Abstract
Objectives:
The exact cause of benign prostatic hyperplasia (BPH) and prostatic carcinoma is unknown. Changes in the level of the trace element zinc (Zn) are known to be associated with the functioning of different organs (breast, colon, stomach, liver, kidney, prostate, and muscle). This study is aimed at estimating and comparing the zinc levels in the prostate tissue, plasma, and urine obtained from patients diagnosed with BPH or prostatic carcinoma.
Materials and Methods:
The prostate tissue zinc, plasma zinc, and urine zinc/creatinine ratio in BPH, prostate cancer, and normal subjects were measured by atomic absorption spectrophotometry.
Results:
In prostate carcinoma, the mean tissue zinc was decreased by 83% as compared to normal tissue and in BPH, there was a 61% decrease in mean tissue zinc as compared to normal tissues. Both these values were statistically significant. The plasma zinc in prostate cancer patients showed a 27% decrease (P < 0.01) as compared to controls and 18% decrease (P < 0.01) as compared to BPH. The urine zinc/creatinine (ratio) was significantly increased to 53% in prostate cancer patients, and a 20% significant increase was observed in BPH as compared to normal subjects.
Conclusions:
It is evident from this study that BPH or prostate carcinoma may be associated with a reduction in the levels of tissue zinc, plasma zinc, and an increase in urine zinc/creatinine.
Keywords: Benign prostatic hyperplasia, plasma zinc, prostate cancer, tissue zinc, urine zinc/creatinine
INTRODUCTION
Prostatitis, benign prostatic hyperplasia (BPH), and prostate cancer (PCa) are the most frequent pathologies of the prostate gland.[1] Prostate cancer is the most common cancer among men in Europe and United States. There is lack of epidemiological data on the exact prevalence of this disease in India. Because the screening for prostate cancer is not routine, the incidence of prostate cancer in India is always under recorded.[2] BPH is a worldwide health problem that causes morbidity in older men.[3] Although the exact cause of BPH is not known, the presence of androgens, especially dihydrotestosterone, and ageing are considered to be the major factors.[4]
Zinc is an important constituent of prostatic fluid and is known to play an important role in the development and normal functioning of prostate.[5,6] Normal prostate tissues from healthy individuals accumulate the highest levels of zinc in the body.[7] In the prostate epithelial cell, the accumulation of high cellular zinc is a specialized function that is necessary for these cells to carry out the major physiological functions of production and secretion of citrate.[8] Malignant prostate cells that develop in the peripheral zone do not contain the high zinc levels that characterize the normal secretory epithelial cells.[9] If this is the case, then one should expect that the zinc-accumulating process that characterizes the normal glandular epithelium is absent or defective in the malignant cells.
Studies consistently show that the zinc levels of malignant prostate tissue are 62%–75% lower than the normal prostate tissue.[10] However, the pathway(s) that leads to lower zinc accumulation in malignant prostate epithelial cells are poorly understood.[9]
The genetic and molecular mechanisms responsible for and associated specifically with the development and progression of malignant prostate cells are largely unidentified. However, the role of altered cellular metabolism as an essential factor in prostate malignancy has been largely ignored. The intermediary metabolism of normal prostate as well as malignant prostate cells are among the least studied and most poorly understood in all mammalian cells. Some important factors, especially the role of zinc, have been identified and implicated in the progression of prostate malignancy.[11]
The utility of urinary zinc determination as a measure of zinc status is also limited by the difficulties encountered in collecting a 24 h urine specimen without exogenous zinc contamination.
In this study, we assessed the changes in zinc in association with the development of BPH or prostate carcinoma in men in our Indian population . We analyzed plasma zinc, urine zinc/creatinine ratio and prostatic tissue zinc to observe whether there was any association between them i.e. as plasma zinc and tissue zinc falls, does the urine zinc excretion increase in prostatic pathologic conditions.
MATERIALS AND METHODS
Patients and protocol
Patients attending the urology clinic of our hospital and presenting with symptoms of BPH or prostatic cancer were enrolled for the study. Chips of prostatic tissue taken from patients in the age group 50–75 years who underwent transurethral resection of prostate for BPH (n = 123) and from tissues of 20 patients in the age group 55–85 years, who underwent radical prostatectomy for prostate carcinoma were sent for histological analysis and for estimation of zinc as described. Blood and random urine samples were also collected in heparinized tubes and zinc-free containers, respectively, from these patients 1 day before surgery, processed and stored at –20°C for analysis of plasma zinc and urine zinc. This study was approved by the Institutional Review board and ethics committee of our Institution.
