, Evans G, Lease H. The influence of product attributes, consumer attitudes and characteristics on the acceptance of: (1) novel bread and milk, and dietary supplements and (2) fish and novel meats as dietary vehicles of long chain omega 3 fatty acids. Food Quality and Preference. 2011;22:205–212. doi:10.1016/j.foodqual.2010.10.003. [Google Scholar]
- Damude H, Kinney A, Ripp K, Zhu Q. Multizymes and their use in making polyunsaturated fatty acids. 2008. Patent WO2008124048.
- Domergue F, Abbadi A, Zähringer U, Moreau H, Heinz E. In vivo characterization of the first acyl-CoA Δ6-desaturase from a member of the plant kingdom, the microalga Ostreococcus tauri. Biochemical Journal. 2005;389:483. doi: 10.1042/BJ20050111. doi:10.1042/BJ20050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization (FAO) State of Food and Agriculture: Agricultural biotechnology: Meeting the needs of the poor. 2004. http://www.fao.org/docrep/006/Y5160E/y5160e11.htm#P15_5987 .
- Freeman M, Hibbeln J, Wisner K, Davis J, Mischoulon D, Peet M, Keck P, Marangell L, Richardson A, Lake J, Stoll A. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. The Journal of Clinical Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. doi:10.4088/JCP.v67n1217. [DOI] [PubMed] [Google Scholar]
- García-Maroto F, Mañas-Fernández A, Garrido-Cárdenas J, Alonso D. Substrate specificity of acyl-Δ6-desaturases from Continental versus Macaronesian Echium species. Phytochemistry. 2006;67:540–544. doi: 10.1016/j.phytochem.2005.12.005. doi:10.1016/j.phytochem.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Hansen J, Holm L, Frewer L, Robinson P, Sandøe P. Beyond the knowledge deficit: recent research into lay and expert attitudes to food risks. Appetite. 2003;41:111–121. doi: 10.1016/s0195-6663(03)00079-5. doi:10.1016/S0195-6663(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Harris W, Lemke S, Hansen S, Goldstein D, DiRienzo M, Su H, Nemeth M, Taylor M, Ahmed G, George C. Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids. 2008;43:805–811. doi: 10.1007/s11745-008-3215-0. doi:10.1007/s11745-008-3215-0. [DOI] [PubMed] [Google Scholar]
- Hicks G, Hironaka C, Dauvillee D, Funke R, D'Hulst C, Waffenschmidt S, Ball S. When simpler is better. Unicellular green algae for discovering new genes and functions in carbohydrate metabolism. Plant Physiology. 2001;127:1334. doi:10.1104/pp.010821. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Wagner M, Abbadi A, Fulda M, Feussner I. Metabolic engineering of ω3-very long chain polyunsaturated fatty acid production by an exclusively acyl-CoA-dependent pathway. The Journal of Biological Chemistry. 2008;283:22352–22362. doi: 10.1074/jbc.M802377200. doi:10.1074/jbc.M802377200. [DOI] [PubMed] [Google Scholar]
- Hossain F, Onyango B. Product attributes and consumer acceptance of nutritionally enhanced genetically modified foods. International Journal of Consumer Studies. 2004;28:255–267. doi:10.1111/j.1470-6431.2004.00352.x. [Google Scholar]
- Hossain F, Onyango B, Schilling B, Hallman W, Adelaja A. Product attributes, consumer benefits and public approval of genetically modified foods. International Journal of Consumer Studies. 2003;27:353–365. doi:10.1046/j.1470-6431.2003.00303.x. [Google Scholar]
- Hölker F, Beare D, Dörner H, di Natale A, Rätz H-J, Temming A, Casey J. Comment on ‘Impacts of biodiversity loss on ocean ecosystem services’. Science. 2007;316:1285. doi: 10.1126/science.1139114. author reply 1285 doi:10.1126/science.1139114. [DOI] [PubMed] [Google Scholar]
- IFIC (International Food Information Council Foundation) Food biotechnology survey questionnaire. 2005. http://www.ific.org/research/qualhealthclaimsres.cfm .
