Abstract
The current clinical guidelines for the management of infertility as presented by the American Urologic Association and European Association of Urology represent consensus opinions for the management of male-factor infertility. The goal of the present study is to define the currently available guidelines for male-factor infertility, provide a rationale for why guidelines should be implemented, and review concerns and shortcomings towards their incorporation into clinical practice. Successfully integrating guidelines into clinical practice offers the potential benefit of creating a standardized, efficient, and cost-effective algorithm for the evaluation of infertility and facilitates future research. Despite their availability and ease of use, many clinicians fail to adopt clinical guidelines for numerous reasons including decreased awareness of available guidelines, insufficient time, lack of interest, and personal financial considerations. The current guidelines are limited by the inability to generalize recommendations to a heterogeneous patient sample, the lack of interdisciplinary adoption of guidelines, and the need for additional emphasis on prevention and lifestyle modifications. Future direction for the current guidelines will likely incorporate a multidisciplinary approach with increasing utilization of genetic analysis and novel treatment strategies. As the field of infertility continues to expand, the utility of guidelines combined with physician clinical judgment will remain prominent in the treatment of male-factor infertility.
Keywords: Consensus, semen analysis, standard
INTRODUCTION
Infertility is defined by the World Health Organization as the “inability of a sexually active, non-contracepting couple to achieve pregnancy in one year.”[1] It is estimated that infertility affects one in seven to one in eight couples of reproductive age, with a male factor being solely responsible in 20% and contributory in an additional 30% of cases.[1–4]
Patients affected by infertility frequently initially present to a variety of medical and surgical specialties including urology, gynecology, primary care, and reproductive endocrinology. Infertility is frequently encountered among practicing US urologists, with 88% having reported treating infertile males regularly in a 2003 survey.[5] Given the range of specialties involved and the frequency of presentation, various guideline bodies have created consensus opinions to better assist practicing physicians in the initial management of infertility.
Consensus statements function both as an aid to the practicing physician as well as to create a standard by which future research may be conducted. Guidelines statements, however, are not intended to be used as a ‘legal standard’ against which physicians should be measured but rather serve to provide a framework of standardized care while maintaining clinical autonomy and physician judgment.
Although no universally accepted consensus exists between specialties on the management of infertility, several algorithms have been devised to provide an initial assessment of the infertile male. These algorithms offer the possibility of creating a standardized and efficient model by which patients may be treated and allow for direct comparisons with future research. However, despite the presence of guidelines, many practitioners elect to evaluate and treat patients outside of the current recommendations for various reasons.
The goal of the present study is to determine the need for guidelines in the management of the infertile male and to discuss the advantages and barriers which influence their incorporation into practice. To accomplish this objective, the currently available guidelines will be critically reviewed, after which the rationale for and arguments against implementation of guidelines will be discussed. The shortcomings of the current guidelines will subsequently be presented with a discussion on guideline implementation in general. Finally, the possibilities for the future role of guidelines will be reviewed.
MATERIALS AND METHODS
The American Urologic Association (AUA) Guidelines (“Report on Optimal Evaluation of the Infertile Male,” “Report on Evaluation of the Azoospermic Male,” “Report on Management of Obstructive Azoospermia,” and “Report on Varicocele and Infertility”) and the European Association of Urology (EAU) Guidelines (“Guidelines on Male Infertility”)[6–10] were reviewed and selected for analysis. Additional guideline statements were not selected for in-depth review due to review length limitations, unpublished status, or decreased applicability to urologic practice. A Pubmed search was conducted utilizing the terms “infertility, guidelines, guideline-based management, semen analysis, with search results analyzed for applicability to the topic of guideline-based management of male infertility.
CURRENT GUIDELINES
Among urologists, two commonly utilized guidelines for the initial management of male factor infertility include the AUA Guidelines and the EAU Guidelines.[6–10] These statements are selected as representative examples of the available guidelines, as several consensus opinions exist from multiple specialties and from other countries. Although neither guideline attempts to provide a comprehensive summary of the management of infertility, both statements provide an excellent review of current clinical practice recommendations and are directly applicable to clinical practice.
In creating the AUA guidelines, the Male Infertility Best Practice Policy Committee of the AUA and the Practice Committee of the American Society for Reproductive Medicine (ASRM) formed a collaboration to include nine urologists, one reproductive endocrinologist, one family physician, and one research andrologist. The intention of the panel was to ‘develop recommendations, based on expert opinion, for optimal clinical practices in the diagnosis and treatment of male infertility.’ One-hundred twenty-five physicians from various specialties reviewed the guidelines with recommendations submitted and approved as appropriate by the Board of Directors of the AUA and ASRM. The EAU guideline was created by a six-member writing panel and was funded by the non-profit EAU.
