Abstract
BACKGROUND
Obesity exacerbates the age-related decline in physical function and causes frailty in older adults; however, the appropriate treatment for obese older adults is controversial.
METHODS
In this 1-year, randomized, controlled trial, we evaluated the independent and combined effects of weight loss and exercise in 107 adults who were 65 years of age or older and obese. Participants were randomly assigned to a control group, a weightmanagement (diet) group, an exercise group, or a weight-management-plus-exercise (diet–exercise) group. The primary outcome was the change in score on the modified Physical Performance Test. Secondary outcomes included other measures of frailty, body composition, bone mineral density, specific physical functions, and quality of life.
RESULTS
A total of 93 participants (87%) completed the study. In the intention-to-treat analysis, the score on the Physical Performance Test, in which higher scores indicate better physical status, increased more in the diet–exercise group than in the diet group or the exercise group (increases from baseline of 21% vs. 12% and 15%, respectively); the scores in all three of those groups increased more than the scores in the control group (in which the score increased by 1%) (P<0.001 for the between-group differences). Moreover, the peak oxygen consumption improved more in the diet–exercise group than in the diet group or the exercise group (increases of 17% vs. 10% and 8%, respectively; P<0.001); the score on the Functional Status Questionnaire, in which higher scores indicate better physical function, increased more in the diet–exercise group than in the diet group (increase of 10% vs. 4%, P<0.001). Body weight decreased by 10% in the diet group and by 9% in the diet–exercise group, but did not decrease in the exercise group or the control group (P<0.001). Lean body mass and bone mineral density at the hip decreased less in the diet–exercise group than in the diet group (reductions of 3% and 1%, respectively, in the diet–exercise group vs. reductions of 5% and 3%, respectively, in the diet group; P<0.05 for both comparisons). Strength, balance, and gait improved consistently in the diet–exercise group (P<0.05 for all comparisons). Adverse events included a small number of exercise-associated musculoskeletal injuries.
CONCLUSIONS
These findings suggest that a combination of weight loss and exercise provides greater improvement in physical function than either intervention alone.
Obesity in older adults is becoming a serious public health problem in the United States.1-4 The number of obese older adults is increasing markedly.5,6 Currently, approximately 20% of adults 65 years of age or older are obese, and the prevalence will continue to rise as more baby boomers become senior citizens.3,7 In older adults, obesity exacerbates the age-related decline in physical function, which causes frailty, impairs quality of life, and results in increases in nursing home admissions.8-12 Given the increasing prevalence of obesity, the most common phenotype of frailty in the future may be an obese, disabled, older adult.4,13
Although obesity is an important cause of disability in older adults,14,15 there is little evidence from clinical trials regarding the benefits and risks of weight-loss interventions to guide the care of this population.16,17 In fact, the clinical approach to obesity in older adults is controversial, given the reduction in relative health risks associated with increasing body-mass index (BMI) in this group.2 It has been suggested that it may be difficult to achieve successful weight loss in older adults because of lifelong diet and activity habits.18 Moreover, there is major concern that weight loss could worsen frailty by accelerating the usual age-related loss of muscle that leads to sarcopenia.4 In a preliminary, short-term study,19 we reported that a combination of weight loss and exercise may ameliorate frailty in obese older adults. We now report the results of a randomized, controlled trial that was designed to determine the independent and combined effects of sustained weight loss and regular exercise on physical function, body composition, and quality of life in obese older adults. We hypothesized that weight loss and exercise would each improve physical function and that the combination of the two would result in the greatest improvement in physical function and amelioration of physical frailty.
METHODS
STUDY OVERSIGHT
We conducted the study from April 2005 through August 2009 at the Washington University School of Medicine. The study was approved by the institutional review board and was monitored by an independent data and safety monitoring board. The protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org. All the authors vouch for the data and analyses, as well as the fidelity of the study to the protocol. The first author wrote the first draft of the manuscript; all the authors participated in writing subsequent drafts and made the decision to submit the manuscript for publication.
PARTICIPANTS
Volunteers were recruited through advertisements, and each participant provided written informed consent. Potential participants underwent a comprehensive medical screening procedure. Volunteers were eligible for inclusion in the study if they were 65 years of age or older and obese (BMI [the weight in kilograms divided by the square of the height in meters] of 30 or more), if they had a sedentary lifestyle, if their body weight had been stable during the previous year (i.e., had not fluctuated more than 2 kg), and if their medications had been stable for 6 months before enrollment. All participants had to have mild-to-moderate frailty, on the basis of meeting at least two of the following operational criteria8,19,20: a score on the modified Physical Performance Test (in which the total score ranges from 0 to 36, with higher scores indicating better physical status) of 18 to 32; a peak oxygen consumption (VO2peak) of 11 to 18 ml per kilogram of body weight per minute; or difficulty in performing two instrumental activities of daily living or one basic activity of daily living. Persons who had severe cardiopulmonary disease; musculoskeletal or neuromuscular impairments that preclude exercise training; visual, hearing, or cognitive impairments; or a history of cancer, as well as persons who were receiving drugs that affect bone health and metabolism or who were current smokers, were excluded.
STUDY OUTCOMES
The primary outcome was the change from baseline in the score on the modified Physical Performance Test. Secondary outcomes included other measures of frailty, body composition, bone mineral density, specific physical functions, and quality of life.
