Abstract
Background: neopterin is a monocyte/macrophage-derived immune activation marker and its levels increase with age. Frailty is an important clinical syndrome of old age. Previous studies have shown significant association between elevated interleukin-6 (IL-6) levels and frailty. The objective of this study was to evaluate IL-6-independent association of serum neopterin levels with prevalent frailty.
Methods: this is a cross-sectional study in community-dwelling older adults recruited from residential and retirement communities in Baltimore, MD, USA. Frailty was determined using validated screening criteria. Serum neopterin and IL-6 levels were measured using standard enzyme-linked immunosorbent assay. Pearson correlation and multivariate linear regression analysis was performed to assess the relationship between log(neopterin) and log(IL-6). Odds ratios (ORs) for frailty were calculated using log(neopterin) and log(IL-6) as continuous measures and across tertiles of neopterin and IL-6 levels, adjusting for age, race, sex, education and body mass index.
Results: one hundred and thirty-three individuals with a mean age of 84 years (range 72–97) completed the study. Neopterin levels were significantly higher in frail older adults than those in non-frail controls [median: 8.94 versus 8.35 nM, respectively, P < 0.001 t-test on log(neopterin)]. Log(neopterin) was significantly associated with prevalent frailty, adjusting for log(IL-6). Participants in the top tertile of neopterin had OR of 3.80 [95% confidence interval (CI) = 1.36–10.6, P < 0.01] for frailty. As expected, participants in the top tertile of IL-6 had OR of 3.29 (95% CI = 1.21–7.86, P < 0.05) for frailty. Log(neopterin) correlated with log(IL-6) (correlation coefficient = 0.19, P < 0.05). Moreover, OR for participants in the top neopterin tertile remained significant after adjusting for IL-6 (OR = 3.97, 95% CI = 1.15–13.72, P < 0.05).
Conclusion: elevated neopterin levels had IL-6-independent association with prevalent frailty, suggesting potential monocyte/macrophage-mediated immune activation in the frail elderly.
Keywords: frailty, neopterin, monocyte/macrophage-mediated immune activation, IL-6, elderly
Introduction
Frailty, increasingly recognised as an important and common geriatric syndrome, is identifiable by a validated set of criteria consisting of three or more of the following five measurable characteristics: slowed motor performance (by walking speed), poor endurance and energy (self-report of exhaustion), weakness (by grip strength), unintentional weight loss and low physical activity [1]. It has been suggested that frailty is a clinical syndrome resulting from dysregulation in multiple physiologic systems, manifested by maladaptive responses to stressors, leading to hospitalisation, mortality and other serious adverse health outcomes [1–4]. Studies have shown that systemic inflammation, as marked by elevated interleukin-6 (IL-6) levels and white blood cell counts, is potentially a key pathophysiological factor for frailty and its associated multisystem dysregulation [5–7]. In addition, we have recently reported that frailty is associated with elevated monocyte counts [8]. Ex vivo studies of purified monocytes with or without lipopolysaccharide (LPS) stimulation have shown that frail older adults have upregulated monocytic expression of stress-responsive inflammatory pathway genes compared with age-, race- and sex-matched non-frail controls [9, 10]. However, potential immune activation and in vivo monocyte/macrophage activity in the syndrome of frailty have not been adequately investigated.
Neopterin, a guanosine triphosphate metabolite, is primarily produced by human monocytes/macrophages and is considered a marker of immune activation and monocyte/macrophage activity [11, 12]. Ageing is associated with increased production and serum concentration of neopterin [11, 13–15] and elevated neopterin level is associated with decreased survival in older adults [14, 16–18]. Elevated neopterin levels have also been documented in conditions with significant immune activation, such as viral infections [19] and autoimmune disease [20], as well as in patients with malignancies [21], acute coronary syndrome and sleep and lung disorders [22–24].