Both the AUA and EAU guidelines provide recommendations based on available literature, and in cases where sufficient data is lacking, utilize expert opinion. The EAU guideline further characterizes the levels of evidence on a scale of one to four with recommendations graded A, B, or C, as modified from Sackett and colleagues.[11] Figures 1–3 summarize management recommendations for the infertile male based on the AUA and EAU guidelines.
Figure 1.
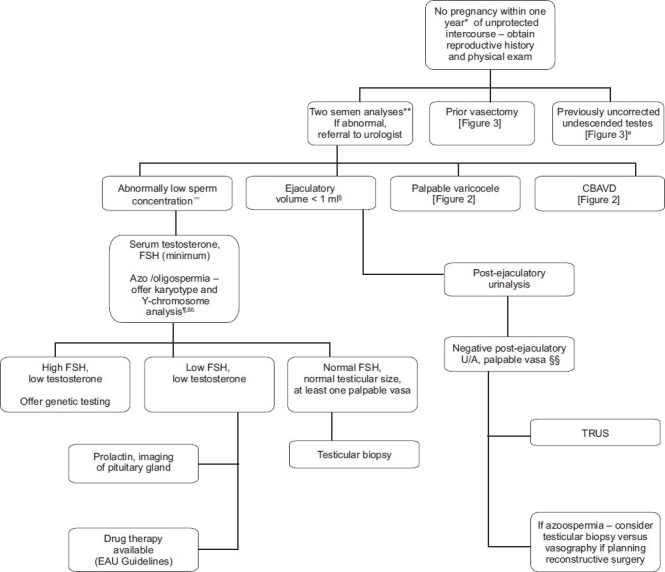
Summary algorithm for initial management of the infertile male as presented in the AUA and EAU guidelines * Earlier evaluation may be warranted if male or female infertility risk factors present or if the male questions his fertility potential. ** EAU guideline recommends second semen analysis if first noted to be abnormal § Except in patients with bilateral vasal agenesis or clinical signs of hypogonadism. §§ Some recommend TRUS for oligospermic patients with low volume ejaculates, palpable vasa and normal testicular size. Ø Ideally treated at less than 1 year of age. ∞ Including azoospermia, impaired sexual function, or clinical findings of possible endocrinopathy
Figure 3.
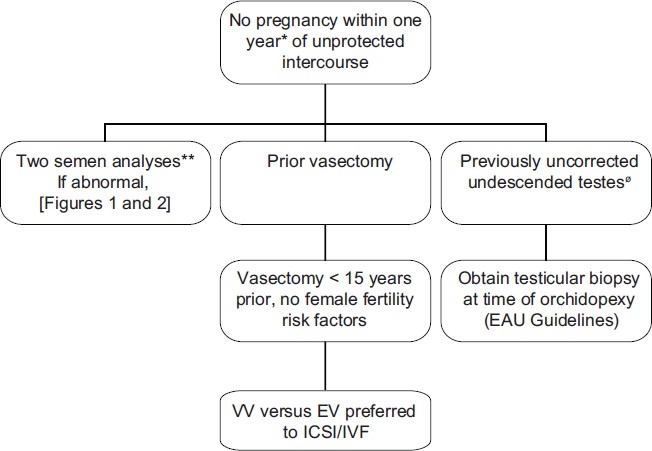
Summary of management guidelines for infertile males with a prior vasectomy or uncorrected undescended testes. *Earlier evaluation may be warranted if male or female infertility risk factors present or if the male questions his fertility potential. ** EAU guideline recommends second semen analysis if first noted to be abnormal. Sperm retrieval / ICSI is preferred to surgical treatment if (1) advanced female age is present, (2) female factors requiring IVF are present (3) the chance for success with sperm retrieval / ICSI exceeds the chance for success with surgical treatment or (4) sperm retrieval / ICSI is preferred by the couple for financial reasons. Ø Ideally treated at less than 1 year of age.
Figure 2.