Tissue specimens from the normal prostate of 20 autopsy cases of patients who died due to illness other than malignancy were included as a control group in this study. Twenty normal healthy males (age between 30 and 50 years) were also included in the study for estimation of plasma zinc and urine zinc/creatinine ratio.
Tissue preparation and trace element zinc analysis
For trace element analysis, prostate tissues of hyperplastic prostate glands weighing about 40–100 mg were used. The tissues were placed in equal volumes 1:1, v/v nitric acid and perchloric acid and digested as described.[12] The digested solution was used to determine the levels of zinc by atomic absorption (Perkin–Elmer analyzer 100). The concentration of tissue zinc is reported in microgram per gram dry weight and wet weight, respectively. Plasma zinc, urine zinc, and urine creatinine were estimated as described.[13,14]
Preparation of plasma
5 ml blood was withdrawn from each patient and transferred into a tube containing 250 IU of sodium heparin. The tube was centrifuged for 10 min at 3500 r/min. Plasma was immediately separated into zinc-free containers, frozen at –20°C until analysis for plasma zinc using A.A.S.
Collection of urine
Random urine samples were collected from each patient on the day before the surgery and from healthy volunteers (control group) in zinc-free polypropylene containers. The urine sample was aspirated in the A.A.S and the reading compared with a standard containing 25 μg zinc/100 ml. Results were reported as μg/l. Urine creatinine (mg%) was estimated using the colorimetric kinetic Jaffe alkaline picrate reaction without deproteinization in the Hitachi 912 analyzer. The urine zinc/creatinie ratio was used to determine the amount of zinc in the random urine sample. The ratio corrects for effects due to concentration or dilution of the urine.
Quality control
Quality control (QC) values for plasma zinc using a commercial assayed chemistry control Biorad UK: are presented. N = 18, mean = 98.2 μg/dl, %CV = 5.6%.
Statistical analysis
Data are expressed as Mean ± SD. Differences between groups were analyzed using paired t-test and ANOVA. A difference was considered statistically significant when the P < 0.05.
RESULTS
Figures 1(a–d) shows the tissue and plasma zinc levels of normal prostate, BPH, and in prostate carcinoma. The mean prostatic tissue zinc concentration in normal prostate from autopsy cases, from patients with BPH or prostate carcinoma was 1009 ± 155, 387 ± 86.1, and 175 ± 58.6 μg/g dry weight, respectively. There was a significant decrease in mean prostatic tissue zinc levels (dry weight) in prostate carcinoma (P < 0.001, 82.6% decrease) and in BPH (P < 0.01, 61% decrease) as compared with controls. Further there was 54.7% decrease (P < 0.01) in prostate carcinoma as compared to BPH.
Figure 1.
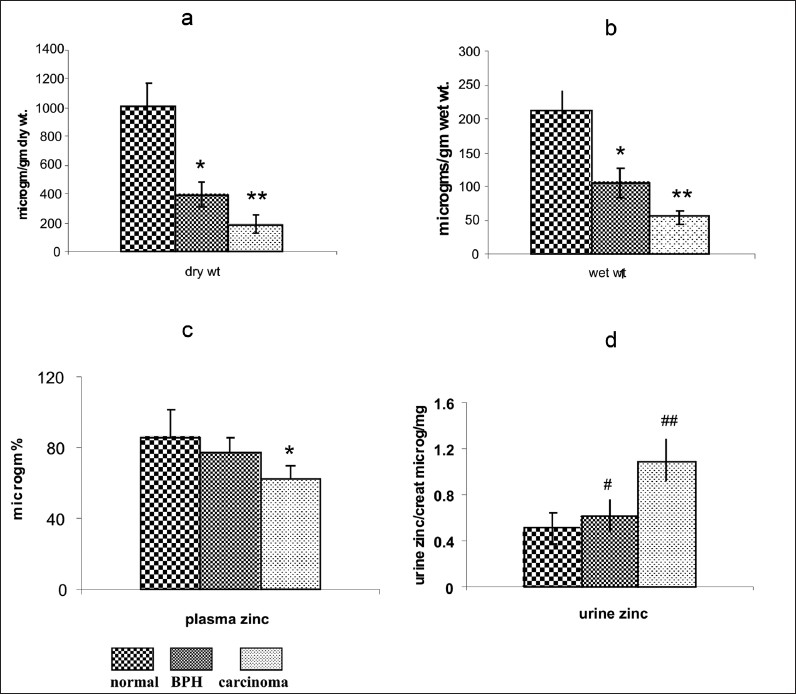
Zinc status in normal subjects, BPH and Prostate carcinoma. (a) Tissue zinc levels (µg/g) dry weight. (b) (µg/g) wet weight. Values are shown as mean ± SD. *P< 0.05, **P< 0.001, as compared with normal. (c) Plasma zinc levels. Values are shown as mean ± SD. *P< 0.01 as compared with normal. (d) Urine zinc/creatinine ratio. Values are shown as mean ± SD. #P< 0.005, ##P< 0.001, as compared with normal healthy person.
