Abstract
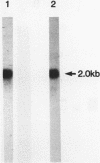
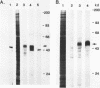
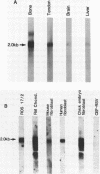
Overlapping cDNA clones encoding bovine osteonectin were isolated from a lambda gt11 expression library constructed from bovine bone cell mRNA. The longest clone, lambda On 17 (insert size 2.0 kb) was studied in detail. The clone was shown to encode osteonectin by hybrid select translation experiments and by DNA sequence analysis. Northern analysis of bone cell RNA showed the length of the osteonectin mRNA to be 2.0 kb. Osteonectin message was found in bone but not in soft tissue (liver and brain) preparations consistent with the distribution of the protein in these tissues. On the other hand, osteonectin message was observed in tendon, a tissue in which little or no osteonectin protein is found in vivo. Hybridization of osteonectin cDNA was detected in cells from a number of species including human, rat, mouse and chick. The level of osteonectin mRNA was drastically decreased in chick embryo fibroblasts transformed by Rous sarcoma virus.
Full text
PDF




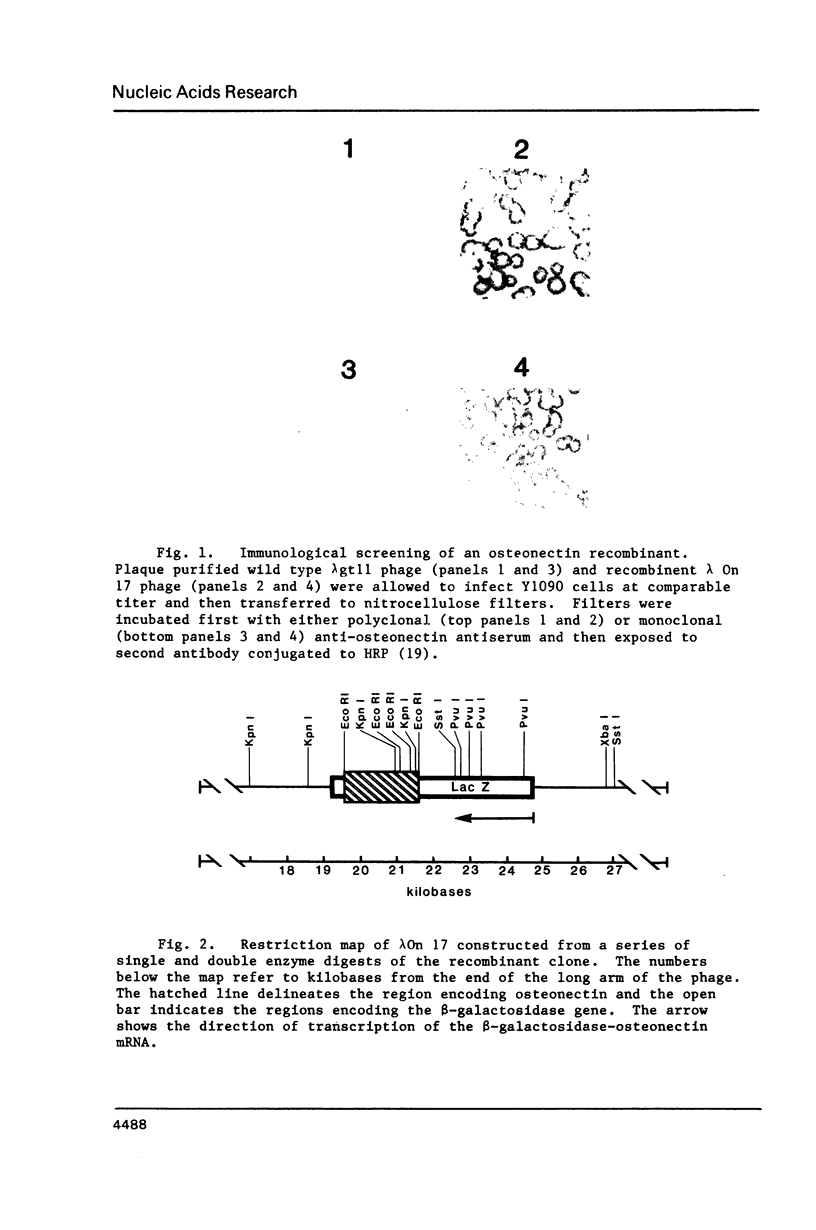

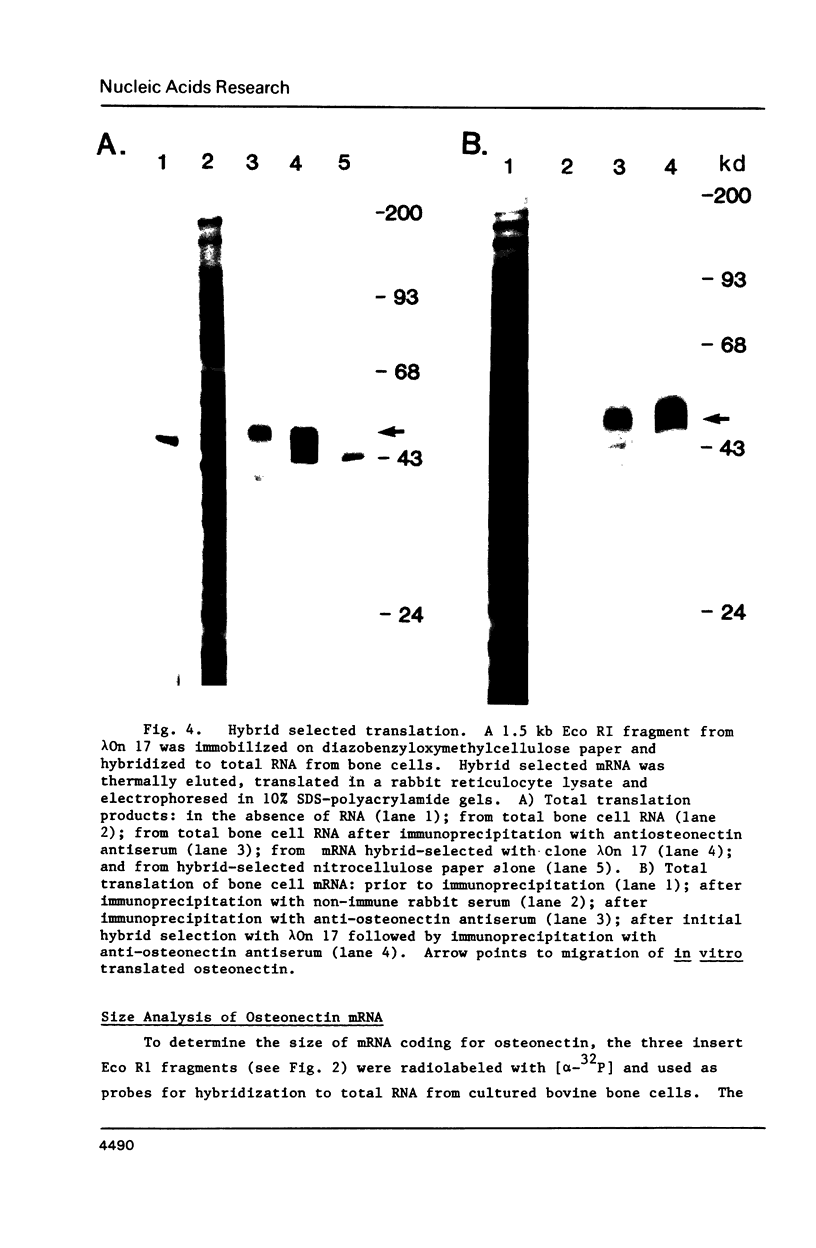

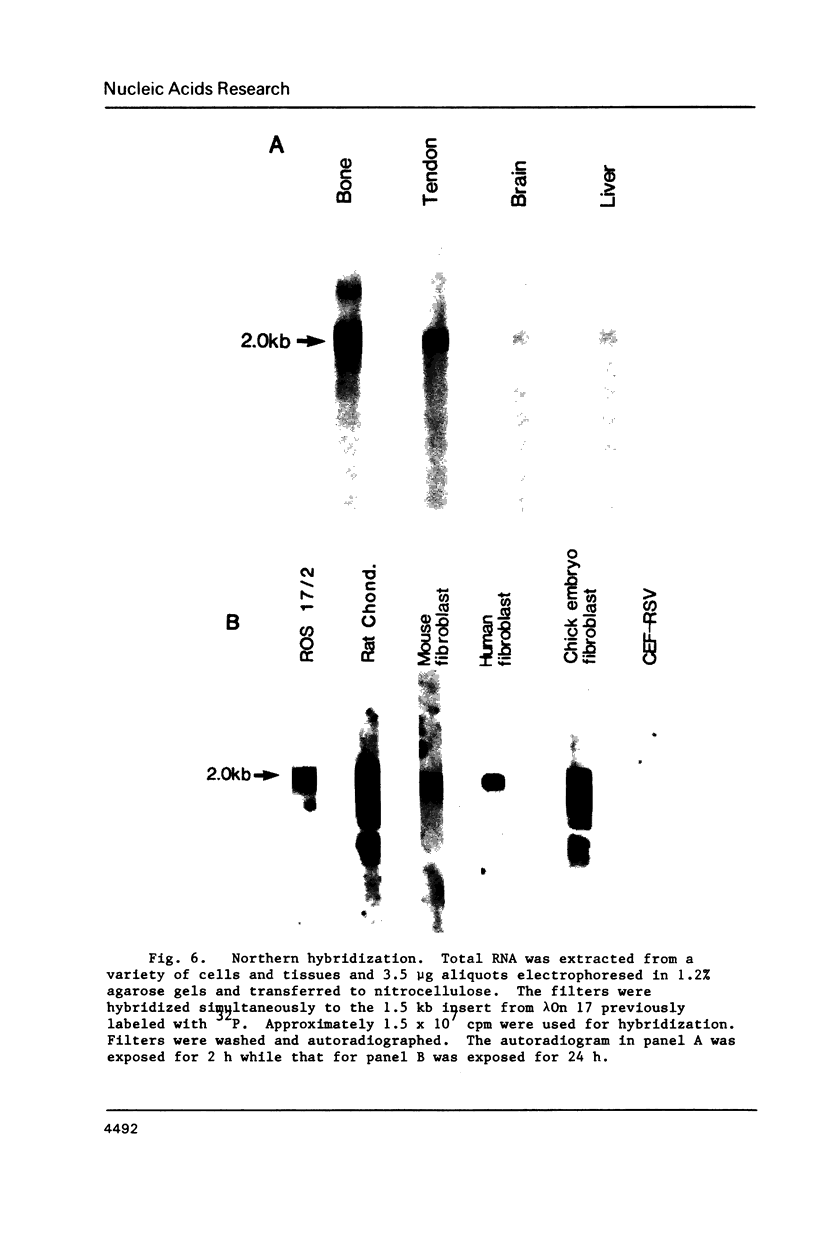





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Boettiger D., Focht R. J., Holtzer H., Pacifici M. Regulation of the synthesis of extracellular matrix components in chondroblasts transformed by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1982 Sep;30(2):373–384. doi: 10.1016/0092-8674(82)90235-5. [DOI] [PubMed] [Google Scholar]