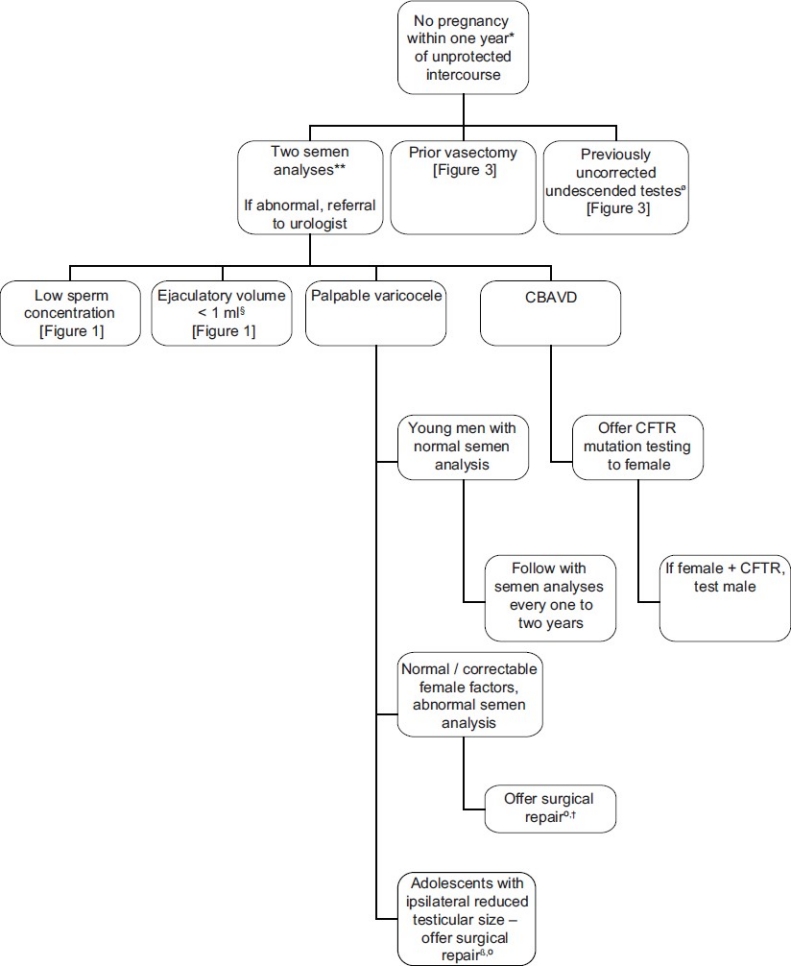
“Summary of management guidelines for infertile males presenting with a palpable varicocele or CBAVD. *Earlier evaluation may be warranted if male or female infertility risk factors present or if the male questions his fertility potential. **EAU guideline recommends second semen analysis if first noted to be abnormal ß If normal ipsilateral testicle size, offer follow-up monitoring with annual objective measurements of testicular size and / or semen analyses. ºFollow-up to include semen analysis at three-month intervals for one year or until pregnancy occurs. † EAU guidelines indicates that varicocele treatment should not be undertaken unless there has been a full discussion with the infertile couple regarding the uncertainties of treatment. Ø Ideally treated at less than 1 year of age.
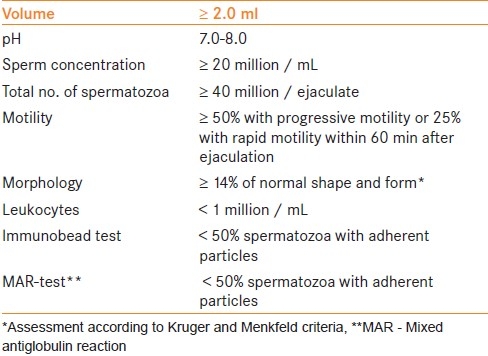
Each of the guidelines utilize the standardized reference values for human semen characteristics as outlined by the World Health Organization [Table 1].[12] The standard values for semen analysis were determined through analyzing 4500 men from 14 countries whose partners had a time-to-pregnancy of ≤ 12 months. Values falling beneath the 5th percentile were reported as the lower limit of normal.
Table 1.
World Health Organization reference values for human semen characteristics
Both the AUA and EAU guidelines offer similar recommendations for management with a few notable exceptions. The EAU panel recommends consideration for varicocele repair only following a full discussion as to the lack of evidence consistently demonstrating a benefit with surgical repair. Additionally, EAU guidelines recommend a single semen analysis during the initial evaluation of the infertile male with a follow-up confirmatory semen analysis if findings of the first test are found to be abnormal. EAU guidelines also incorporate recommendations for patients with a history of or current cryptorchidism, genitourinary infections, abnormalities of testicular ultrasound (microlithiasis), ejaculatory disorders, premature ejaculation, obtaining samples in spinal cord patients, and semen preservation prior to chemo/surgical therapy.