- Jaenike J. Comment on ‘Impacts of biodiversity loss on ocean ecosystem services’. Science. 2007;316:1285. doi: 10.1126/science.1137730. author reply 1285 doi:10.1126/science.1137730. [DOI] [PubMed] [Google Scholar]
- James M, Ursin V, Cleland L. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. American Journal of Clinical Nutrition. 2003;77:1140–1145. doi: 10.1093/ajcn/77.5.1140. [DOI] [PubMed] [Google Scholar]
- Kajikawa M, Yamato KT, Kohzu Y, Nojiri M, Shimizu S, Sakai Y, Fukuzawa H. Isolation and characterization of Δ6-desaturase, an ELO-like enzyme and Δ5-desaturase from the liverwort Marchantia polymorpha and production of arachidonic and eicosapentaenoic acids in the methylotrophic yeast Pichia pastoris. Plant Molecular Biology. 2004;54:335–352. doi: 10.1023/B:PLAN.0000036366.57794.ee. doi:10.1023/B:PLAN.0000036366.57794.ee. [DOI] [PubMed] [Google Scholar]
- Kinney A, Cahoon E, Damude H, Hitz W, Kolar CLZ. Production of very long chain polyunsaturated fatty acids in oilseed plants. 2004. Patent US20040057.
- Kremer J, Lawrence D, Petrillo G, Litts L, Mullaly P, Rynes R, Stocker R, Parhami N, Greenstein N, Fuchs B. Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates. Arthritis and Rheumatism. 1995;38:1107–1114. doi: 10.1002/art.1780380813. doi:10.1002/art.1780380813. [DOI] [PubMed] [Google Scholar]
- Lippmeier J, Crawford K, Owen C, Rivas A, Metz J, Apt K. Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp. Lipids. 2009;44:621–630. doi: 10.1007/s11745-009-3311-9. doi:10.1007/s11745-009-3311-9. [DOI] [PubMed] [Google Scholar]
- Metz J, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science. 2001;293:290. doi: 10.1126/science.1059593. doi:10.1126/science.1059593. [DOI] [PubMed] [Google Scholar]
- Metz J, Flatt J, Kuner J. PUFA polyketide synthases and uses thereof. 2006. Patent WO2006135866.
- Metz J, Kuner J, Rosenzweig B, Lippmeier JC, Roessler P, Zirkle R. Biochemical characterization of polyunsaturated fatty acid synthesis in Schizochytrium: release of the products as free fatty acids. Plant Physiology and Biochemistry. 2009;47:472–478. doi: 10.1016/j.plaphy.2009.02.002. doi:10.1016/j.plaphy.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Miller R, Wu G, Deshpande R, Vieler A, Gaertner K, Li X, Moellering E, Zauner S, Cornish A, Liu B, Bullard B, Sears BB, Kuo M-H, Hegg E, Shachar-Hill Y, Shiu S-H, Benning C. Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen-deprivation predict diversion of metabolism. Plant Physiology. 2010;154:1737–1752. doi: 10.1104/pp.110.165159. doi:10.1104/pp.110.165159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa N. Pathway engineering of plants toward astaxanthin production. Plant Biotechnology. 2009;26:93–99. doi:10.5511/plantbiotechnology.26.93. [Google Scholar]
- Moellering E, Benning C. RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryotic Cell. 2010;9:97–106. doi: 10.1128/EC.00203-09. doi:10.1128/EC.00203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Bao D, Burke V, Puddey I, Beilin L. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–260. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- Myers R, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. doi:10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- Nagel G, Nieters A, Becker N, Linseisen J. The influence of the dietary intake of fatty acids and antioxidants on hay fever in adults. Allergy. 2003;58:1277–1284. doi: 10.1046/j.1398-9995.2003.00296.x. doi:10.1046/j.1398-9995.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- Oh D, Talukdar S, Bae E, Imamura T, Morinaga H, Fan W, Li P, Lu W, Watkins S, Olefsky J. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. doi:10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Gibson N, Brotchie H, Heruc G, Rees A-M, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. The American Journal of Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. doi:10.1176/appi.ajp.163.6.969. [DOI] [PubMed] [Google Scholar]