The objective of this study was to investigate the relationship between serum neopterin and frailty. We hypothesised that elevated neopterin levels would have a significant association with prevalent frailty, independent of IL-6. Addressing these hypotheses will provide initial evidence for the involvement of monocyte/macrophage-mediated immune activation in the geriatric syndrome of frailty. To test these hypotheses, we conducted a case-control study in community-dwelling older men and women, evaluating serum neopterin and IL-6 levels and their relationships with prevalent frailty.
Methods
Human subjects
Community-dwelling older adults aged 70 years and older were recruited from outpatient medical clinics, senior centres and residential retirement communities in Baltimore, MD. Potential candidates were identified by physicians and nursing staff, or responded to ads posted in local newspapers. These individuals were screened by trained clinical research coordinators. Study eligibility criteria included the validated frailty screening tool (see below) and exclusion criteria. Exclusion criteria included Parkinson's disease, stroke with residual hemiparesis, symptomatic congestive heart failure, uncompensated endocrine disorders, active malignancy, rheumatoid arthritis or any other inflammatory conditions, or use of immune-modulating drugs including oral corticosteroids. These exclusion criteria were intended to minimise the impact of one single disease in mimicking or initiating the presence of the clinical phenotype of frailty or potential effects on monocyte/macrophage activities from inflammatory/autoimmune diseases or immune-modulating medications. Persons with significant cognitive deficit (Folstein mini-mental status exam score <18/30) were also excluded because this study was focused on physical frailty (based on the frailty criteria described below) rather than cognitive dysfunction. In addition, most individuals who were excluded by mini-mental status exam scores had difficulties in providing informed consent. Those who qualified came to the Clinical Research Unit at Johns Hopkins Institute for Clinical and Translational Research on Johns Hopkins Bayview Medical Center campus for a medical history and physical examination by a physician member of the research team to ensure that they met the eligibility criteria and did not have an acute illness. The Johns Hopkins Institutional Review Board approved the study protocol. Written informed consent was obtained from all participants.
Determination of the syndrome of frailty
Validated and widely utilised frailty screening criteria were employed for the determination of the syndrome of frailty [1]. These criteria are based on the presence or absence of five measurable characteristics: slowed motor performance (walking speed), poor endurance and energy (self-report of exhaustion), weakness (grip strength), shrinking (unintentional weight loss) and low level of physical activity. Individuals with a critical mass of three or more of the five components were defined as frail, those with one or two components as prefrail and those with none of the components as non-frail.
Measurements of serum neopterin and IL-6 levels
Peripheral venous blood samples were collected into serum separation tubes (Becton Dickinson, Mountain View, CA, USA). Sera were obtained after a 20-min centrifugation, aliquoted and stored at −80°C until analysis. Serum neopterin was measured using a commercially available competitive enzyme-linked immunosorbent assay (ELISA) (ALPCO Diagnostics; Salem, NH, USA). This immunoassay has a sensitivity of 0.8 nM and an interassay coefficient of variance of 5.29%. Serum IL-6 was measured using an ultra-sensitive sandwich-based ELISA human cytokine kit (Meso Scale Discovery, Gaithersburg, MD, USA), which has a sensitivity of 0.05 pg/l and an interassay coefficient of variance of 3.92% for serum IL-6 measurement.
Data analyses
Summary statistics of demographic characteristics and serum neopterin and IL-6 levels were constructed for all study participants and distributions of these characteristics and study variables were summarised according to the frailty status. Log-transformed values of neopterin and IL-6 levels were used in our statistical analyses (except for models using tertiles, see below). Frailty was modelled as a three-level outcome (frail versus prefrail versus non-frail) and analysis of variance (ANOVA) was employed to assess the relationship between frailty and log(neopterin). Pearson's correlation and multivariate linear regression analysis were used to evaluate the relationship between log(IL-6) and log(neopterin). Multinomial logistic regression models were used to assess effects of serum neopterin and IL-6 levels on the risk of being frail or prefrail versus non-frail. Log(neopterin) and log(IL-6) were initially analysed as continuous covariates for maximal efficiency; the estimated effects were expressed as odds ratios (ORs) of being frail versus non-frail or prefrail versus non-frail for every one standard deviation (SD) unit increase of log(neopterin) or log(IL-6) such that the relative contributions of neopterin and IL-6 levels could be readily compared. Because of the wide 95% confidence intervals (CIs) from these analyses as well as potential non-linear associations between frailty and neopterin and IL-6 levels suggested by our exploratory analyses, neopterin and IL-6 levels were then modelled as tertiles in association with frailty for the ease of interpretation. Models I and II assessed the individual effects of neopterin or IL-6, respectively, while model III evaluated the independent effect of neopterin and IL-6 on frailty. All analyses were adjusted for age, race, sex, education and body mass index (BMI). Intercooled Stata software, version 9 was used for model estimation and diagnostics (Stata Corporation, College Station, TX, USA).