Additional male fertility guidelines are available and are not included in the current review due to space limitations. The Royal College of Obstetricians and Gynecologists′ guidelines, for example, provide additional recommendations with regards to lifestyle counseling as well as focusing on evaluating both partners simultaneously.[3,13]
RATIONALE FOR IMPLEMENTATION OF GUIDELINES
The use of guideline-based models for the management of male infertility arises from implementation of evidence-based medicine principles with a goal of identifying the best external evidence by which standard treatment algorithms may be devised.[11] The “best” available evidence frequently does not include randomized-controlled trials in the case of infertility management and most often is based around prospective or retrospective reviews of available data. And as all aspects of the evaluation of male-factor infertility are not subject to literary review, the role for expert opinion and clinical experience is significant.
The available guidelines for management of male-factor infertility provide a framework algorithm upon which practicing physicians may base clinical decision-making. The purpose of these guidelines is not to be a rigid standard by which a physician must practice, but rather to select an appropriate, cost-effective, and risk-benefit approach to care.
Although it may be more efficient and complete for the treating clinician to obtain semen analyses, complete hormone profiles, cystoscopy, scrotal/transrectal ultrasound, and testicular biopsy at the time of initial consultation, such an approach would be inefficient, costly, and would subject patients to unnecessary and potentially harmful procedures. In attempting to identify the most efficacious tests and interventions, frequently, guidelines result in improved cost and time savings for both patients and practitioners.
For example, previous reports have estimated that 20-25% of provided healthcare is unnecessary in the United States, and up to 40% of patients do not receive care based on available evidence.[14–17] Although these findings do not evaluate the management of infertility specifically, they demonstrate significant opportunities for quality improvement in delivered care.
With regards to guideline implementation, Knuppel and colleagues noted that the introduction of a case management program with female-factor infertility resulted in decreasing infertility costs by 30-40% and a decrease in suboptimal outcomes.[18] Such a program utilizes a standardized approach to initial patient evaluation and bases subsequent evaluations on accepted guidelines for management. Deviations from the routine care were performed as felt to be appropriate by the treating physician and resulted in a tailoring of the initial treatment algorithm to the patients’ needs.
Given that few insurance companies cover infertility treatments, cost savings benefits the patients directly. And although cost savings are not the primary goal of guidelines, frequently they are a secondary benefit which increases efficiency and maximizes the overall quality of patient care delivered.
In addition to the above factors, guideline-based treatment strategies create a standardization by which future research may be conducted. The creation of measurable, standardized variables in urology such as the AUA symptom score, international index of erection function (IIEF), and World Health Organization (WHO) semen value references permits facilitated comparisons among studies despite varied patient populations. Similarly, a standardized approach to the initial evaluation and management of a patient with infertility allows for improved epidemiological reporting, comparison of outcomes (particularly in cases of rare findings), and further refinement of treatment algorithms. Such an approach of standardized management permits advances in research analogous to the growth of industrialization resulting from the adoption of assembly lines and interchangeable parts.
One further unintended benefit of the implementation of guidelines is the decreased liability associated with following accepted practice standards. Guidelines may therefore function as a safety net standard of care. It is, however, important to note that deviation from the established guidelines due to individualized patient needs does not constitute a deviation from the standard of care. The purpose of the current guidelines is not to develop criteria by which physicians are to be measured, but rather to provide a framework upon which astute physicians may base evaluation and treatment strategies.
BARRIERS TOWARDS IMPLEMENTING GUIDELINES
Despite availability and ease of use, many providers fail to follow guidelines for various reasons. One possible factor impeding adoption of the current guidelines is patient or physician expectations of results at the time of initial consultation. This may result in ordering a "battery" of studies prior to the initial consultation including blood and urine tests, semen analyses, and imaging. Although this may decrease the number of patient visits, it ultimately results in performing unnecessary and occasionally costly or invasive exams.
A second factor which may impede guideline implementation is the lack of awareness of available recommendations. With an overabundance of information and limited resources of time, some providers may simply lack the time to review available guidelines. Other providers may prefer to approach clinical scenarios from a bottom-up approach with testing performed according to clinical suspicion alone.
