Abstract
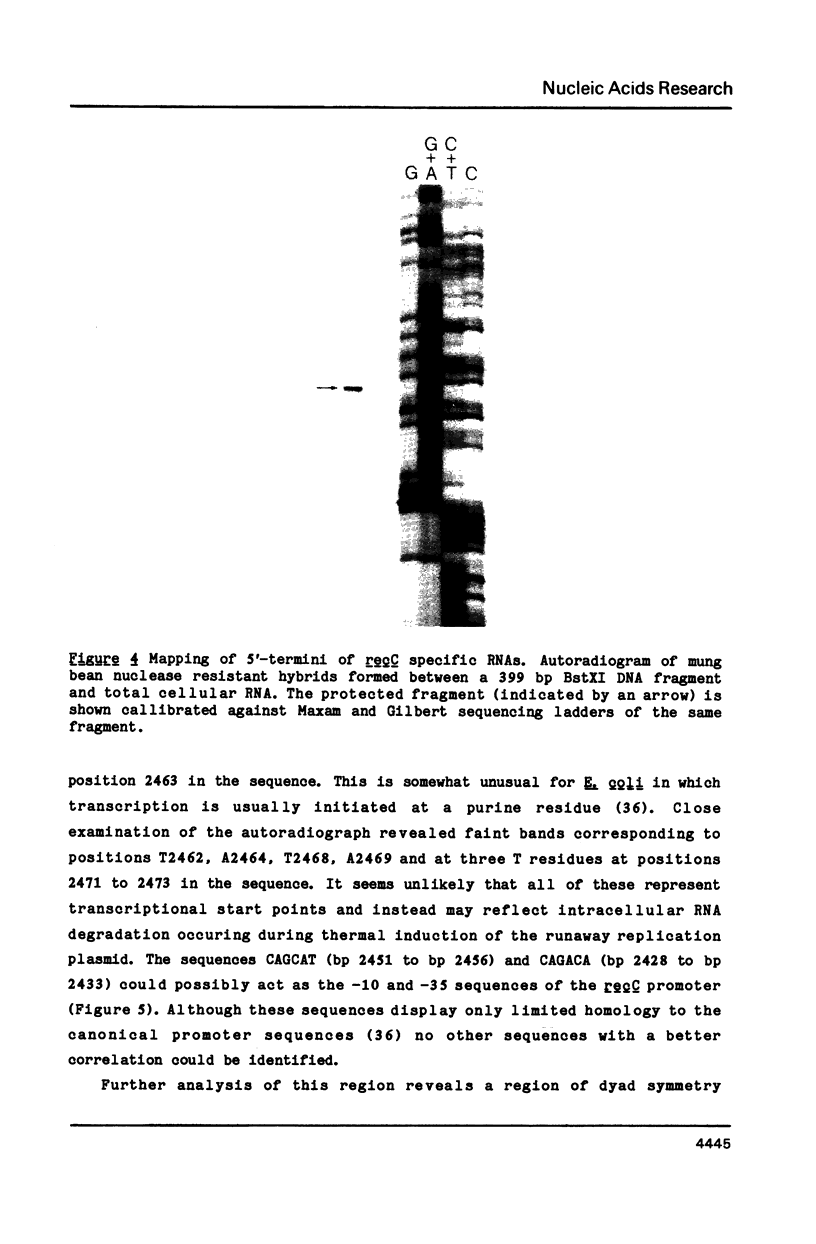
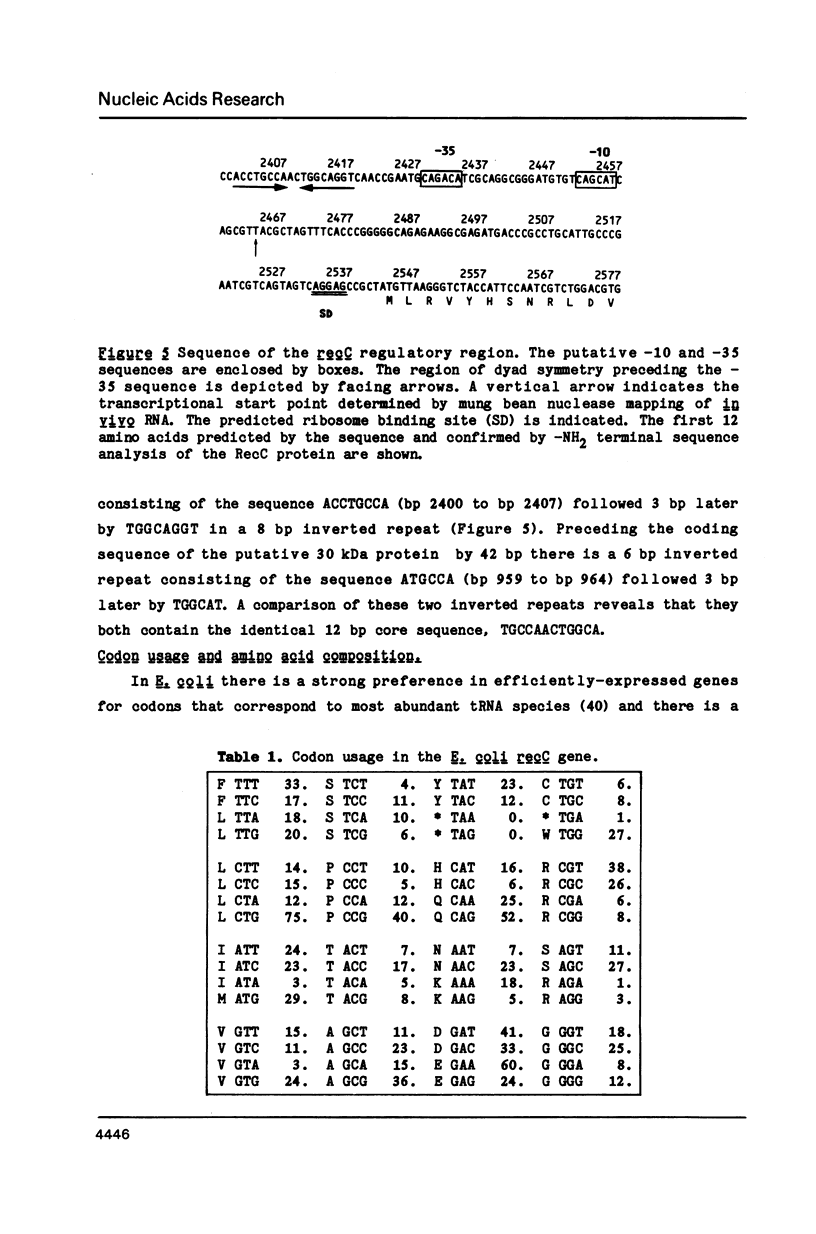
The nucleotide sequence of a 6,000 bp region of the E. coli chromosome that includes the 3' end of the coding region for the thyA gene and the entire recC gene has been determined. The proposed coding region for the RecC protein is 3369 nucleotides long, which would encode a polypeptide consisting of 1122 amino acids with a calculated molecular mass of 129 kDa. Mung bean nuclease mapping of a recC specific transcript produced in vivo indicates that transcription of recC is initiated 80 bp upstream of the translational start point. A weak promoter sequence situated 5' to the transcription initiation point has been identified. In the 1953 bp thyA-recC intergenic region there are three open reading frames that would code for polypeptides of molecular mass 30 kDa, 13.5 kDa and 12 kDa, respectively. Although the first and third of these open reading frames are preceded by possible ribosome binding sites, no obvious promoter sequences could be identified. Moreover, transcripts for these reading frames could not be detected.