Results
Characteristics of the study participants
Approximately 300 community-dwelling men and women aged 70 years and older were screened. Approximately one-quarter of those met the entry criteria. One hundred and thirty-three participants with complete data on frailty measurements and serum neopterin and IL-6 levels were included in the study. As shown in Table 1, participants had a mean age of 84 years with a range of 72–97. The majority of them were female and Caucasians. According to the frailty criteria described above, 50 participants were frail, 32 were prefrail and 51 were non-frail. There were significant differences in race, sex, education or BMI across the frailty categories, whereas the difference in age approached statistical significance (P = 0.07) (Table 1). Overall, participants had an average of three medical diagnoses including the following common diseases: hypertension, osteoarthritis, hypercholesterolemia, osteoporosis, diabetes mellitus, etc. On average, they took three medications including antihypertensive medications (thiazide diuretics, beta-blockers, calcium-channel blockers, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, etc). No significant difference in clinical diagnosis or medication use was observed across the frailty categories (data not shown). None of the participants was a current smoker, or reported illicit drug use or heavy alcohol consumption.
Table 1.
Demographic characteristics and serum neopterin and IL-6 levels in all study participants and across frailty categories
| Variables | All subjects (n = 133) | Non-frail (n = 51) | Prefrail (n = 32) | Frail (n = 50) | P value |
|---|---|---|---|---|---|
| Age, mean ± SD (year) | 84.0 ± 4.7 | 82.7 ± 5.2 | 85.4 ± 4.1 | 84.4 ± 4.1 | 0.07 |
| Race, white (%) | 95.7 | 98 | 93.8 | 93.6 | 0.31 |
| Sex, female (%) | 80 | 84.3 | 68.7 | 84.3 | 0.34 |
| Education (high school or above) (%) | 91.0 | 94.1 | 90.6 | 88.0 | 0.21 |
| BMI, mean ± SD (kg/m2) | 25.5 ± 5.6 | 25.0 ± 4.1 | 25.0 ± 3.7 | 26.2 ± 7.8 | 0.62 |
| Neopterin levels (nM) | |||||
| Mean ± SD | 9.66 ± 4.21 | 8.56 ± 1.80 | 10.08 ± 4.37 | 10.53 ± 5.49 | <0.001a |
| Median | 8.65 | 8.35 | 8.89 | 8.94 | |
| (Range) | (5.25–36.12) | (5.25–13.43) | (5.53–28.16) | (6.09–36.12) | |
| Tertiles (%) | <0.05 | ||||
| Bottom (<7.89) | 44.4 | 28.1 | 23.4 | ||
| Middle (7.89–9.64) | 35.2 | 31.3 | 34.0 | ||
| Top (>9.64) | 20.4 | 40.6 | 42.6 | ||
| IL-6 levels (pg/l) | |||||
| Mean ± SD | 2.15 ± 1.72 | 1.78 ± 1.86 | 1.99 ± 1.54 | 2.60 ± 1.63 | <.01a |
| Median | 1.56 | 1.22 | 1.49 | 2.17 | |
| (Range) | (0.38–9.19) | (0.39–7.72) | (0.44–8.49) | (0.64–9.19) | |
| Tertiles (%) | <0.05 | ||||
| Bottom (<1.12) | 36.0 | 46.9 | 18.6 | ||
| Middle (1.12–2.25) | 46.9 | 36.0 | 25.0 | 34.9 | |
| Top (>2.25) | 25.0 | 28.0 | 28.1 | 46.5 |
aDetermined using ANOVA test on log-transformed values across the frailty categories.