- Petrie J, Liu Q, Mackenzie A, Shrestha P, Mansour P, Robert S, Frampton D, Blackburn S, Nichols P, Singh S. Isolation and characterisation of a high-efficiency desaturase and elongases from microalgae for transgenic LC-PUFA production. Marine Biotechnology. 2009 doi: 10.1007/s10126-009-9230-1. doi:10.1007/s10126-009-9230-1. [DOI] [PubMed] [Google Scholar]
- Petrie J, Shrestha P, Mansour M, Nichols P, Liu Q, Singh S. Metabolic engineering of omega-3 long-chain polyunsaturated fatty acids in plants using an acyl-CoA Δ6-desaturase with ω3-preference from the marine microalga Micromonas pusilla. Metabolic Engineering. 2010a;12:233–240. doi: 10.1016/j.ymben.2009.12.001. doi:10.1016/j.ymben.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Petrie J, Mackenzie A, Shrestha P, Liu Q, Frampton D, Robert S, Singh S. Isolation of three novel long-chain polyunsaturated fatty acid Δ9-elongases and the transgenic assembly of the entire Pavlova salina docosahexaenoic acid pathway in Nicotiana benthamiana. Journal of Phycology. 2010b;46:917–925. doi:10.1111/j.1529-8817.2010.00870.x. [Google Scholar]
- Petrie J, Shrestha P, Liu Q, Mansour M, Wood C, Zhou X, Nichols P, Green A, Singh S. Rapid expression of transgenes driven by seed-specific constructs in leaf tissue: DHA production. Plant Methods. 2010c;6:8. doi: 10.1186/1746-4811-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi B, Beaudoin F, Fraser T, Stobart A, Napier J, Lazarus C. Identification of a cDNA encoding a novel C18-Δ9 polyunsaturated fatty acid-specific elongating activity from the docosahexaenoic acid (DHA)-producing microalga, Isochrysis galbana. FEBS Letters. 2002;510:159–165. doi: 10.1016/s0014-5793(01)03247-1. doi:10.1016/S0014-5793(01)03247-1. [DOI] [PubMed] [Google Scholar]
- Qi B, Fraser T, Mugford S, Dobson G, Sayanova O, Butler J, Napier J, Stobart K, Lazarus C. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nature Biotechnology. 2004;22:739–745. doi: 10.1038/nbt972. doi:10.1038/nbt972. [DOI] [PubMed] [Google Scholar]
- Robert S, Singh S, Zhou X, Petrie J, Blackburn S, Mansour P, Nichols P, Liu Q, Green A. Metabolic engineering of Arabidopsis to produce nutritionally important DHA in seed oil. Functional Plant Biology. 2005;32:473. doi: 10.1071/FP05084. doi:10.1071/FP05084. [DOI] [PubMed] [Google Scholar]
- Ruiz-López N, Haslam RP, Venegas-Calerón M, Larson TR, Graham IA, Napier JA, Sayanova O. The synthesis and accumulation of stearidonic acid in transgenic plants: a novel source of “heart-healthy”omega-3 fatty acids. Plant Biotechnology Journal. 2009;7:704–716. doi: 10.1111/j.1467-7652.2009.00436.x. doi:10.1111/j.1467-7652.2009.00436.x. [DOI] [PubMed] [Google Scholar]
- Sayanova OV, Beaudoin F, Michaelson LV, Shewry PR, Napier J. Identification of Primula fatty acid delta 6-desaturases with n-3 substrate preferences. FEBS Letters. 2003;542:100–104. doi: 10.1016/s0014-5793(03)00358-2. doi:10.1016/S0014-5793(03)00358-2. [DOI] [PubMed] [Google Scholar]