BASELINE ASSESSMENTS
Physical Function
Frailty was assessed with the use of the modified Physical Performance Test, the measurement of VO2peak, and the Functional Status Questionnaire. The modified Physical Performance Test includes seven standardized tasks (walking 50 ft, putting on and removing a coat, picking up a penny, standing up from a chair, lifting a book, climbing one flight of stairs, and performing a progressive Romberg test) plus two additional tasks (climbing up and down four flights of stairs and performing a 360-degree turn). The score for each task ranges from 0 to 4; a perfect score is 36.20-23 A low score on the Physical Performance Test is associated with a high BMI,8,24 and the score increases in response to weight-loss therapy.19 VO2peak was assessed during graded treadmill walking, as described previously. 8 Information regarding the ability to perform activities of daily living was obtained with the use of the Functional Status Questionnaire (on which scores range from 0 to 36, with higher scores indicating better functional status).25 We also assessed specific physical functions such as strength, balance, and gait and determined one-repetition maximums (the maximal weight a person can lift at one time). We assessed static balance by measuring the time the participant could stand on a single leg8 and dynamic balance by measuring the time needed to complete an obstacle course.20 Fast gait speed was determined by a measurement of the time needed to walk 25 ft.
Body Composition and Bone Mineral Density
Fat mass, lean body mass, and bone mineral density of the whole body and at the lumbar spine and total hip were measured with the use of dual-energy x-ray absorptiometry (Delphi 4500/w, Hologic), as described previously.19,26 Thigh muscle and fat volumes were measured with the use of magnetic resonance imaging (MRI) (Siemens), as described previously.27
Health-Related Quality of Life
The Medical Outcomes 36-Item Short-Form Health Survey (SF-36) was used to evaluate quality of life.28 The subscales we used were those for the physical component summary and the mental component summary.29 Scores on these two subscales range from 0 to 100, with higher scores indicating better health status.
FOLLOW-UP ASSESSMENTS
All baseline assessments were repeated at 6 months and 12 months, with the exception of the MRI, which was repeated only at 12 months. The personnel who conducted the assessments were not aware of the group assignments.
INTERVENTION
For this 52-week study, participants were randomly assigned, with stratification according to sex, to one of four groups: a control group, a group that participated in a weight-management program (diet group), a group that received exercise training (exercise group), and a group that received both weight-management instruction and exercise training (diet–exercise group).
Participants assigned to the control group did not receive advice to change their diet or activity habits and were prohibited from participating in any weight-loss or exercise program. They were provided general information about a healthy diet during monthly visits with the staff.
Participants assigned to the diet group were prescribed a balanced diet that provided an energy deficit of 500 to 750 kcal per day from their daily energy requirement.2 The diet contained approximately 1 g of high-quality protein per kilogram of body weight per day.2 Participants met weekly as a group with a dietitian for adjustments of their caloric intake and for behavioral therapy. They were instructed to set weekly behavioral goals and attend weekly weigh-in sessions. Food diaries were reviewed, and new goals were set on the basis of diary reports. The goal was to achieve a weight loss of approximately 10% of their baseline body weight at 6 months and to maintain that weight loss for an additional 6 months.
Participants in the exercise group were given information regarding a diet that would maintain their current weight and participated in three group exercise-training sessions per week. Each session was approximately 90 minutes in duration and consisted of aerobic exercises, resistance training, and exercises to improve flexibility and balance. The exercise sessions were led by a physical therapist. The aerobic exercises included walking on a treadmill, stationary cycling, and stair climbing. The participants exercised so that their heart rate was approximately 65% of their peak heart rate and gradually increased the intensity of exercise so that their heart rate was between 70 and 85% of their peak heart rate. The progressive resistance training included nine upper-extremity and lower-extremity exercises with the use of weight-lifting machines. Participants performed 1 or 2 sets at a resistance of approximately 65% of their one-repetition maximum, with 8 to 12 repetitions of each exercise; they gradually increased the intensity to 2 to 3 sets at a resistance of approximately 80% of their one-repetition maximum, with 6 to 8 repetitions of each exercise. Participants in the diet–exercise group participated in both the weight-management and exercise programs described above. All participants were given supplements to ensure an intake of approximately 1500 mg of calcium per day and approximately 1000 IU of vitamin D per day.2
STATISTICAL ANALYSIS
We estimated that with 26 to 28 participants in each group, the study would have more than 80% power to detect a clinically important difference among the groups in the change in the score on the Physical Performance Test, assuming a mean between-group difference in the score of 1.7 points, with a pooled standard deviation of 2.1 (on the basis of preliminary data), at an alpha level of 5%.