The mean plasma zinc concentrations in normal healthy controls (n = 20) and in patients with BPH (n = 45) and prostate carcinoma (n = 18) were 86.5 ± 15.2, 77 ± 9.5, and 63 ± 6.4 μg/dl, respectively. A significant mean 27% decrease in plasma zinc levels was observed in prostate carcinoma patients as compared with controls and a mean decrease of 18% (P < 0.05) in plasma zinc was observed in prostate carcinoma as compared with BPH. There was no significant decrease in plasma zinc between BPH and normal controls as shown in Figure 1c
As shown in Figure 1d the mean urine zinc/creatinine ratio in normal healthy controls (n = 20), and patients with BPH (n = 45) and prostate carcinoma (n = 18), was 0.513 ± 0.101, 0.615 ± 0.144, and 1.099 ± 0.190 μg/mg, respectively. A significant mean 44% increase was observed in the urine zinc/creatinine in prostate carcinoma as compared to BPH. Further a significant increase (P < 0.01, mean 53%) was observed in urine zinc/creatinine (ratio) in prostate carcinoma as compared with controls. There was significant increase in mean urine zinc/creatinine ratio in BPH as compared to normal prostate (P < 0.05, mean 19.8%).
DISCUSSION
The human prostate gland contains a higher level of zinc than most other tissues.[15] The most consistent and persistent biochemical characteristic of prostate cancer (PCa) is the marked decrease in zinc in the malignant cells. Using biochemical methods (A.A.S), this study of zinc in pathologic conditions such as BPH and prostatic carcinoma showed a significant difference in concentration of tissue zinc, plasma zinc, and urine zinc/creatinine ratio in these patients as compared with normal healthy persons.
We observed a significant decrease in mean tissue zinc levels in prostatic carcinoma and BPH as compared to normal prostate from autopsy cases. It was observed that plasma zinc levels before surgery from patients presenting with prostatic carcinoma showed a significant decrease as compared to normal healthy volunteers.
Our study is consistent with earlier studies that were reported separately, in which the level of plasma or tissue zinc is substantially lowered in the cancerous prostate and BPH than in normal prostate.[16,17] In contrast, an increase in tissue zinc in BPH and a decrease in tissue zinc in prostatic carcinoma as compared to controls has been reported in another study.[18]
Neoplastic diseases and infectious diseases have been reported to lead to changes in zinc metabolism as evidenced by alterations in plasma zinc.[19] The presence of this condition that alters plasma zinc content may lead to accumulative zinc depletion in the tissues.
Changes in only plasma zinc concentrations are an insensitive indicator as hypozincemia may occur when the dietary intake is very low. Lowered plasma zinc or urine zinc have been documented in patients with gastrointestinal disorders, Crohn's disease, regional enteritis, and deficient dietary intake.[20,21] Hence, plasma zinc alone cannot be an indicator of possible malignancy as there could be a zinc deficient status due to diet.
It has been observed that zinc deficiencies in patients with neoplastic diseases and inflammatory diseases have been attributed to loss of zinc from catabolic tissue and increased urinary excretion of zinc subsequent to its mobilization from interleukins.[20]
Although an increase in renal zinc excretion in cancer patients is well documented,[22] the mechanisms involved are still disputed. While urine-zinc concentrations can be affected in various diseases, the measurement of zinc in a sample of urine collected during 24 h can be helpful for the diagnosis of zinc deficiency in healthy individuals. However, there is no data so far regarding zinc in basal urine (random urine sample) expressed as Zn/creatinine ratio. Our results showed the Zn/creatinine ratio in basal urine was an important indicator of the increased excretion of zinc in prostate cancer patients as compared to normal healthy males.