Association between neopterin and frailty
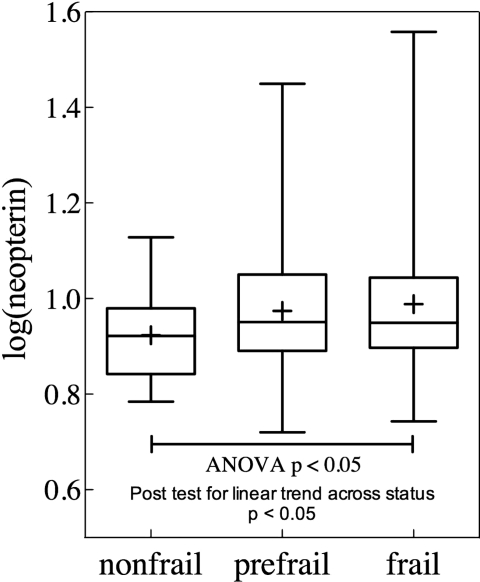
As shown in Table 1, the mean and median of serum neopterin levels in this study population were 9.66 and 8.65 nM, respectively. Among the frailty categories, mean, median and percentages of participants in top tertiles of neopterin levels had stepwise increase from the non-frail to prefrail and frail groups. Figure 1 shows log(neopterin) values across the frailty groups with the median value in each group illustrated by a horizontal bar and mean value by a ‘+’, further demonstrating higher neopterin in frail and prefrail participants than that in non-frail ones (P < 0.05, ANOVA trend test). In multinomial logistic regression analyses, higher log(neopterin) was associated with significantly increased OR of being frail (OR = 94.9, 95% CI = 1.7–5,312, P < 0.01), adjusting for age, race, sex, education and BMI (Table 2, Model 1). With neopterin levels being modelled in tertiles, OR of being frail compared with non-frail was 3.8 times greater (95% CI = 1.36–10.6, P < 0.01) in the top tertile of neopterin levels compared with the bottom tertile, adjusting for age, race, sex, education and BMI (Table 2, Model I).
Figure 1.
Log(neopterin) across frailty groups. Study participants were segregated into non-frail, prefrail and frail groups. The horizontal bar represents the median value and ‘+’ sign represents the mean value for each group.
Table 2.
Odds (95% CIs) of being frail versus non-frail or prefrail versus non-frail using continuous measures of log-transformed neopterin and IL-6 values or comparing top and middle tertiles with bottom tertile of serum neopterin and IL-6 levels in community-dwelling older adults
| Analysis | Model Ia |
Model IIa |
Model IIIa |
|||
|---|---|---|---|---|---|---|
| Prefrail | Frail | Prefrail | Frail | Prefrail | Frail | |
| Using continuous measures | ||||||
| Log(neopterin) | 27.8 (0.99–2,311) | 94.9 (1.7–5,312)** | 27.8 (0.99–2,309) | 70.9 (1.08–4,662)* | ||
| Log (IL-6) | 0.4 (0.1–1.9) | 5.1 (1.05–24.75)* | 0.3 (0.1–1.81) | 3.7 (0.74–18.62) | ||
| Using tertiles | ||||||
| Neopterin tertiles | ||||||
| Middle | 1.5 (0.5–4.5) | 1.97 (0.73–5.3) | 1.33 (0.39–4.53) | 3.11 (0.99–9.78) | ||
| Top | 3.02 (0.99–9.19) | 3.8 (1.36–10.6)** | 3.85 (1.23–11.95)* | 3.97 (1.15–13.72)* | ||
| IL-6 tertiles | ||||||
| Middle | 0.53 (0.18–1.57) | 1.88 (0.64–5.51) | 0.37 (0.11–1.3) | 1.57 (0.46–5.37) | ||
| Top | 0.83 (0.28–2.48) | 3.29 (1.21–7.86)* | 0.45 (0.13–1.53) | 3.14 (0.96–10.23) | ||
aModels I and II were multinomial logistic regression analysis including indicator variables neopterin or IL-6 alone as continuous measures (log-transformed values) or for middle and top tertiles (using bottom tertile as the reference group) of serum neopterin or IL-6 levels, respectively. Model III was multiple logistic regression analysis including both neopterin and IL-6 as continuous measures or for middle and top tertiles. All models were adjusted for age, race, sex, education and BMI.