Intention-to-treat analyses were performed with the use of SAS software, version 9.2. Baseline characteristics were compared with the use of analysis of variance or Fisher’s exact test. Longitudinal changes between groups were tested with the use of mixed-model repeated-measures analysis of variance, with adjustment for baseline values and sex. The primary focus of the analyses was the 12-month change in outcome in the four groups. When the overall P value for the interaction between group and time was less than 0.05, prespecified contrast statements were used to test three hypotheses: first, that changes in the diet group were different from those in the control group; second, that changes in the exercise group were different from those in the control group; and third, that changes in the diet–exercise group were different from those in the diet group and from those in the exercise group. For the scores on the Physical Performance Test, Bonferroni’s correction was used to adjust for these four comparisons, which were prespecified. Changes within a group were analyzed with the use of repeatedmeasures analysis of variance. Supplementary analyses that validated the statistical approach taken included a comparison of changes in the diet–exercise group with those in the control group, a three-way analysis of variance (with factors for diet, exercise, and time) to determine any synergistic effects, logistic regression to determine whether data were consistent with an assumption that missing data were missing completely at random, and verification by analyses of data with the last value carried forward. (There was no significant evidence of an interaction effect, and the data were consistent with the assumption that missing data were missing completely at random.) Data are presented as mean percentage change ±SD, unless otherwise specified. P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
STUDY POPULATION
A total of 107 volunteers underwent randomization; 93 (87%) completed the study (Fig. 1). Fourteen participants discontinued the intervention and were included in the intention-to-treat analyses (13 provided follow-up data at 6 months and 1 at approximately 12 months). There were no significant between-group differences in baseline characteristics (Table 1).
Figure 1.
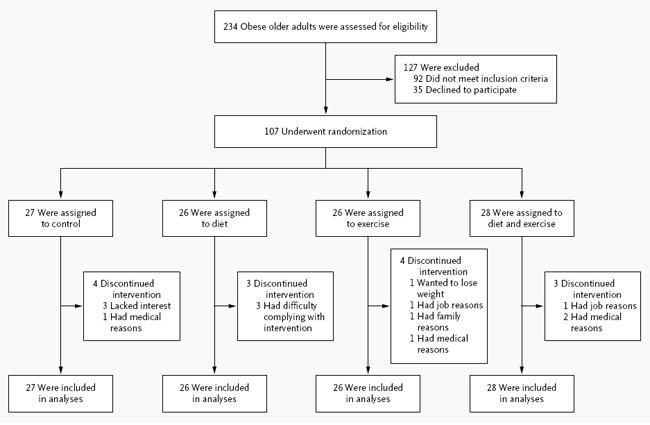
Screening, Randomization, and Follow-up.
Table 1.
Baseline Characteristics of Participants.✲
| Characteristic | Control (N = 27) | Diet (N = 26) | Exercise (N = 26) | Diet–Exercise (N = 28) | P Value |
|---|---|---|---|---|---|
| Age — yr | 69±4 | 70±4 | 70±4 | 70±4 | 0.85 |
| Sex — no. (%) | |||||
| Male | 9 (33) | 9 (35) | 10 (38) | 12 (43) | 0.89 |
| Female | 18 (67) | 17 (65) | 16 (62) | 16 (57) | |
| Race — no. (%)† | |||||
| White | 22 (81) | 23 (88) | 21 (81) | 25 (89) | 0.78 |
| Black | 4 (15) | 3 (12) | 4 (15) | 3 (11) | |
| Other | 1 (4) | 0 | 1 (4) | 0 | |
| Education — no. (%) | |||||
| Less than college degree | 9 (33) | 7 (27) | 7 (27) | 9 (32) | 0.85 |
| College degree | 13 (48) | 15 (58) | 10 (38) | 9 (32) | |
| Graduate school | 5 (19) | 4 (15) | 9 (35) | 10 (36) | |
| Marital status — no. (%) | |||||
| Single | 1 (4) | 3 (12) | 2 (8) | 2 (7) | 0.73 |
| Married | 19 (70) | 19 (73) | 13 (50) | 16 (57) | |
| Divorced | 2 (8) | 2 (8) | 6 (23) | 5 (18) | |
| Widowed | 5 (19) | 2 (8) | 5 (19) | 5 (18) | |
| Weight — kg | 101.0±16.3 | 104.1±15.3 | 99.2±17.4 | 99.1±16.8 | 0.66 |
| Body-mass index‡ | 37.3±4.7 | 37.2±4.5 | 36.9±5.4 | 37.2±5.4 | 0.93 |
| Chronic diseases — no. | 2.2±1.2 | 2.2±1.4 | 2.0±1.3 | 2.2±1.3 | 0.93 |
| Routine medications — no. | 4.6 ±2.6 | 3.3±2.3 | 4.7±2.5 | 4.1±2.8 | 0.24 |
Plus–minus values are means ±SD.
Race was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The median attendance at diet-therapy sessions was 83% (interquartile range, 79 to 89) among participants in the diet group and 82% (interquartile range, 76 to 89) among those in the diet–exercise group. The median attendance at exercise sessions was 88% (interquartile range, 85 to 92) among participants in the exercise group and 83% (interquartile range, 80 to 88) among those in the diet–exercise group.
ADVERSE EVENTS
One participant fell during testing of physical function, and the fall resulted in an ankle fracture. A summary of adverse events is provided in Table 1 in the Supplementary Appendix, available at NEJM.org.
PHYSICAL PERFORMANCE TEST AND OTHER MEASURES OF FRAILTY
The mean (±SD) scores on the Physical Performance Test (the primary outcome) increased more in the diet–exercise group than in the diet group or the exercise group: an increase of 5.4±2.4 points in the diet–exercise group (a 21% change from baseline), as compared with increases of 3.4±2.4 points in the diet group (a 12% change) and 4.0±2.5 points in the exercise group (a 15% change) (Table 2 and Fig. 2). In addition, the VO2peak improved more in the diet–exercise group than in the diet group or the exercise group: an increase of 3.1±2.4 ml per kilogram per minute in the diet–exercise group (a 17% change from baseline), as compared with increases of 1.7±2.3 ml per kilogram per minute in the diet group (a 10% change) and 1.4±1.0 ml per kilogram per minute in the exercise group (an 8% change). The scores on the Functional Status Questionnaire increased more in the diet–exercise group than in the diet group (an increase of 2.7±2.6 points [a 10% change from baseline] vs. 1.3±1.5 points [a 4% change]).