Patients with cancer can excrete as much as three times more zinc in their urine than normal persons.[22] Increased urinary zinc excretion in cancer patients may be linked to immune activation and renal-tubular-cell dysfunction and skeletal-muscle catabolism.[23]
We have found a 2.1 times increase in urinary zinc excretion in prostate cancer patients as compared to normal healthy controls in this study. It was observed in our study that the mean urine zinc/creatinine ratio shows a significant increase (53%) in prostate carcinoma group as compared to the normal control group, whereas a 44% increase in urine zinc/creatinine ratio of prostatic carcinoma as compared to the BPH group.
As discussed, studies reported earlier have measured either tissue zinc or plasma zinc in prostate carcinoma or BPH. The present study, on the other hand has measured all the three parameters related to zinc levels in pathological conditions of the prostate and compared with controls. To our knowledge, this is the first study showing the association between the levels of tissue zinc, plasma zinc and urine zinc in BPH, and carcinoma. This relationship may suggest that the lost ability of the malignant cells to accumulate zinc is an important factor in the development of prostate malignancy. The presence of the diseased status, i.e., prostate carcinoma or BPH, which alters plasma-zinc level may be responsible for the observed decrease in prostate tissue zinc levels.
The data presented in this study, therefore, suggest that pathological conditions of the prostate gland in patients with BPH or carcinoma may be associated with an alteration in biochemical parameters such as reduction in the level of tissue zinc, plasma zinc, and an increase in urinary zinc excretion.
It is well documented that tumor cells undergo metabolic transformations that are essential for their malignant existence but are not the cause of malignancy. The specialized function of the normal prostate glandular epithelium to produce and secrete enormously high levels of citrate requires unique intermediary metabolic activities that are not generally associated with other normal mammalian cells. The accumulation of zinc in normal prostate glandular epithelial cells results in two important effects, a metabolic effect, and a proliferative effect. Its metabolic effect is the inhibition of citrate oxidation, which is necessary for prostate function. A second effect of zinc is its inhibition on prostate-cell proliferation.[24]
In malignancy, the normal zinc-accumulating citrate-producing epithelial cells are metabolically transformed to citrate-oxidizing cells that lose the ability to accumulate zinc. A genetic alteration in the expression of ZIP1 zinc transporter is associated with this metabolic transformation. These genetic/metabolic relationships have important consequences on citrate-related metabolism, bioenergetics, cell proliferation, and invasive capabilities of the malignant cells, which result in tumor-suppression characteristics. Zinc is critical to these relationships.[11]
There appears to be an association between plasma zinc, tissue zinc, and urine zinc in prostatic diseases in our study. This pattern of change in zinc concentrations (i.e., low plasma zinc and tissue zinc in combination with high urine zinc) is typical of patients with zinc deficiency due to disease and suggests that a chronic zinc deficiency status may be present in some of these patients as the disease progresses.[25]
Further studies will help clarify whether, along with clinical diagnosis, the zinc concentration in prostate biopsy specimens, plasma zinc and urine zinc/creatinine ratio of patients presenting with symptoms of prostatic diseases, are useful in the differential diagnosis of diagnosed prostatic carcinoma and BPH.