A third barrier which may impact guideline integration is financial considerations. In attempting to achieve a cost-effective treatment algorithm, the current guidelines lead to multiple patient follow-ups for review of each new finding and further recommendations. This may result in decreased time available for other patients and decreased office visit reimbursements. In contrast, while the yield for performing a transrectal ultrasound and cystoscopy to assess for obstructive causes of infertility may be low, it results in a significantly higher reimbursement with minimal investment of time when compared to approaching a patient as indicated in the current guidelines.
Whatever the underlying motives, widespread adoption of guideline-based practices continues to face multiple barriers. Mourad and colleagues reported on variations in the management of 2698 infertile couples in the Netherlands.[19] Findings demonstrated that 30-40% of patients did not receive care based on the available scientific evidence. Among 39 guideline-based performance indicators selected, 14 scored < 50% adherence, with the initial assessment of infertility revealing the lowest rate of adherence.
LIMITATIONS WITH THE CURRENT GUIDELINES
In addition to physician factors which may impede implementation of established recommendations, the infertility guidelines themselves have several limitations which prevent them from becoming universally applicable.
Indeed, the goal of the current AUA and EAU guidelines for infertility is not to provide a complete reference for all aspects of infertility, but rather to provide a baseline template. Some topics which are not discussed or minimally reviewed include guideline applicability to alternative populations (age, prior testicular surgery, concomitant cancer diagnoses), integration of guidelines with existing statements (gynecology and family practice), discussion of prevention of infertility, and lifestyle counseling and ethics considerations.
As men presenting with infertility are a heterogeneous group with varied etiologies for their underlying infertility, treating physicians frequently will deviate from guideline-based care as it is felt to not be applicable to many patient clinical scenarios. For example, an adolescent with bilateral Grade three varicoceles and borderline abnormal semen analysis should likely be managed differently than a 40-year-old male with an abnormal semen analysis, unilateral Grade one varicocele, and history of cryptorchidism. The current guidelines fail to stratify men with regards to prior medical and surgical history, age, lifestyle, current symptomatology, as well as various genetic, social, and family risk factors. Each of these variables should be taken into account when deciding an appropriate evaluation/treatment regimen.
In addition to the above factors, there are numerous challenges to creating a guideline which is universally applicable to the broad range of specialties which evaluate and treat patients with infertility. Possibly due to a combination of physician and cultural biases, it is not uncommon for an initial infertility evaluation to be performed by family practice and gynecologic surgeons with referral to urology upon completion of a negative female evaluation/abnormal male screening evaluation. As such, the current gynecologic guidelines include algorithms for both male and female evaluations. The urology recommendations, in contrast, are focused on male factors contributing to infertility. An ideal set of guidelines would integrate and be accepted by all specialties treating infertility and therefore result in referrals to other specialties in a timely and appropriate manner, particularly given the rapid nature of advancements in the field of infertility.
One of the most important aspects of infertility which is either not or minimally addressed in the current guidelines is that of prevention of or planning for infertility. Numerous factors have been associated with increased risk of infertility including but not limited to genetic anomalies, reproductive diseases, and cancer diagnoses requiring chemotherapy, radiation or surgery.[20] Iatrogenically induced infertility is one area where appropriate pretreatment counseling is essential. Prostate and testicular cancers, in particular, often lead to treatments which may at a minimum impact future fertility and potentially render the patient completely infertile.
Similarly, with regard to pretreatment counseling, one recent study reported that fewer than one-half of US physicians refer patients of childbearing age to infertility specialists prior to receiving chemotherapy despite established recommendations for referral.[21] Further, less than one-half of patients recall having a discussion with their oncologist regarding fertility preservation prior to treatment. This study highlights the significant ongoing need for improvement in physician counseling prior to receiving gonadotoxic therapy.
Other factors which are not addressed in the current guidelines and which are essential to prevention of infertility include discussion of the appropriate age for orchidopexy, emergent management of testicular torsion, effect of chronic orchitis as well as a broader discussion of reducing exposure to occupational/environmental gonadotoxins.[22–26]
One final consideration which receives limited discussion in the current guidelines is the need for counseling on modifiable lifestyle factors, ethical dilemmas, and genetic concerns. Numerous studies have described the relationship between tobacco/alcohol and infertility.[27,28] These studies are particularly applicable given their prevalence and cumulative exposures in the reproductive-age population.[29] In addition to known ingested and inhaled gonadotoxins, other lifestyle factors such as diabetes and obesity are associated with changes in sperm quality.[30] To date, the effect of modifying patient factors (diabetes, weight loss, and discontinuing use of alcohol and tobacco) on fertility potential has not been elucidated. However, it is likely that modification of one or several of these factors would result in improved overall fertility.