Table 2.
Effect of Diet, Exercise, or Both on Primary and Secondary Outcome Variables in Obese Older Adults.✲
| Outcome Variable | Control (N = 27) | Diet (N = 26) | Exercise (N = 26) | Diet–Exercise (N = 28) | P Value† | ||||
|---|---|---|---|---|---|---|---|---|---|
| Interaction between Group and Time | Diet vs. Control | Exercise vs. Control | Diet–Exercise vs. Diet | Diet–Exercise vs. Exercise | |||||
| Primary outcome | |||||||||
|
| |||||||||
| PPT score† | |||||||||
|
| |||||||||
| Baseline | 26.8±4.5 | 28.6±1.9 | 27.1±3.1 | 28.0±2.9 | |||||
|
| |||||||||
| Change at 6 mo | 0.6±1.7 | 2.3±1.8‡ | 3.4±2.4‡ | 4.7±2.4‡ | |||||
|
| |||||||||
| Change at 1 yr | 0.2±1.8 | 3.1±1.4‡ | 4.0±2.5‡ | 5.4±2.4‡ | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 |
|
| |||||||||
| Secondary outcomes | |||||||||
|
| |||||||||
| Other frailty measures | |||||||||
|
| |||||||||
| VO2peak (ml/kg/min) | |||||||||
|
| |||||||||
| Baseline | 16.3±3.8 | 17.6±2.2 | 17.4±3.5 | 17.3±3.5 | |||||
|
| |||||||||
| Change at 6 mo | −0.7±2.3 | 1.4±1.7‡ | 1.3±1.0‡ | 2.8±2.3‡ | |||||
|
| |||||||||
| Change at 1 yr | −0.9±1.5 | 1.7±2.3‡ | 1.4±1.0‡ | 3.1±2.4‡ | <0.001 | <0.001 | <0.001 | 0.01 | 0.002 |
|
| |||||||||
| FSQ score | |||||||||
|
| |||||||||
| Baseline | 30.5±3.2 | 31.6±2.0 | 29.8±3.3 | 30.0±3.5 | |||||
|
| |||||||||
| Change at 6 mo | −0.1±3.1 | 0.9±1.5‡ | 1.9±2.9‡ | 2.4±2.3‡ | |||||
|
| |||||||||
| Change at 1 yr | −0.2±2.4 | 1.3±1.5‡ | 1.8±2.7§ | 2.7±2.6‡ | <0.001 | 0.05 | 0.005 | 0.04 | 0.19 |
|
| |||||||||
| Body weight and composition | |||||||||
|
| |||||||||
| Weight (kg) | |||||||||
|
| |||||||||
| Baseline | 101.0±16.3 | 104.1±15.3 | 99.2±17.4 | 99.1±16.8 | |||||
|
| |||||||||
| Change at 6 mo | 0.9±2.8 | −9.0±5.4‡ | −0.3±2.3 | −7.7±4.2‡ | |||||
|
| |||||||||
| Change at 1 yr | −0.1±3.5 | −9.7±5.4‡ | −0.5±3.6 | −8.6±3.8‡ | <0.001 | <0.001 | 0.71 | 0.67 | <0.001 |
|
| |||||||||
| Lean body mass (kg) | |||||||||
|
| |||||||||
| Baseline | 57.3±11.5 | 61.4±13.0 | 57.6±13.7 | 57.2±10.3 | |||||
|
| |||||||||
| Change at 6 mo | −0.7±2.3 | −3.5±2.7‡ | 1.1±2.1¶ | −1.7±1.6‡ | |||||
|
| |||||||||
| Change at 1 yr | −0.8±2.5 | −3.2±2.0‡ | 1.3±1.6¶ | −1.8±1.7‡ | <0.001 | <0.001 | <0.001 | 0.04 | <0.001 |
|
| |||||||||
| Fat mass (kg) | |||||||||
|
| |||||||||
| Baseline | 43.8±9.9 | 42.8±6.6 | 41.6±9.4 | 41.9±11.5 | |||||
|
| |||||||||
| Change at 6 mo | −0.3±3.4 | −6.0±3.8‡ | −1.2±2.0 | −5.6±3.2‡ | |||||
|
| |||||||||
| Change at 1 yr | 1.2±5.1 | −7.1±3.9‡ | −1.8±1.9 | −6.3±2.8‡ | <0.001 | <0.001 | 0.004 | 0.57 | <0.001 |
|
| |||||||||
| Thigh muscle (cm3) | |||||||||
|
| |||||||||
| Baseline | 1138±290 | 1271±280 | 1188±234 | 1261±253 | |||||
|
| |||||||||
| Change at 1 yr | −7±54 | −81±63‡ | 30±34‡ | −28±63¶ | <0.001 | <0.001 | 0.045 | <0.001 | <0.001 |
|
| |||||||||
| Thigh fat (cm3) | |||||||||
|
| |||||||||
| Baseline | 1813±773 | 1553±529 | 1423±541 | 1472±718 | |||||
|
| |||||||||
| Change at 1 yr | −0.5±158 | −255±179‡ | −76±97§ | −247±217‡ | <0.001 | <0.001 | 0.19 | 0.44 | 0.02 |
|
| |||||||||
| Bone mineral density at total hip (g/cm2) | |||||||||
|
| |||||||||
| Baseline | 0.962±0.132 | 1.021±0.139 | 0.958±0.151 | 1.014±0.151 | |||||