P value *<0.05 or **<0.01.
Association of neopterin with frailty: independent of IL-6
To gain initial insight into the question of whether the observed association between elevated neopterin levels and frailty is dependent on elevated IL-6, a pro-inflammatory cytokine that is known to be associated with frailty, we first evaluated the relationship between neopterin and IL-6 levels in the study population. There was a significant correlation between log(neopterin) and log(IL-6) with Pearson's correlation coefficient of 0.19 (P < 0.05). As expected, serum IL-6 levels had significant association with prevalent frailty when analysed as a continuous variable [log(IL-6): OR = 5.01, 95% CI = 1.05–24.75, P < 0.05) or in tertiles (top tertile versus bottom tertile: OR = 3.8, 95% CI = 1.36–10.6, P < 0.05), both after adjusting for age, race, sex, education and BMI (Table 2, Model II). Neopterin and IL-6 levels were entered in the same model and the OR for association between neopterin and frailty was 70.9 (95% CI = 1.08–4662, P < 0.05) when log(neopterin) was modelled as a continuous variable, and 3.97 (95% CI = 1.15–13.72) when serum neopterin levels were modelled in tertiles; both after adjusting for IL-6. Of interest, the association between IL-6 and frailty was no longer statistically significant after adjusting for neopterin (Table 2, Model III).
Discussion
In this study, we have observed, for the first time, a significant association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults, adjusting for age, race, sex, education and BMI. Moreover, the observed association is independent of IL-6 levels, a pro-inflammatory cytokine that is known for its association with frailty.
Neopterin is a marker of monocyte/macrophage-mediated immune activation in humans. We have recently shown that neopterin level increases with age [13]. An Austrian study in a large cohort of healthy blood donors has suggested the mean value ±standard deviation (SD) of serum neopterin levels of 9.7 ±5.0 nM in persons aged 75 years and above [12]. Consistent with these reports, older adults included in the present study had a mean ±SD of 9.66 ±4.21 nM. The currently observed results—that frail participants had significantly higher neopterin levels than non-frail controls after adjusting for age and other potential confounding factors—indicate further elevation of neopterin levels in the syndrome of frailty above and beyond age-related changes. The observed elevation in neopterin levels suggests immune activation and increased monocyte/macrophage activities in frailty. Such monocyte–macrophage-mediated immune activation is supported, at least in part, by our previous findings that compared with their age-, race- and sex-matched non-frail controls; frail older adults had high monocyte counts and upregulated ex vivo expression of pro-inflammatory chemokine CXCL10 by unstimulated monocytes and several additional stress-responsive genes after brief ex vivo stimulation with LPS [8–10]. The neopterin levels observed in the frail elderly in this study appeared to be not as markedly elevated as those reported in acute viral infections, such as acute cytomegalovirus infection (>13 nM) [25] and human immunodeficiency virus infection (>16 nM) [26] or in active systemic lupus erythematosus (>15 nM) [27]. Additional studies are needed to further investigate the mechanism and regulation of neopterin production and monocyte/macrophage-mediated immune regulation in frailty.