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho S., Tate V., Boedtker H. Multiple 3' ends of the chicken pro alpha 2(I) collagen gene. Nucleic Acids Res. 1983 Aug 25;11(16):5443–5450. doi: 10.1093/nar/11.16.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Delclos K. B., Blumberg P. M. Decrease in collagen production in normal and Rous sarcoma virus-transformed chick embryo fibroblasts induced by phorbol myristate acetate. Cancer Res. 1979 May;39(5):1667–1672. [PubMed] [Google Scholar]
- Fagan J. B., Sobel M. E., Yamada K. M., de Crombrugghe B., Pastan I. Effects of transformation on fibronectin gene expression using cloned fibronectin cDNA. J Biol Chem. 1981 Jan 10;256(1):520–525. [PubMed] [Google Scholar]
- Kuwata F., Yao K. L., Sodek J., Ives S., Pulleyblank D. Identification of pre-osteonectin produced by cell-free translation of fetal porcine calvarial mRNA. J Biol Chem. 1985 Jun 10;260(11):6993–6998. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- Muto M., Yoshimura M., Okayama M., Kaji A. Cellular transformation and differentiation. Effect of Rous sarcoma virus transformation on sulfated proteoglycan synthesis by chicken chondrocytes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4173–4177. doi: 10.1073/pnas.74.10.