|
| |||||||||
| Change at 6 mo | −0.003±0.016 | −0.015±0.017¶ | 0.008±0.012‡ | −0.010±0.024¶ | |||||
|
| |||||||||
| Change at 1 yr | −0.007±0.019¶ | −0.027±0.021¶ | 0.013±0.014‡ | −0.011±0.026¶ | <0.001 | 0.001 | 0.001 | 0.005 | <0.001 |
|
| |||||||||
| Strength, balance, and gait | |||||||||
|
| |||||||||
| Total 1RM (lb)∣ | |||||||||
|
| |||||||||
| Baseline | 505±143 | 607±213 | 519±187 | 539±218 | |||||
|
| |||||||||
| Change at 6 mo | −16±78 | 8±60 | 110±138§ | 96±108§ | |||||
|
| |||||||||
| Change at 1 yr | −6±101 | 1±85 | 174±166‡ | 164±124‡ | <0.001 | 0.90 | <0.001 | <0.001 | 0.32 |
|
| |||||||||
| Obstacle course (sec) | |||||||||
|
| |||||||||
| Baseline | 11.6±3.3 | 11.0±2.2 | 10.9±3.3 | 10.7±3.3 | |||||
|
| |||||||||
| Change at 6 mo | −0.1±1.2 | −0.7±1.3 | −1.6 ±1.6‡ | −1.1±2.2 | |||||
|
| |||||||||
| Change at 1 yr | 0.0±1.0 | −1.1±1.1 | −1.5±1.4‡ | −1.7±2.2‡ | 0.002 | 0.03 | 0.004 | 0.18 | 0.68 |
|
| |||||||||
| One-leg stance (sec) | |||||||||
|
| |||||||||
| Baseline | 10.7±10.6 | 11.7±8.7 | 13.4±10.4 | 10.5±9.5 | |||||
|
| |||||||||
| Change at 6 mo | −2.4±8.2 | 0.8±6.1 | 1.4±7.7 | 6.3±7.6‡ | |||||
|
| |||||||||
| Change at 1 yr | −2.3±9.4 | 4.7±5.0‡ | 3.4±5.9¶ | 7.9±7.8‡ | <0.001 | 0.001 | 0.02 | 0.18 | 0.04 |
|
| |||||||||
| Gait speed (m/min) | |||||||||
|
| |||||||||
| Baseline | 75.5±17.6 | 87.5±15.8 | 76.0±18.3 | 72.9±14.9 | |||||
|
| |||||||||
| Change at 6 mo | −3.0±10.5 | 1.7±5.4 | 7.6±14.8§ | 5.5±7.6§ | |||||
|
| |||||||||
| Change at 1 yr | 1.1±11.0 | 4.7±5.2 | 8.2±15.5§ | 16.9±42.3§ | 0.02 | 0.45 | 0.003 | 0.04 | 0.39 |
Plus–minus values are means ±SD. Scores on the modified Physical Performance Test (PPT, the primary outcome) range from 0 to 36, with higher scores indicating better physical function. Peak oxygen consumption (VO2peak) was assessed during graded treadmill walking. Scores on the Functional Status Questionnaire (FSQ) range from 0 to 36, with higher scores indicating better functional status.
P values for the comparison among the groups of changes from baseline to 1 year were calculated with the use of mixed-model repeated-measures analysis of variance (with baseline values and sex as covariates) and are reported when the overall P value was less than 0.05 for the interaction among the four groups over time. Bonferroni correction was used to adjust for the prespecified comparisons in the PPT score; these P values have been multiplied by 4 for the comparison to alpha value of 0.05. Secondary analyses included a comparison between diet–exercise and control; all P values were less than 0.05.
P<0.001 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
P<0.01 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
P<0.05 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
One-repetition maximum (1RM) is the maximal weight lifted at one time; the totals listed here are the sum of the maximal weights lifted in the biceps curl, bench press, seated row, knee extension, knee flexion, and leg press exercises. To convert the values to kilograms, divide by 2.2.
Figure 2. Mean Percentage Changes in Objective and Subjective Measures of Frailty during the 1-Year Intervention.