A number of studies have observed significant association of elevated IL-6 levels with frailty in older adults [5–7, 28, 29]. Results from the present study were consistent with this observation (Table 2, Model II). IL-6 is a well-known pro-inflammatory that is produced by the immune system cells including monocytes and macrophages and a variety of other cell types [29]. Significant correlation between neoperin and IL-6 levels identified in this study, coupled with age-related increase in neopterin or IL-6 levels observed in other studies [13, 29], may suggest monocyte/macrophage activation leading to increased production of both neopterin and IL-6 in older adults. The results that the association of neopterin with frailty remained significant after adjusting for IL-6 (Table 2, Model III) indicates that such association is independent of IL-6. On the other hand, the data that the association between IL-6 and frailty was no longer statistically significant after adjusting for neopterin may suggest monocyte/macrophage-mediated immune activation marked by elevated neopterin levels as a potentially important mechanism that mediates IL-6-marked inflammation and its contribution to frailty.
The following limitations should be considered. First, the relatively small sample size may limit the study power. For example, that the ORs of being frail had large 95% CIs when log(neopterin) and log(IL-6) modelled as continuous variables is likely due to a limited sample size and data variability. This was partly addressed by modelling neopterin and IL-6 levels in tertiles. In addition, that the OR of being prefrail for top tertile of neopterin was marginally significant is also likely due to the limited sample size. Secondly, except for the pre-established exclusion criteria, other medical conditions that are common in older adult population were not excluded and they were not included in the statistical models. However, as this study was designed to evaluate associations of immune activation and inflammation with frailty, it was not intended to adjust for common comorbid conditions, because they may have significant contributions to immune activation and inflammation in frail older adults. Lastly, the directionality of the observed associations between elevated neopterin and frailty could not be determined in this cross-sectional study. Despite these limitations, results from this study support the original hypothesis and suggest significant monocyte/macrophage-mediated immune activation in frail elderly living in the community, independent of IL-6 levels. These findings provide a basis for further mechanistic and longitudinal investigations into the underlying immune mechanisms that contribute to frailty, based on which potential interventional strategies can be developed for the prevention and treatment of this important and common geriatric syndrome in the future.
Conclusion
In the study sample of community-dwelling older adults, serum neopterin levels had stepwise increase across the spectrum of the frailty syndrome, from non-frail to prefrail and frail. Multivariate regression analyses demonstrated that elevated neopterin levels conferred significantly higher ORs for prevalent frailty, independent of IL-6 levels. These findings provide initial evidence supportive of potential monocyte/macrophage-mediated immune activation in this clinical syndrome of old age.
Key points.
This study demonstrates significant cross-sectional association between elevated neopterin levels and prevalent frailty in a sample of community-dwelling older adults.
The association between neopterin and frailty is independent of IL-6 levels.
Neopterin is a marker of monocyte/macrophage-mediated immune activation and its elevation may suggest such immune activation in the geriatric syndrome of frailty.
Conflict of interest
None declared.
Funding
S.X.L. is a current recipient of the Paul Beeson Career Development Award in Aging Research, K23 AG028963, (supported by the National Institute on Aging, the American Federation for Aging Research, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation and an anonymous donor). A.M. was supported by training award T32 AG000120 from the National Institute on Aging. N.S.F. is supported by United States Department of Defense grant W81XWH-11-1-0239 and National Institutes of Health grant UL1 RR025005. The Clinical Research Unit at Johns Hopkins Bayview Medical Center is supported by grant UL1 RR025005 from the National Center for Research Resources, a component of the NIH and NIH Roadmap for Medical Research. The funding bodies had no role in study design, data collection and analysis, writing of the manuscript, or in the decision to submit the paper for publication.