Patient counseling further encompasses discussions regarding several ethical and genetic issues including the goals of treatment (number of desired children), welfare of the future child, and individual patient characteristics (maternal/paternal age). Further, as patients with male-factor infertility are at an increased risk of having chromosomal abnormalities, patients should be counseled both to the risks of transference to offspring as well as increased rates of congenital abnormalities in children born through selected assisted reproductive technologies.[31] Given the ethical nature of these discussions, these topics are inherently limited in guideline recommendations and should rather be reviewed with individual ethics committees as appropriate.[32,33]
CONCERNS WITH GUIDELINE-BASED MANAGEMENT
In addition to the inherent limitations of guidelines in creating generalized treatment strategies, several concerns exist regarding guideline implementation.[11] One common misconception with guideline creation is development of "cook-book" medicine with predefined steps and algorithms dictating management decisions. However, as the current guidelines are created from a bottom-up approach to incorporate available data with clinical experience, physician experience will dictate when a particular patient's condition is applicable to the currently available evidence. In this manner, the physician retains the clinical decision-making role and autonomy to assess the how the patient should be treated within the current guideline framework.
One additional concern is that the current guidelines will be utilized by policy-makers to reduce the costs of healthcare delivery. However, as the guidelines are formulated on evidence-based outcomes, the primary objective of established guidelines becomes to maximize the quality of the treatment, without specific consideration to cost.
FUTURE DIRECTION AND ROLE FOR INFERTILITY GUIDELINES
As the rate of growth of medical knowledge continues to increase, the role for and utility of expert guidelines will become increasingly important. Future guidelines will likely reflect novel treatment modalities and will continue to provide a basic framework upon which physicians may base clinical judgments. Several areas for future direction include increasing cooperation between specialties, further integrating genetic analysis into treatment algorithms, addition of novel treatments, and increasing focus on patient counseling.
Given the numerous specialties involved in the management of infertility there is a need to integrate consensus guidelines between areas of expertise. This would provide for a unified approach in which every aspect of infertility is addressed by the appropriate specialties. Ideally, such an approach would include pre-surgical/pre-chemotherapy counseling for patients undergoing potentially fertility-altering treatments. This would further permit a timely, efficient, and appropriate workup of the infertile couple as well as provide appropriate prophylactic counseling.
The role for genetic testing with regards to male-factor infertility is currently limited to one of providing appropriate counseling and recommendations and is related to detecting germ-line chromosomal abnormalities.[34] Future genetic analysis will likely include direct evaluation of sperm DNA integrity to determine damage sustained and risks associated with fertilization.[1,35–37] Appropriate selection of sperm for assisted reproductive techniques would be an expected benefit as well as a more accurate determination as to underlying direct causes for infertility (environmental exposures, etc.). A more accurate understanding of a patient's infertility would potentially create a streamlined and less invasive approach to management. Additionally, the ability to directly evaluate sperm DNA for damage allows for interval assessments to determine the efficacy of therapeutic interventions.
Future guidelines will continue to incorporate new discoveries for treatment in the management of infertility. Numerous investigations are ongoing into determining predictive associations between semen findings and overall sperm quality beyond the currently accepted WHO semen characteristics. The role of macrophage activity, oxidative stress, DNA fragmentation, and additional factors in semen as well as their predictive value and implications of treatment will be further elucidated with new potential avenues for treatment created.[38–40] Similarly, the role of nitric oxide in spermatogenesis and the potential therapeutic benefit of phosphodiesterase inhibitors is currently being investigated for a possible therapeutic role.[41,42]
Among the future potential additions to the current infertility guidelines, integrating patient counseling into current practice does not require any novel discoveries and would likely result in immediate improvements in sperm quality. Future guidelines will likely emphasize the importance of modifying patient behaviors and characteristics such as controlling obesity, diabetes and discontinuing smoking/alcohol intake. Further investigations will likely continue to enforce the association between patient variables and semen characteristics.