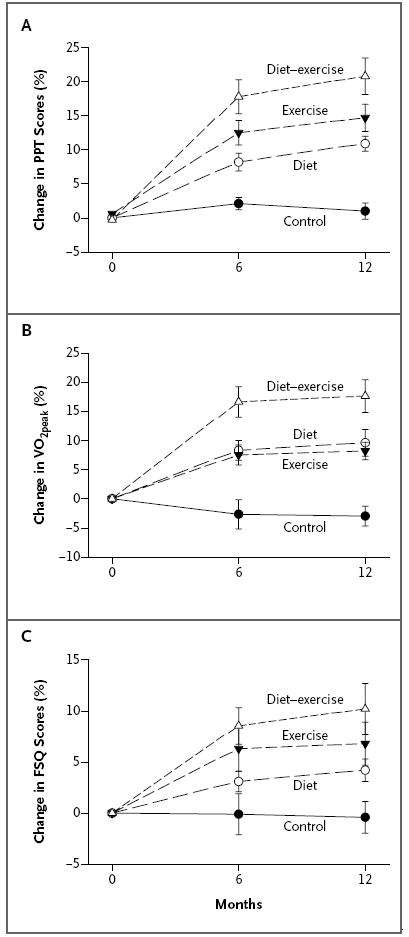
The objective measures of frailty included the scores on the Physical Performance Test (PPT), which range from 0 to 36, with higher scores indicating better physical status (Panel A), and the peak oxygen consumption (VO2peak) (Panel B). The scores on the Functional Status Questionnaire (FSQ), which range from 0 to 36, with higher scores indicating better functional status, were used as a subjective measure of frailty (Panel C). The change in the scores on the PPT was the primary outcome. In Panels A and B, the change in the diet–exercise group differed significantly from the changes in the exercise group and in the diet group, and the changes in the exercise group and in the diet group differed significantly from that in the control group. In Panel C, the change in the diet–exercise group differed significantly from that in the diet group, and the changes in the exercise group and in the diet group differed significantly from that in the control group. I bars indicate standard errors.
BODY WEIGHT AND COMPOSITION
There was a substantial decrease in body weight in the diet group (a weight loss of 9.7±5.4 kg, representing a 10% decrease from baseline) and in the diet–exercise group (a weight loss of 8.6±3.8 kg, representing a 9% decrease), but not in the exercise group (a weight loss of 1.8±2.7 kg, representing a 1% decrease) or the control group (a weight loss of 0.9±1.5 kg, representing <1% decrease) (Table 2). The time-course of weight loss is shown in Figure 3. Lean body mass decreased less in the diet–exercise group than in the diet group (a decrease of 1.8±1.7 kg, representing a 3% change from baseline, vs. a decrease of 3.2±2.0 kg, representing a 5% change). The lean body mass increased by 1.3±1.6 kg in the exercise group (a 2% increase from baseline). Fat mass decreased by 6.3±2.8 kg in the diet–exercise group (a 16% change from baseline), by 7.1±3.9 kg in the diet group (a 17% change), and by 1.8±1.9 kg in the exercise group (a 5% change). Similar changes were observed with respect to thigh muscle and fat.
Figure 3. Mean Percentage Changes in Body Weight during the 1-Year Intervention.
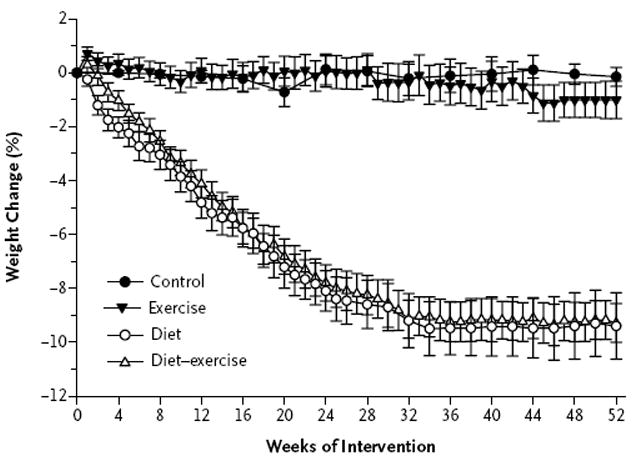
I bars indicate standard errors.
BONE MINERAL DENSITY
Bone mineral density at the total hip decreased by 0.011±0.026 g per square centimeter (a decrease of 1.1% from baseline) in the diet–exercise group, as compared with 0.027±0.021 g per square centimeter (a decrease of 2.6%) in the diet group, whereas it increased, by 0.013±0.014 g per square centimeter (a 1.5% increase), in the exercise group (Table 2). There were no significant changes in bone mineral density of the whole body or at the lumbar spine (Table 2 in the Supplementary Appendix).
STRENGTH, BALANCE, GAIT, AND QUALITY OF LIFE
The total one-repetition maximum (i.e., the sum of the maximal weights lifted in the biceps curl, bench press, seated row, knee extension, knee flexion, and leg press exercises) increased in the diet–exercise group (an increase of 164±124 lb [75±56 kg], representing a 35% change from baseline) and in the exercise group (an increase of 174±166 lb [79±75 kg], representing a 34% change), whereas it was maintained in the diet group (an increase of 1±85 lb [0.5±39 kg], representing a 3% change) (Table 2). The time needed to complete the obstacle course was reduced by 1.7±2.2 seconds in the diet–exercise group (a reduction of 12%), by 1.1±1.1 seconds in the diet group (a reduction of 10%), and by 1.5±1.4 seconds in the exercise group (a reduction of 13%). The duration of time the participant could stand on a single leg increased by similar amounts in those groups. Gait-speed increased in the diet–exercise group (an increase of 16.9±42.3 seconds, representing a 23% change from baseline) and in the exercise group (an increase of 8.2±15.5 seconds, representing a 14% change).The physical-component summary score of the SF-36 (which was used to measure quality of life) increased by 8.6±9.3 points in the diet–exercise group (a 15% increase from baseline), by 8.4±10.1 points in the diet group (a 14% increase), and by 5.7±8.0 points in the exercise group (a 10% increase) (Table 2 in the Supplementary Appendix).
DISCUSSION
Obesity in older adults is a public health problem that challenges our health care professionals and health care delivery systems.1-3,10-12 In this 1-year, randomized, controlled trial involving obese older adults, weight loss plus exercise improved physical function and ameliorated frailty more than either weight loss or exercise alone, although each of those was beneficial.