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka K., Yao K. L., Wasi S., Tung P. S., Aubin J. E., Sodek J., Termine J. D. Biosynthesis of osteonectin by fetal porcine calvarial cells in vitro. J Biol Chem. 1984 Aug 10;259(15):9805–9812. [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Romberg R. W., Werness P. G., Lollar P., Riggs B. L., Mann K. G. Isolation and characterization of native adult osteonectin. J Biol Chem. 1985 Mar 10;260(5):2728–2736. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. D., Martin G. R., Miller E. J., Dorfman A., Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975 Jan;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- Sobel M. R., Yamamoto T., de Crombrugghe B., Pastan I. Regulation or procollagen messenger ribonucleic acid levels in Rous sarcoma virus transformed chick embryo fibroblasts. Biochemistry. 1981 Apr 28;20(9):2678–2684. doi: 10.1021/bi00512a049. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Conn K. M., Kleinman H. K. Mineral and collagen-binding proteins of fetal calf bone. J Biol Chem. 1981 Oct 25;256(20):10403–10408. [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Wasi S., Otsuka K., Yao K. L., Tung P. S., Aubin J. E., Sodek J., Termine J. D. An osteonectinlike protein in porcine periodontal ligament and its synthesis by periodontal ligament fibroblasts. Can J Biochem Cell Biol. 1984 Jun;62(6):470–478. doi: 10.1139/o84-064. [DOI] [PubMed] [Google Scholar]
- Young M. F., Vogeli G., Nunez A. M., Fernandez M. P., Sullivan M., Sobel M. E. Isolation of cDNA and genomic DNA clones encoding type II collagen. Nucleic Acids Res. 1984 May 25;12(10):4207–4228. doi: 10.1093/nar/12.10.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]