Full text
PDF










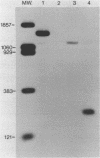
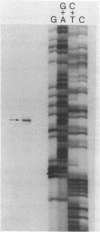
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfort M., Maley G., Pedersen-Lane J., Maley F. Primary structure of the Escherichia coli thyA gene and its thymidylate synthase product. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4914–4918. doi: 10.1073/pnas.80.16.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Dykstra C. C., Prasher D., Kushner S. R. Physical and biochemical analysis of the cloned recB and recC genes of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):21–27. doi: 10.1128/jb.157.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Emmerson P. T. The nucleotide sequence of the uvrD gene of E. coli. Nucleic Acids Res. 1984 Jul 25;12(14):5789–5799. doi: 10.1093/nar/12.14.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. An endonuclease activity from Escherichia coli absent from certain rec- strains. Proc Natl Acad Sci U S A. 1970 Sep;67(1):434–441. doi: 10.1073/pnas.67.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell. 1980 Nov;22(1 Pt 1):231–242. doi: 10.1016/0092-8674(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel C., Irminger J. C., Bucher P., Birnstiel M. L. Sea urchin histone mRNA termini are located in gene regions downstream from putative regulatory sequences. Nature. 1980 May 15;285(5761):147–151. doi: 10.1038/285147a0. [DOI] [PubMed] [Google Scholar]
- Hickson I. D., Atkinson K. E., Hutton L., Tomkinson A. E., Emmerson P. T. Molecular amplification and purification of the E. coli recC gene product. Nucleic Acids Res. 1984 May 11;12(9):3807–3819. doi: 10.1093/nar/12.9.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson I. D., Emmerson P. T. Identification of the Escherichia coli recB and recC gene products. Nature. 1981 Dec 10;294(5841):578–580. doi: 10.1038/294578a0. [DOI] [PubMed] [Google Scholar]
- Hickson I. D., Robson C. N., Atkinson K. E., Hutton L., Emmerson P. T. Reconstitution of RecBC DNase activity from purified Escherichia coli RecB and RecC proteins. J Biol Chem. 1985 Jan 25;260(2):1224–1229. [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Godson G. N. Evidence for use of rare codons in the dnaG gene and other regulatory genes of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):687–691. doi: 10.1073/pnas.80.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S. T., Stahl M. M., McMilin K. D., Stahl F. W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974 Jul;77(3):425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Thomas A. On the nature of the RecBC and RecF pathways of conjugal recombination in Escherichia coli. Mol Gen Genet. 1983;190(1):156–161. doi: 10.1007/BF00330339. [DOI] [PubMed] [Google Scholar]
- Lovett S. T., Clark A. J. Genetic analysis of regulation of the RecF pathway of recombination in Escherichia coli K-12. J Bacteriol. 1983 Mar;153(3):1471–1478. doi: 10.1128/jb.153.3.1471-1478.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli A. S., Schultz D. W., Taylor A. F., Smith G. R. Chi-dependent DNA strand cleavage by RecBC enzyme. Cell. 1985 May;41(1):145–151. doi: 10.1016/0092-8674(85)90069-8. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Fujiyoshi T., Shimada K., Takagi Y. Fine structure of the recB and recC gene region of Escherichia coli. Biochem Biophys Res Commun. 1982 Nov 30;109(2):414–422. doi: 10.1016/0006-291x(82)91737-5. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Measurements of the effects that coding for a protein has on a DNA sequence and their use for finding genes. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):551–567. doi: 10.1093/nar/12.1part2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Smith G. R. Unwinding and rewinding of DNA by the RecBC enzyme. Cell. 1980 Nov;22(2 Pt 2):447–457. doi: 10.1016/0092-8674(80)90355-4. [DOI] [PubMed] [Google Scholar]
- Umeno M., Anai M., Sasaki M., Takagi Y. Purification and subunit structure of recBC DNase from Escherichia coli harboring a recB and recC genes-inserted plasmid. J Biochem. 1985 Sep;98(3):681–685. doi: 10.1093/oxfordjournals.jbchem.a135325. [DOI] [PubMed] [Google Scholar]
- Umeno M., Sasaki M., Anai M., Takagi Y. Properties of the recB and recC gene products of Escherichia coli. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1144–1150. doi: 10.1016/s0006-291x(83)80262-9. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Takagi T. Overproduction of Escherichia coli replication proteins by the use of runaway-replication plasmids. J Bacteriol. 1983 Jun;154(3):1153–1161. doi: 10.1128/jb.154.3.1153-1161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Konings R. N., Veltkamp E., Nijkamp H. J. Transcription of bacteriocinogenic plasmid CloDF13 in vivo and in vitro: structure of the cloacin immunity operon. J Bacteriol. 1980 Nov;144(2):579–591. doi: 10.1128/jb.144.2.579-591.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]