It also appears that the development of malignancy in prostate cancer may involve an essential metabolic transformation that results in the lost capability of malignant cells to accumulate zinc. Consideration of the clinical and biochemical evidence presented here leads to the conclusion that an altered zinc metabolism may play an important role in the pathogenesis of prostate malignancy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bracarda S, Ottaviao De Cobelli, Greco, Galleti C, Valdagni T, Gatta R, et al. Cancer of the Prostate, Critical Reviews In Oncology /Hematology. 2005;56:379–96. doi: 10.1016/j.critrevonc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Platz EA, Yeole BB, Cho E, Jussawalla DJ, Giovannuci E, Ascherio A. 1997 Vasectomy and Prostate Cancer. Int J Epidemiol. 1997;26:933–8. doi: 10.1093/ije/26.5.933. [DOI] [PubMed] [Google Scholar]
- 3.Mc Connell JD, Barry MJ, Bruskewitz RC. Benign Prostatic Hyperplasia: Diagnosis and Treatment.Agency for health care policy and research. Clin. 1994;8:1–17. [PubMed] [Google Scholar]
- 4.Kobayashi M. Studies on trace elements in cancerous stomach tissue of the patients with stomach cancer. Hokkaido Igaku Zasshi. 1990;65:320–35. [PubMed] [Google Scholar]
- 5.Platz EA, Helzlsouer KJ. Selenium, Zinc and Prostate Cancer. Epidemiol Rev. 2001;23:93–101. doi: 10.1093/oxfordjournals.epirev.a000801. [DOI] [PubMed] [Google Scholar]
- 6.Gómez Y, Arocha F, Espinoza F, Fernández D, Vásquez A, Granadillo V. Zinc levels in prostatic fluid of patients with prostate pathologies. Invest Clin. 2007;48:287–94. [PubMed] [Google Scholar]
- 7.Costella LC, Franklin RB. Novel role of zinc in the regulation of prostate.Citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–96. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci. 2005;10:2230–9. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Kirschke CP, Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int. 2006;31:6–10. doi: 10.1186/1475-2867-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, et al. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;15:5–17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahnke MJ. Atomic Absorption Spectrophotometry applied to determination of zinc in formalinised human tissue. At Absorption News. 1966;5:7. [Google Scholar]
- 13.Rosner F, Garfien PC. Erythrocytes and plasma zinc and magnesium levels in health and disease. J Lab Clin Med. 1968;72:213. [PubMed] [Google Scholar]
- 14.Seation B, Ali A. Simplified manual high performance clinical chemistry methods for developing countries. Med lab SCI. 1984;41:327–36. [PubMed] [Google Scholar]
- 15.Li XM, Zhang L, Li J, Li Y, Wang HL, Ji GY, et al. Measurement of serum zinc improves prostate cancer detection efficiency in patients with PSA levels between 4 ng/mL and 10 ng/m. Asian J Androl. 2005;7:323–8. doi: 10.1111/j.1745-7262.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- 16.Malm J, Helman J, Hogg P, Lilja H. Enzymatic Action of prostate specific antigen PSA or Hk3 : substrate specificity and regulation by Zn, a tight binding inhibitor. Prostate. 2000;45:132–9. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Gómez Y, Arocha F, Espinoza F, Fernández D, Vásquez A, Granadillo V. Zinc levels in prostatic fluid of patients with prostate pathologies. Invest Clin. 2007;48:287–94. [PubMed] [Google Scholar]
- 18.Zaichick VY, Sriirdoya TV, Zaichick SV. Zinc in human prostate gland: normal, hyperplastic and cancerous. Int Urol Nepfhrol. 1997;29:565–74. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 19.Vallee BL, Wacker WE, Bartholomay AF, Hoch FL. zinc metabolism in hepatic dysfunctioni, serum zinc concentration in laennec's cirrhosis and their validation by sequential analysis. New Engl J Med. 1957;257:1055. doi: 10.1056/NEJM195711282572201. [DOI] [PubMed] [Google Scholar]
- 20.Prasad AS. Clinical manifestations of zinc deficiency. Ann Rev Nutr. 1985;4:591–8. doi: 10.1146/annurev.nu.05.070185.002013. [DOI] [PubMed] [Google Scholar]
- 21.Prasad A S, Miale A, Farid H H. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism. J Lab Chin Med. 1963;64:537. [PubMed] [Google Scholar]
- 22.Schwartz Norton K. Role of trace elements in cancer. Clinical Res. 1975;35:3847–941. [PubMed] [Google Scholar]
- 23.Melichar B, Malí F, Jandík P, Malíova E, Vavrova J, Mergancova JK, et al. Increased urinary zinc excretion in cancer patients is linked to immune activation and renal tubular cell dysfunction. BioMetals. 1995;8:205–8. doi: 10.1007/BF00143377. [DOI] [PubMed] [Google Scholar]
- 24.Costello LC, Feng P, Franklin RB. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005;5:143–53. doi: 10.1016/j.mito.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melichar B, Jandik P, Tichy M, Malir F, Mergancova J, Voboril Z. Urinary zinc excretion and acute phase response in cancer patients. Clin Investig. 1994;72:1012–4. doi: 10.1007/BF00577746. [DOI] [PubMed] [Google Scholar]