SUMMARY
The current clinical guidelines provide a framework upon which the practicing physician may approach male patients presenting with infertility. Implementation of guidelines offers advantages of standardization of care, improved efficiency, enhanced research opportunities, and creation of a cost-effective treatment algorithm. Although many physicians fail to adopt published guidelines for various reasons, the role for guideline-based management combined with physician judgment will likely continue to represent the standard of care.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Smit M, Romijn JC, Wildhagen MF, Weber RF, Dohle GR. Sperm chromatin structure is associated with the quality of spermatogenesis in infertile patients. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 2.De Kretser DM. Male infertility. Lancet. 1997;349:787–90. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 3.Royal College of Obstetricians and Gynaecologists Evidence-based Clinical Guidelines. Guideline Summary No. 2: The initial investigation and management of the infertile couple. BJU Int. 1999;83:636–40. [PubMed] [Google Scholar]
- 4.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 5.O’leary MP, Baum NH, Bohnert WW, Blizzard R, Bonney WW, Cooper TP, et al. 2003 American Urological Association Gallup survey: Physician practice patterns, cryosurgery/brachytherapy, male infertility, female urology and insurance/professional liability. J Urol. 2004;171:2363–5. doi: 10.1097/01.ju.0000127745.26501.5e. [DOI] [PubMed] [Google Scholar]
- 6.Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86:S202–9. doi: 10.1016/j.fertnstert.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Report on evaluation of the azoospermic male. Fertil Steril. 2006;86:S210–5. doi: 10.1016/j.fertnstert.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Report on varicocele and infertility. Fertil Steril. 2008;90:S247–9. doi: 10.1016/j.fertnstert.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Report on management of obstructive azoospermia. Fertil Steril. 2006;86:S259–63. doi: 10.1016/j.fertnstert.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, Weidner W. EAU Working Group on Male Infertility. EAU guidelines on male infertility. Eur Urol. 2005;48:703–11. doi: 10.1016/j.eururo.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21:3–5. doi: 10.1016/s0146-0005(97)80013-4. [DOI] [PubMed] [Google Scholar]
- 12.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 13.Royal College of Obstetricians and Gynaecologists Evidence-based Clinical Guidelines. Guideline Summary No. 3: the management of infertility in secondary care. BJU Int. 1999;83:641–5. [PubMed] [Google Scholar]
- 14.Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? Milbank Q. 1998;76:517–63,509. doi: 10.1111/1468-0009.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:246–54. doi: 10.1097/00005650-200108002-00003. [DOI] [PubMed] [Google Scholar]
- 16.McGlynn EA. Quality assessment of reproductive health services. West J Med. 1995;163:19–27. [PubMed] [Google Scholar]
- 17.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 18.Knuppel RA, Knuppel RE, Campbell DD, Marcus L. Infertility case management: Improving both short-term and long-term outcomes while reducing costs. Prof Case Manag. 2007;12:232–8. doi: 10.1097/01.PCAMA.0000282910.43908.a6. [DOI] [PubMed] [Google Scholar]
- 19.Mourad SM, Nelen WL, Hermens RP, Bancsi LF, Braat DD, Zielhuis GA, et al. Variation in subfertility care measured by guideline-based performance indicators. Hum Reprod. 2008;23:2493–500. doi: 10.1093/humrep/den281. [DOI] [PubMed] [Google Scholar]
- 20.O’Flynn O’Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: A review. Fertil Steril. 2010;93:1–12. doi: 10.1016/j.fertnstert.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 21.Quinn GP, Vadaparampil ST, Lee JH, Jacobsen PB, Bepler G, Lancaster J, et al. Physician referral for fertility preservation in oncology patients: A national study of practice behaviors. J Clin Oncol. 2009;27:5952–7. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
- 22.Kollin C, Hesser U, Ritzén EM, Karpe B. Testicular growth from birth to two years of age, and the effect of orchidopexy at age nine months: A randomized, controlled study. Acta Paediatr. 2006;95:318–24. doi: 10.1080/08035250500423812. [DOI] [PubMed] [Google Scholar]
- 23.Kollin C, Karpe B, Hesser U, Granholm T, Ritzén EM. Surgical treatment of unilaterally undescended testes: Testicular growth after randomization to orchiopexy at age 9 months or 3 years. J Urol. 2007;178:1589–93. doi: 10.1016/j.juro.2007.03.173. [DOI] [PubMed] [Google Scholar]