Currently, evidence-based data to guide the treatment of obese older adults are limited.16,17 The few clinical trials that have been conducted typically addressed cardiovascular risk factors rather than physical function.16 However, frailty is an important problem in the elderly because it leads to loss of independence and increased morbidity and mortality.30,31 Physical frailty is common in obese older adults,8,9 and obesity is associated with increased admissions to nursing homes.10-12 Four previous randomized, controlled trials examined the effect of weight loss on physical function in obese older adults,14 but these studies were either short-term19,32,33 or limited to participants with specific health conditions.34 The current study suggests that weight loss alone or exercise alone can reverse frailty but that the combination of weight loss and exercise is more effective than either individual intervention. Therefore, weight loss and exercise may be an important therapy for frail, obese older adults. Moreover, one study has shown that weight loss and exercise reduce knee pain and improve physical function in overweight and obese older adults with osteoarthritis of the knee.34 Our data suggest that a major objective of weight-loss therapy in older adults may be to improve physical function, and we speculate that doing so may be at least as important as treating obesity-associated medical complications, which is often the main goal in treating obese younger adults.35
Physical frailty in obese older adults is associated with low muscle mass relative to body weight (relative sarcopenia) despite a greater absolute amount of muscle mass.4,8 In the current study, relative sarcopenia was reduced in all the intervention groups — owing to the larger reduction in fat mass relative to lean body mass in the diet and diet–exercise groups and owing to the decrease in fat mass and increase in lean body mass in the exercise group. These positive changes in body composition could underlie the improvement in physical function in the participants.4,8 However, because the greatest improvement occurred in the diet–exercise group, adding an exercise program to a diet regimen, which results in the preservation of lean body mass in addition to the reduction in fat mass induced by a diet, may be the best approach. Accordingly, the diet–exercise group had not only the greatest increase in scores on the Physical Performance Test but also the most consistent improvements in strength, balance, and gait.
The improvements that were seen in the objective measures of frailty among the participants in this trial have important implications for the ability of older adults to maintain their independence. The functional items in the Physical Performance Test simulate activities of daily living, and the Physical Performance Test has been used to monitor physical performance and predict disability, loss of independence, and death.20,36,37 Moreover, the VO2peak relative to body weight is the standard measure for assessing cardiovascular fitness,38 and the VO2peak is important for assessing the ability to perform activities that require movement of increased body weight.8,39 The improvements in scores on the Physical Performance Test and in VO2peak among the participants in this study were accompanied by improvements in scores on the Functional Status Questionnaire and in the physical-component summary score of the SF-36 (measuring quality of life), both of which indicate subjective improvements in the ability of the participants to function.
A potential adverse effect of our interventions was the reduction in lean body mass and bone mineral density at the hip in the diet groups. However, the addition of exercise to diet attenuated the losses of lean tissue and further augmented physical function. Although the clinical importance of the modest loss of bone mineral density is unclear, strategies to prevent this loss in participants involved in future studies might include prescribing higher doses of calcium and vitamin D than those used in this study, having participants perform endurance exercise alone or resistance exercise alone (rather than both endurance and resistance exercises), and perhaps antiresorptive therapy. Exercise was also associated with musculoskeletal injuries; careful screening and safeguards before and during exercise are needed to decrease the risk of these adverse events. An additional health concern is raised by findings from observational studies that suggest that weight loss may be associated with an increased risk of death.2 However, these studies did not rigorously distinguish intentional from nonintentional weight loss. Follow-up data from a randomized, controlled trial involving overweight and obese older adults suggest that intentional weight loss may reduce the risk of death.40
The strengths of our study include the randomized, controlled design, the long duration of the intervention, the comprehensive diet and exercise programs, the high rate of adherence to the interventions, and the use of objective and subjective measures of physical function. A limitation of our study is that it was not powered to determine potential differences in the outcomes between sexes. Because we selected volunteers who were able to participate in a lifestyle program, the results may not necessarily apply to the general obese, older adult population. Nonetheless, they provide evidence that successful weight loss is achievable in this population. Further studies are needed to determine whether weight loss can be maintained beyond 1 year and prevent institutionalization of obese older adults. Our sample size was small, and most of the participants were women, white, well educated, and older (70±4 years of age) with mild-to-moderate frailty (and sarcopenic obesity4), thus limiting broader inferences of our results. Our study did not address the usefulness or safety of these interventions for markedly obese older persons with severe frailty.
In conclusion, our findings suggest that weight loss alone or exercise alone improves physical function and ameliorates frailty in obese older adults; however, a combination of weight loss and regular exercise may provide greater improvement in physical function and amelioration of frailty than either intervention alone. Therefore, weight loss combined with regular exercise may be beneficial in helping obese older adults maintain their functional independence.
Supplementary Material
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Supported by grants (RO1-AG025501 and P30-DK56341 [Clinical Nutrition Research Unit], UL1-RR024992 [a Clinical and Translational Science Award], and DK20579 [Diabetes Research and Training Center]) from the National Institutes of Health. Dr. Hilton was supported by a postdoctoral fellowship (HD007434) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and a New Investigator Fellowship Initiative grant from the Foundation for Physical Therapy.