- von Schacky C. A review of omega-3 ethyl esters for cardiovascular prevention and treatment of increased blood triacylglycerol levels. Vascular Health and Risk Management. 2006;2:251–262. doi: 10.2147/vhrm.2006.2.3.251. doi:10.2147/vhrm.2006.2.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer E, Bongard V, Beiser A, Lamon-Fava S, Robins S, Au R, Tucker K, Kyle D, Wilson P, Wolf P. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Archives of Neurology. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. doi:10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- Simopoulos A. Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- Tocher D, Dick J, MacGlaughlin P, Bell J. Effect of diets enriched in Δ6 desaturated fatty acids (18:3n-6 and 18:4n-3), on growth, fatty acid composition and highly unsaturated fatty acid synthesis in two populations of Arctic charr (Salvelinus alpinus L.) Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2006;144:245–253. doi: 10.1016/j.cbpb.2006.03.001. doi:10.1016/j.cbpb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Ueshima H, Stamler J, Elliott P, Chan Q, Brown I, Carnethon M, Daviglus M, He K, Moag-Stahlberg A, Rodriguez B, Steffen L, Van Horn L, Yarnell J, Zhou B. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension. 2007;50:313–319. doi: 10.1161/HYPERTENSIONAHA.107.090720. doi:10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas-Calerón M, Sayanova O, Napier JA. An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Progress in Lipid Research. 2010;49:108–119. doi: 10.1016/j.plipres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Wang C, Harris W, Chung M, Lichtenstein A, Balk E, Kupelnick B, Jordan H, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. The American Journal of Clinical Nutrition. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ullrich N, Joo S, Waffenschmidt S, Goodenough U. Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryotic Cell. 2009;8:1856–1868. doi: 10.1128/EC.00272-09. doi:10.1128/EC.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J. Dietary stearidonic acid is a long chain (n-3) polyunsaturated fatty acid with potential health benefits. Journal of Nutrition. 2009;139:5–10. doi: 10.3945/jn.108.094268. doi:10.3945/jn.108.094268. [DOI] [PubMed] [Google Scholar]
- Wijffels R, Barbosa M. An outlook on microalgal biofuels. Science. 2010;329:796–799. doi: 10.1126/science.1189003. doi:10.1126/science.1189003. [DOI] [PubMed] [Google Scholar]
- Wilberg M, Miller T. Comment on ‘Impacts of biodiversity loss on ocean ecosystem services’. Science. 2007;316:1285. doi: 10.1126/science.1137946. doi:10.1126/science.1137946. [DOI] [PubMed] [Google Scholar]
- Wood C, Petrie J, Shrestha P, Mansour M, Nichols P, Green A, Singh S. A leaf-based assay using interchangeable design principles to rapidly assemble multistep recombinant pathways. Plant Biotechnology Journal. 2009;7:914–924. doi: 10.1111/j.1467-7652.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- Worm B, Barbier E, Beaumont N, Duffy J, Folke C, Halpern B, Jackson J, Lotze H, Micheli F, Palumbi S, Sala E, Selkoe K, Stachowicz J, Watson R. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. doi:10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- Wu G, Truksa M, Datla N, Vrinten P, Bauer J, Zank T, Cirpus P, Heinz E, Qiu X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nature Biotechnology. 2005;23:1013–1017. doi: 10.1038/nbt1107. doi:10.1038/nbt1107. [DOI] [PubMed] [Google Scholar]
- Zhou XR, Robert SS, Petrie JR, Frampton DMF, Mansour MP, Blackburn SI, Nichols PD, Green AG, Singh SP. Isolation and characterization of genes from the marine microalga Pavlova salina encoding three front-end desaturases involved in docosahexaenoic acid biosynthesis. Phytochemistry. 2007;68:785–796. doi: 10.1016/j.phytochem.2006.12.016. doi:10.1016/j.phytochem.2006.12.016. [DOI] [PubMed] [Google Scholar]