- 24.Lamar CA, DeCherney AH. Fertility preservation: State of the science and future research directions. Fertil Steril. 2009;91:316–9. doi: 10.1016/j.fertnstert.2008.08.133. [DOI] [PubMed] [Google Scholar]
- 25.Bonde JP. Male reproductive organs are at risk from environmental hazards. Asian J Androl. 2010;12:152–6. doi: 10.1038/aja.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodruff DY, Horwitz G, Weigel J, Nangia AK. Fertility preservation following torsion and severe ischemic injury of a solitary testis. Fertil Steril. 2010;94:352:e4–5. doi: 10.1016/j.fertnstert.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 27.Gaur DS, Talekar MS, Pathak VP. Alcohol intake and cigarette smoking: Impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010;53:35–40. doi: 10.4103/0377-4929.59180. [DOI] [PubMed] [Google Scholar]
- 28.Calogero A, Polosa R, Perdichizzi A, Guarino F, La Vignera S, Scarfia A, et al. Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod Biomed Online. 2009;19:564–71. doi: 10.1016/j.rbmo.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Dorfman SF. Tobacco and fertility: Our responsibilities. Fertil Steril. 2008;89:502–4. doi: 10.1016/j.fertnstert.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Kriegel TM, Heidenreich F, Kettner K, Pursche T, Hoflack B, Grunewald S, et al. Identification of diabetes- and obesity-associated proteomic changes in human spermatozoa by difference gel electrophoresis. Reprod Biomed Online. 2009;19:660–70. doi: 10.1016/j.rbmo.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Hindryckx A, Peeraer K, Debrock S, Legius E, de Zegher F, Francois I, et al. Has the Prevalence of Congenital Abnormalities after Intracytoplasmic Sperm Injection Increased.The Leuven Data 1994-2000 and a Review of the Literature? Gynecol Obstet Invest. 2010;70:11–22. doi: 10.1159/000279323. [DOI] [PubMed] [Google Scholar]
- 32.Milliez J, Milliez J. Fertility centers and who they should treat: FIGO committee for the ethical aspects of human reproduction and women's health. Int J Gynaecol Obstet. 2009;107:166. [Google Scholar]
- 33.Frith L. The use of clinical ethics committees in infertility clinics. Hum Fertil (Camb) 2009;12:89–94. doi: 10.1080/14647270902787745. [DOI] [PubMed] [Google Scholar]
- 34.McLachlan RI, O’Bryan MK. State of the Art for Genetic Testing of Infertile Men. J Clin Endocrinol Metab. 2010;95:1013–24. doi: 10.1210/jc.2009-1925. [DOI] [PubMed] [Google Scholar]
- 35.Barratt CL, Aitken RJ, Björndahl L, Carrell DT, de Boer P, Kvist U, et al. Sperm DNA: Organization, protection and vulnerability: From basic science to clinical applications--a position report. Hum Reprod. 2010;25:824–38. doi: 10.1093/humrep/dep465. [DOI] [PubMed] [Google Scholar]
- 36.Berlinguer F, Madeddu M, Pasciu V, Succu S, Spezzigu A, Satta V, et al. Semen molecular and cellular features: These parameters can reliably predict subsequent ART outcome in a goat model. Reprod Biol Endocrinol. 2009;7:125. doi: 10.1186/1477-7827-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poplinski A, Tüttelmann F, Kanber D, Horsthemke B, Gromoll J. Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int J Androl. 2010;33:642–9. doi: 10.1111/j.1365-2605.2009.01000.x. [DOI] [PubMed] [Google Scholar]
- 38.Tremellen K, Tunc O. Macrophage activity in semen is significantly correlated with sperm quality in infertile men. Int J Androl. 2010;33:823–31. doi: 10.1111/j.1365-2605.2009.01037.x. [DOI] [PubMed] [Google Scholar]
- 39.Lanzafame FM, La Vignera S, Vicari E, Calogero AE. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 2009;19:638–59. doi: 10.1016/j.rbmo.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Mehdi M, Khantouche L, Ajina M, Saad A. Detection of DNA fragmentation in human spermatozoa: Correlation with semen parameters. Andrologia. 2009;41:383–6. doi: 10.1111/j.1439-0272.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee NP, Cheng CY. Nitric oxide and cyclic nucleotides: Their roles in junction dynamics and spermatogenesis. Adv Exp Med Biol. 2008;636:172–85. doi: 10.1007/978-0-387-09597-4_10. [DOI] [PubMed] [Google Scholar]
- 42.Dimitriadis F, Tsounapi P, Saito M, Watanabe T, Sylakos A, Tsabalas S. Is there a role for PDE5 inhibitors in the management of male infertility due to defects in testicular or epididymal function? Curr Pharm Des. 2009;15:3506–20. doi: 10.2174/138161209789207015. [DOI] [PubMed] [Google Scholar]