We thank the participants; the staff of the Intensive Research Unit of the Institute of Clinical and Translational Sciences; Nicole Wright for study coordination; Stacie Metzger, Amelia Grant, and Ellen Frye for exercise training; and Kathy Obert, Laura Weber, and Cindi Inman for weight-loss training.
Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT00146107.
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.van Baak MA, Visscher TL. Public health success in recent decades may be in danger if lifestyles of the elderly are neglected. Am J Clin Nutr. 2006;84:1257–8. doi: 10.1093/ajcn/84.6.1257. [DOI] [PubMed] [Google Scholar]
- 2.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–34. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 3.Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–12. doi: 10.1111/j.1532-5415.2004.52517.x. [DOI] [PubMed] [Google Scholar]
- 4.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–8. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Fisher KJ, Harmer P. Prevalence of overweight and obesity in older U.S. adults: estimates from the 2003 Behavioral Risk Factor Surveillance System survey. J Am Geriatr Soc. 2005;53:737–9. doi: 10.1111/j.1532-5415.2005.53228_10.x. [DOI] [PubMed] [Google Scholar]
- 8.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 9.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–34. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 10.Zizza CA, Herring A, Stevens J, Popkin BM. Obesity affects nursing-care facility admission among whites but not blacks. Obes Res. 2002;10:816–23. doi: 10.1038/oby.2002.110. [DOI] [PubMed] [Google Scholar]
- 11.Lapane KL, Resnik L. Obesity in nursing homes: an escalating problem. J Am Geriatr Soc. 2005;53:1386–91. doi: 10.1111/j.1532-5415.2005.53420.x. [DOI] [PubMed] [Google Scholar]
- 12.Elkins JS, Whitmer RA, Sidney S, Sorel M, Yaffe K, Johnston SC. Midlife obesity and long-term risk of nursing home admission. Obesity (Silver Spring) 2006;14:1472–8. doi: 10.1038/oby.2006.167. [DOI] [PubMed] [Google Scholar]
- 13.Alley DE, Ferrucci L, Barbagallo M, Studenski SA, Harris TB. A research agenda: the changing relationship between body weight and health in aging. J Gerontol A Biol Sci Med Sci. 2008;63:1257–9. doi: 10.1093/gerona/63.11.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010;11:671–85. doi: 10.1111/j.1467-789X.2009.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13:46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 16.Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing. 2010;39:176–84. doi: 10.1093/ageing/afp251. [DOI] [PubMed] [Google Scholar]
- 17.Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc. 2008;9:302–12. doi: 10.1016/j.jamda.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Elia M. Obesity in the elderly. Obes Res. 2001;9(Suppl 4):244S–248S. doi: 10.1038/oby.2001.126. [DOI] [PubMed] [Google Scholar]
- 19.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–6. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 20.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–M355. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 21.Host H, Sinacore D, Brown M, Holloszy J. Reliability of the modified physical performance test in older adults. Phys Ther. 1996;76(Suppl):S23–S24. [Google Scholar]
- 22.Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81:960–5. doi: 10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- 23.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–8. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 24.Apovian CM, Frey CM, Rogers JZ, McDermott EA, Jensen GL. Body mass index and physical function in obese older women. J Am Geriatr Soc. 1996;44:1487–8. doi: 10.1111/j.1532-5415.1996.tb04082.x. [DOI] [PubMed] [Google Scholar]
- 25.Jette AM, Cleary PD. Functional disability assessment. Phys Ther. 1987;67:1854–9. doi: 10.1093/ptj/67.12.1854. [DOI] [PubMed] [Google Scholar]
- 26.Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166:2502–10. doi: 10.1001/archinte.166.22.2502. Erratum, Arch Intern Med 2007;167:452. [DOI] [PubMed] [Google Scholar]
- 27.Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102:634–40. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons RA, Perry HM, Littlepage BN. Evidence for the validity of the Short-form 36 Questionnaire (SF-36) in an elderly population. Age Ageing. 1994;23:182–4. doi: 10.1093/ageing/23.3.182. [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. 2001;12:2797–806. doi: 10.1681/ASN.V12122797. [DOI] [PubMed] [Google Scholar]
- 30.Hamerman D. Toward an understanding of frailty. Ann Intern Med. 1999;130:945–50. doi: 10.7326/0003-4819-130-11-199906010-00022. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. Am J Med. 2007;120:748–53. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16:66–72. doi: 10.1097/gme.0b013e31817dacf7. [DOI] [PubMed] [Google Scholar]
- 33.Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silver Spring) 2006;14:1219–30. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 34.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 35.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastro enterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. Erratum, Gastroenterology 2002;123:1752. [DOI] [PubMed] [Google Scholar]
- 36.Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. J Gerontol. 1992;47:M106–M110. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]
- 37.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 38.Binder EF, Birge SJ, Spina R, et al. Peak aerobic power is an important component of physical performance in older women. J Gerontol A Biol Sci Med Sci. 1999;54:M353–M356. doi: 10.1093/gerona/54.7.m353. [DOI] [PubMed] [Google Scholar]
- 39.Shah K, Wingkun NJ, Lambert CP, Villareal DT. Weight-loss therapy improves endurance capacity in obese older adults. J Am Geriatr Soc. 2008;56:1157–9. doi: 10.1111/j.1532-5415.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shea MK, Houston DK, Nicklas BJ, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT Study. J Gerontol A Biol Sci Med Sci. 2010;65:519–25. doi: 10.1093/gerona/glp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