References
- 1.Fried LP, Tangen C, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M1–11. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:115–25. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–57. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 5.Leng S, Chaves P, Koenig K, et al. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–71. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 6.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–7. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 7.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–71. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 8.Leng S, Xue Q, Tian J. Association of neutrophil and monocyte counts with fraily in community-dwelling older women. Exp Gerontol. 2009;44:511–6. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Qu T, Yang H, Walston JD, et al. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009;46:319–24. doi: 10.1016/j.cyto.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu T, Walston JD, Yang H, et al. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130:161–6. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murr C, Widner B, Wirleitner B, et al. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–87. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 12.Prof Dietmar Fuchs. Immune Activation Marker Neopterin. scitopoics com Available at <www.scitopics.com/Immune_activation_marker_neopterin.html. > (accessed 7 August 2008)
- 13.Spencer ME, Jain A, Matteini A, et al. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI and percentage body fat. J Gerontol A Biol Sci Med Sci. 2010;65:858–65. doi: 10.1093/gerona/glq066. 10.1093/gerona/glq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reibnegger G, Huber LA, Jurgens G, et al. Approach to define ‘normal aging' in man. Immune function, serum lipids, lipoproteins and neopterin levels. Mech Ageing Dev. 1988;46:67–82. doi: 10.1016/0047-6374(88)90115-7. [DOI] [PubMed] [Google Scholar]
- 15.Frick B, Schroecksnadel K, Neurauter G, et al. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–7. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Fahey JL, Schnelle JF, Boscardin J, et al. Distinct categories of immunologic changes in frail elderly. Mech Ageing Dev. 2000;115:1–20. doi: 10.1016/s0047-6374(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 17.Solichova D, Melichar B, Blaha V, et al. Biochemical profile and survival in nonagenarians. Clin Biochem. 2001;34:563–9. doi: 10.1016/s0009-9120(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 18.Murr C, Hainz U, Asch E, et al. Association of increased neopterin production with decreased humoral immunity in the elderly. Exp Gerontol. 2003;38:583–7. doi: 10.1016/s0531-5565(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 19.Nubling CM, Chudy M, Volkers P, et al. Neopterin levels during the early phase of human immunodeficiency virus, hepatitis C virus, or hepatitis B virus infection. Transfusion. 2006;46:1886–91. doi: 10.1111/j.1537-2995.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 20.Nasonov EL, Samsonov MI, Tilz G, et al. [Neopterin: new immunological marker of autoimmune rheumatic disease] Klin Med (Mosk) 2000;78:43–6. [PubMed] [Google Scholar]
- 21.von Ingersleben G, Souchon R, Fitzner R. Serum neopterin levels in lung and breast cancer patients undergoing radiotherapy and/or chemotherapy. Int J Biol Markers. 1988;3:135–9. [PubMed] [Google Scholar]
- 22.Punjabi NM, Beamer BA, Jain A, et al. Elevated levels of neopterin in sleep-disordered breathing. Chest. 2007;132:1124–30. doi: 10.1378/chest.07-0743. [DOI] [PubMed] [Google Scholar]
- 23.Avanzas P, rroyo-Espliguero R, Cosin-Sales J, et al. Markers of inflammation and multiple complex stenoses (pancoronary plaque vulnerability) in patients with non-ST segment elevation acute coronary syndromes. Heart. 2004;90:847–52. doi: 10.1136/hrt.2003.015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takabatake N, Sata M, Abe S, et al. Impaired systemic cell-mediated immunity and increased susceptibility to acute respiratory tract infections in patients with COPD. Respir Med. 2005;99:485–92. doi: 10.1016/j.rmed.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Schennach H, Hessenberger G, Mayersbach P, et al. Acute cytomegalovirus infections in blood donors are indicated by increased serum neopterin concentrations. Med Microbiol Immunol. 2002;191:115–8. doi: 10.1007/s00430-002-0148-8. [DOI] [PubMed] [Google Scholar]
- 26.Mildvan D, Spritzler J, Grossberg SE, et al. Serum neopterin, an immune activation marker, independently predicts disease progression in advanced HIV-1 infection. Clin Infect Dis. 2005;40:853–8. doi: 10.1086/427877. [DOI] [PubMed] [Google Scholar]
- 27.Samsonov MY, Tilz GP, Egorova O, et al. Serum soluble markers of immune activation and disease activity in systemic lupus erythematosus. Lupus. 1995;4:29–32. doi: 10.1177/096120339500400107. [DOI] [PubMed] [Google Scholar]
- 28.Schmaltz HN, Fried LP, Xue QL, et al. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–54. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 29.Maggio M, Guralnik JM, Longo DL, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]